Spatiotemporal Variation in Environmental Vibrio cholerae in an Estuary in Southern Coastal Ecuador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Collection of Water Samples and Environmental Measurements
2.3. Laboratory Analyses
2.4. DNA Isolation and PCR
2.5. Statistical Analyses
3. Results
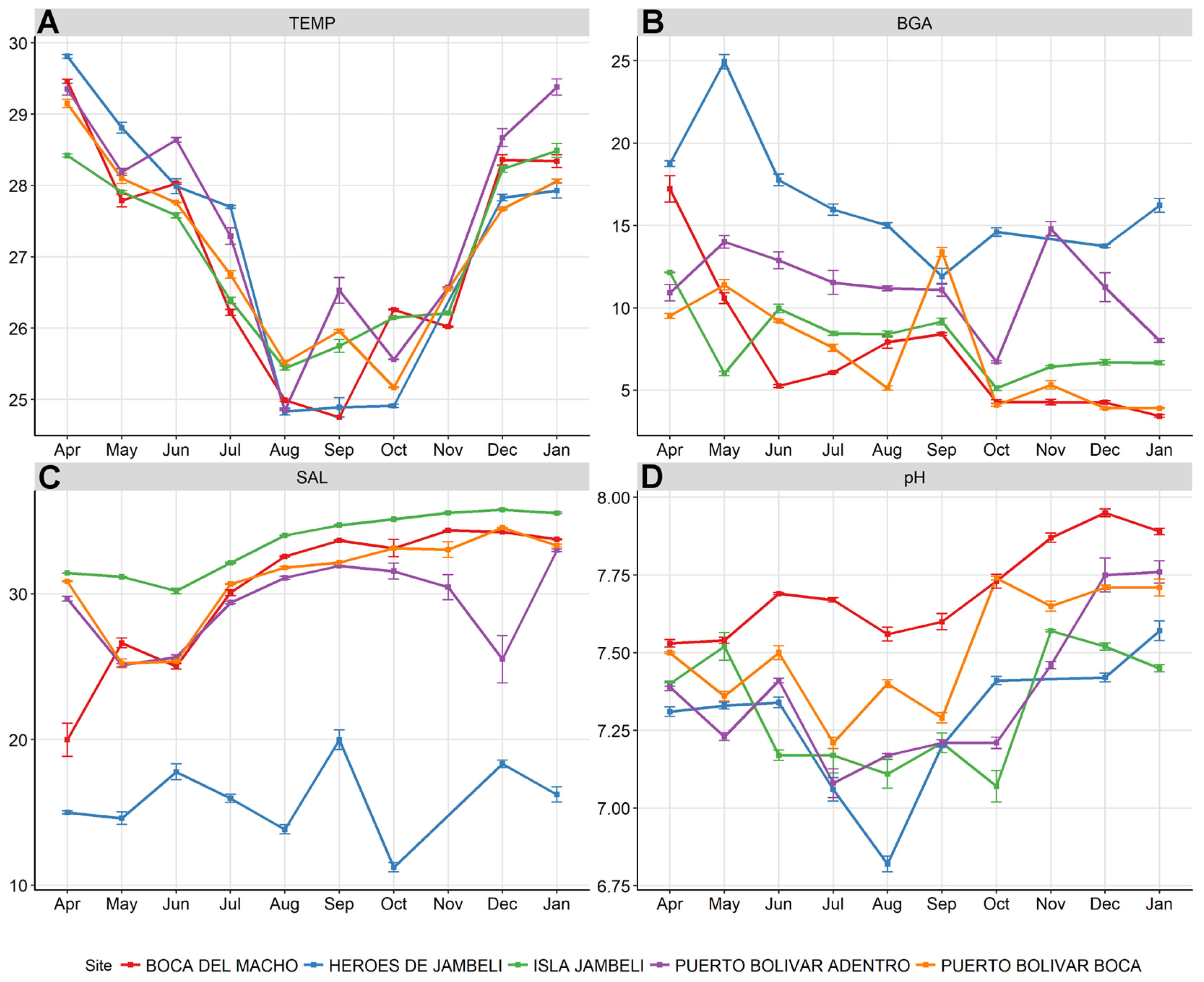
3.1. Water Sample Environmental Measurements
3.2. Laboratory Analyses
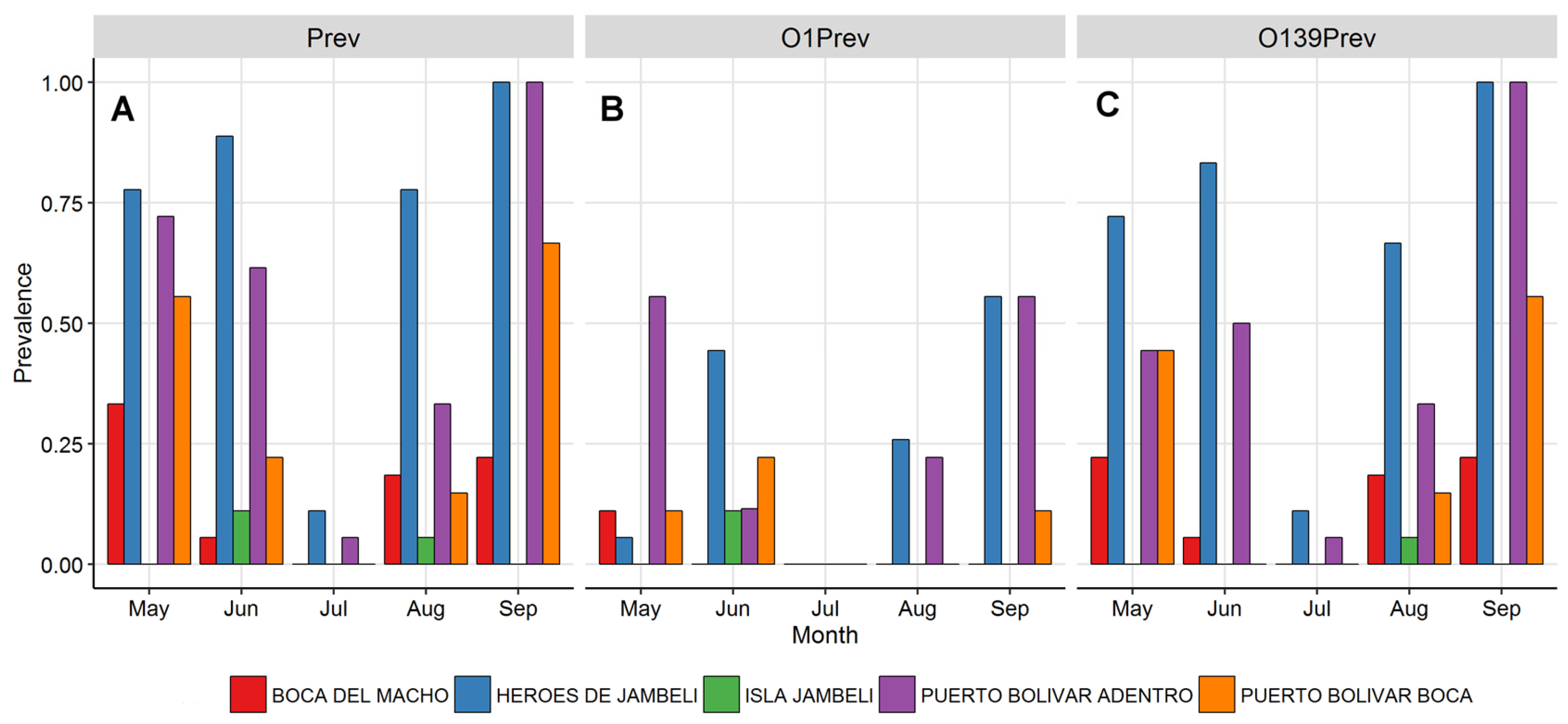
3.3. Vibrio cholerae Prevalence
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO. Cholera Annual Report. Wkly. Epidemiol. Rec. 2013, 88, 321–336. [Google Scholar]
- Ali, M.; Nelson, A.R.; Lopez, A.L.; Sack, D.A. Updated global burden of cholera in endemic countries. PLoS Negl. Trop. Dis. 2015, 9, E0003832. [Google Scholar] [CrossRef] [PubMed]
- Jutla, A.S.; Akanda, A.S.; Islam, S. Tracking Cholera in Coastal Regions Using Satellite Observations1. J. Am. Water Resour. Assoc. 2010, 46, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Jutla, A.; Akanda, A.S.; Huq, A.; Faruque, A.S.G.; Colwell, R.R.; Islam, S. A water marker monitored by satellites to predict seasonal endemic cholera. Remote Sens. Lett. 2013, 4, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Islam, M.S.; Mahmud, Z.H.; Cairncross, S.; Clemens, J.D.; Collins, A.E. Role of phytoplankton in maintaining endemicity and seasonality of cholera in Bangladesh. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Lipp, E.K.; Huq, A.; Colwell, R.R. Effects of global climate on infectious disease: The cholera model. Clin. Microbiol. Rev. 2002, 15, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.E.; Ryan, S.J.; Stewart-Ibarra, A.M.; Finkelstein, J.L.; King, C.A.; Qiao, H.; Polhemus, M.E. A global map of suitability for coastal Vibrio cholerae under current and future climate conditions. Acta Trop. 2015, 149, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.R.; Nur, Z.; Hassan, N.; von Seidlein, L.; Dunachie, S. Pandemics, pathogenicity and changing molecular epidemiology of cholera in the era of global warming. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Hallegatte, S.; Green, C.; Nicholls, R.J.; Corfee-Morlot, J. Future flood losses in major coastal cities. Nat. Clim. Chang. 2013, 3, 802–806. [Google Scholar] [CrossRef]
- Hanson, S.; Nicholls, R.; Ranger, N.; Hallegatte, S.; Corfee-Morlot, J.; Herweijer, C.; Chateau, J. A global ranking of port cities with high exposure to climate extremes. Clim. Chang. 2011, 104, 89–111. [Google Scholar] [CrossRef] [Green Version]
- De Sherbinin, A.; Schiller, A.; Pulsipher, A. The vulnerability of global cities to climate hazards. Environ. Urban. 2007, 19, 39–64. [Google Scholar] [CrossRef]
- Dixon, T.H.; Amelung, F.; Ferretti, A.; Novali, F.; Rocca, F.; Dokka, R.; Sella, G.; Kim, S.-W.; Wdowinski, S.; Whitman, D. Space geodesy: Subsidence and flooding in New Orleans. Nature 2006, 441, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, R.J. Coastal megacities and climate change. GeoJournal 1995, 37, 369–379. [Google Scholar] [CrossRef]
- Cai, W.; Borlace, S.; Lengaigne, M.; Van Rensch, P.; Collins, M.; Vecchi, G.; Timmermann, A.; Santoso, A.; McPhaden, M.J.; Wu, L.; et al. Increasing frequency of extreme El Niño events due to greenhouse warming. Nat. Clim. Chang. 2014, 4, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Colwell, R.R. Infectious disease and environment: Cholera as a paradigm for waterborne disease. Int. Microbiol. 2004, 7, 285–289. [Google Scholar] [PubMed]
- Colwell, R.R.; Huq, A. Environmental reservoir of Vibrio cholerae the causative agent of cholera. Ann. N. Y. Acad. Sci. 1994, 740, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Mutreja, A.; Kim, D.W.; Thomson, N.R.; Connor, T.R.; Lee, J.H.; Kariuki, S.; Croucher, N.J.; Choi, S.Y.; Harris, S.R.; Lebens, M.; et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 2011, 477, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.R. Global climate and infectious disease: The cholera paradigm. Science 1996, 274, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Pruzzo, C.; Huq, A.; Colwell, R.R. Environmental reservoirs of Vibrio cholerae and their role in cholera. Environ. Microbiol. Rep. 2010, 2, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rossel, F.; Cadier, E.; Gómez, G. Las inundaciones en la zona costera ecuatoriana: Causas—Obras de protección existentes y previstas. Bull. Inst. Fr. Ét. Andines 1996, 25, 399–420. [Google Scholar]
- Rossel, F.; Cadier, E. El Niño and prediction of anomalous monthly rainfalls in Ecuador. Hydrol. Process. 2009, 23, 3253–3260. [Google Scholar] [CrossRef]
- Lacey, S.W. Cholera: Calamitous past, ominous future. Clin. Infect. Dis. 1995, 20, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Malavade, S.S.; Narvaez, A.; Mitra, A.; Ochoa, T.; Naik, E.; Sharma, M.; Galwankar, S.; Breglia, M.; Izurieta, R. Cholera in Ecuador: Current relevance of past lessons learnt. J. Glob. Infect. Dis. 2011, 3, 189–194. [Google Scholar] [PubMed]
- Swerdlow, D.L.; Mintz, E.D.; Rodriguez, M.; Tejada, E.; Ocampo, C.; Espejo, L.; Barrett, T.J.; Petzelt, J.; Bean, N.H.; Seminario, L.; et al. Severe life-threatening cholera associated with blood group 0 in Peru: Implications for the Latin American epidemic. J. Infect. Dis. 1994, 170, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.J.; Harris, J.B.; Morris, J.G.; Calderwood, S.B.; Camilli, A. Cholera transmission: The host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 2009, 7, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.N.; Rombo, L.; Fisch, A. The true burden and risk of cholera: Implications for prevention and control. Lancet Infect. Dis. 2007, 7, 521–530. [Google Scholar] [CrossRef]
- WHO. Cholera Unveiled; Global Task Force on Cholera Control, World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Sedas, V.T.P. Influence of environmental factors on the presence of Vibrio cholerae in the marine environment: A climate link. J. Infect. Dev. Ctries. 2007, 1, 224–241. [Google Scholar] [PubMed]
- Akanda, A.S.; Jutla, A.S.; Gute, D.M.; Sack, R.B.; Alam, M.; Huq, A.; Colwell, R.R.; Islam, S. Population vulnerability to biannual cholera outbreaks and associated macro-scale drivers in the Bengal delta. Am. J. Trop. Med. Hyg. 2013, 89, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Yamasaki, S.; Mukhopadhyay, A.K.; Chakraborty, S.; Basu, A.; Bhattacharya, S.K.; Nair, G.B.; Shimada, T.; Takeda, Y. Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139. FEMS Immunol. Med. Microbiol. 1998, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Peter, W.A.; Thomas, S. Rapid detection of virulence-associated genes in environmental strains of Vibrio cholerae by multiplex PCR. Curr. Microbiol. 2010, 60, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.V.; Isac, S.R.; Colwell, R.R. Development of a hexaplex PCR assay for rapid detection of virulence and regulatory genes in Vibrio cholerae and Vibrio mimicus. J. Clin. Microbiol. 2002, 40, 4321–43224. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Maechler, M.; Bolker, B.M. lme4: Linear Mixed-Effects Models Using S4 Classes (R Package Version 0.99999-0). 2012. Available online: http://cran.r-project.org/package=lme4 (accessed on 29 January 2018).
- Faruque, S.M.; Ahmed, K.M.; Abdul, A.R.A.; Qadri, F.; Siddique, A.K.; Albert, M.J. Emergence of a new clone of toxigenic Vibrio cholerae O1 biotype ElTor displacing V. cholerae O139 Bengal in Bangladesh. J. Clin. Microbiol. 1997, 35, 624–630. [Google Scholar] [PubMed]
- Jiang, S.C.; Matte, M.; Matte, G.; Huq, A.; Colwell, R.R. Genetic diversity of clinical and environmental isolates of Vibrio cholerae determined by amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 2000, 66, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Strutton, P.G.; Ryan, J.P.; Chavez, F.P. Enhanced chlorophyll associated with tropical instability waves in the equatorial Pacific. Geophys. Res. Lett. 2001, 28, 2005–2008. [Google Scholar] [CrossRef]
- Montilla, R.; Chowdhury, M.; Huq, A.; Xu, B.; Colwell, R.R. Serogroup conversion of Vibrio cholerae non-O1 to Vibrio cholerae O1: Effect of growth state of cells, temperature, and salinity. Can. J. Microbiol. 1996, 42, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.; Bazine, G. Phototrophic prokaryotes: The cyanobacteria. Annu. Rev. Microbiol. 1977, 31, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Epstein, P.R. Algal blooms in the spread and persistence of cholera. Biosystems 1993, 31, 209–221. [Google Scholar] [CrossRef]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Elliott, J. The seasonal sensitivity of cyanobacteria and other phytoplankton to changes in flushing rate and water temperature. Glob. Chang. Biol. 2010, 16, 864–876. [Google Scholar] [CrossRef]
- Huber, V.; Wagner, C.; Gerten, D.; Adrian, R. To bloom or not to bloom: Contrasting responses of cyanobacteria to recent heat waves explained by critical thresholds of abiotic drivers. Oecologia 2012, 169, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A. Predicting the impact of changing nutrient load and temperature on the phytoplankton of England’s largest lake, Windermere. Freshw. Biol. 2012, 57, 400–413. [Google Scholar] [CrossRef] [Green Version]
- Robarts, R.D.; Zohary, T. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. N. Z. J. Mar. Freshw. Res. 1987, 21, 391–399. [Google Scholar] [CrossRef]
- Stal, L.J.; Albertano, P.; Bergman, B.; von Bröckel, K.; Gallon, J.R.; Hayes, P.K.; Sivonen, K.; Walsby, A.E. BASIC: Baltic Sea cyanobacteria. An investigation of the structure and dynamics of water blooms of cyanobacteria in the Baltic Sea—responses to a changing environment. Cont. Shelf Res. 2003, 23, 1695–1714. [Google Scholar] [CrossRef]
- Paerl, H.W. Nuisance phytoplankton blooms in coastal, estuarine, and inland waters. Limnol. Oceanogr. 1988, 33, 823–843. [Google Scholar] [CrossRef]
- Kanoshina, I.; Lips, U.; Leppänen, J.-M. The influence of weather conditions (temperature and wind) on cyanobacterial bloom development in the Gulf of Finland (Baltic Sea). Harmful Algae 2003, 2, 29–41. [Google Scholar] [CrossRef]
- Suikkanen, S.; Laamanen, M.; Huttunen, M. Long-term changes in summer phytoplankton communities of the open northern Baltic Sea. Estuar. Coast. Shelf Sci. 2007, 71, 580–592. [Google Scholar] [CrossRef]
- Wasmund, N. Occurrence of cyanobacterial blooms in the Baltic Sea in relation to environmental conditions. Int. Rev. Gesamten. Hydrobiol. Hydrogr. 1997, 82, 169–184. [Google Scholar] [CrossRef]
- Pan American Health Organization/World Health Organization. Epidemiological Update: Cholera; PAHO/WHO: Washington, DC, USA, 2016. [Google Scholar]


| Set | Primer | Sequence | Product | Reference |
|---|---|---|---|---|
| 1 | O1F2-1 | GTT TCA CTG AAC AGA TGG G | 192 bp | Hoshino et al. [30] |
| O1R2-2 | CGG TCA TCT GTA AGT ACA AC | |||
| 2 | O139F2 | AGC CTC TTT ATT ACG GGT GG | 449 bp | |
| O139R2 | GTC AAA CCC GAT CGT AAA GG | |||
| 3 | tcpA-F | ATG CAA TTA TTA AAA CAG CTT TTT AAG | 675 bp | Kumar et al. [31] |
| tcpA-R | TTA GCT GTT ACC AAA TGC AAC AG | |||
| 4 | ctxA-F | CGG GCA GAT TCT AGA CCT CCT G | 564 bp | Singh et al. [32] |
| ctxA-R | CGA TGA TCT TGG AGC ATT CCC AC |
| Environmental Variable | Site | χ2 | DF | p-Value |
|---|---|---|---|---|
| Temperature | Boca de Macho | 832.65 | 9 | <0.0001 |
| Héroes de Jambelí | 643.85 | 8 | <0.0001 | |
| Isla de Jambelí | 622.85 | 9 | <0.0001 | |
| Puerto Bolívar Adentro | 445.99 | 9 | <0.0001 | |
| Puerto Bolívar Boca | 625.44 | 9 | <0.0001 | |
| Salinity | ||||
| Boca de Macho | 837.16 | 9 | <0.0001 | |
| Héroes de Jambelí | 230.17 | 8 | <0.0001 | |
| Isla de Jambelí | 671.41 | 9 | <0.0001 | |
| Puerto Bolívar Adentro | 464.85 | 9 | <0.0001 | |
| Puerto Bolívar Boca | 619.21 | 9 | <0.0001 | |
| pH | ||||
| Boca de Macho | 534.3 | 9 | <0.0001 | |
| Héroes de Jambelí | 431.66 | 8 | <0.0001 | |
| Isla de Jambelí | 245.91 | 9 | <0.0001 | |
| Puerto Bolívar Adentro | 378.53 | 9 | <0.0001 | |
| Puerto Bolívar Boca | 416.76 | 9 | <0.0001 | |
| BGA | ||||
| Boca de Macho | 650.84 | 9 | <0.0001 | |
| Héroes de Jambelí | 309.2 | 8 | <0.0001 | |
| Isla de Jambelí | 469.75 | 9 | <0.0001 | |
| Puerto Bolívar Adentro | 219.78 | 9 | <0.0001 | |
| Puerto Bolívar Boca | 519.81 | 9 | <0.0001 |
| Environmental Variable | Prevalence | Estimate (SE) | z | p-Value |
|---|---|---|---|---|
| Temperature | V. cholerae | −0.60 (0.19) | −3.20 | 0.001 |
| Strain O1 | −0.21 (0.22) | −0.93 | 0.35 | |
| Strain O139 | −0.68 (0.19) | −3.67 | <0.001 | |
| Salinity | V. cholerae | −0.13 (0.06) | −2.27 | 0.02 |
| Strain O1 | −0.10 (0.07) | −1.47 | 0.14 | |
| Strain O139 | −0.09 (0.06) | −1.45 | 0.15 | |
| pH | V. cholerae | 3.45 (1.33) | 2.60 | 0.01 |
| Strain O1 | 1.14 (1.65) | 0.68 | 0.49 | |
| Strain O139 | 3.86 (1.35) | 2.86 | 0.004 | |
| BGA | V. cholerae | 0.27 (0.06) | 4.24 | <0.0001 |
| Strain O1 | 0.005 (0.07) | 0.07 | 0.94 | |
| Strain O139 | 0.23 (0.06) | 3.70 | <0.0001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryan, S.J.; Stewart-Ibarra, A.M.; Ordóñez-Enireb, E.; Chu, W.; Finkelstein, J.L.; King, C.A.; Escobar, L.E.; Lupone, C.; Heras, F.; Tauzer, E.; et al. Spatiotemporal Variation in Environmental Vibrio cholerae in an Estuary in Southern Coastal Ecuador. Int. J. Environ. Res. Public Health 2018, 15, 486. https://doi.org/10.3390/ijerph15030486
Ryan SJ, Stewart-Ibarra AM, Ordóñez-Enireb E, Chu W, Finkelstein JL, King CA, Escobar LE, Lupone C, Heras F, Tauzer E, et al. Spatiotemporal Variation in Environmental Vibrio cholerae in an Estuary in Southern Coastal Ecuador. International Journal of Environmental Research and Public Health. 2018; 15(3):486. https://doi.org/10.3390/ijerph15030486
Chicago/Turabian StyleRyan, Sadie J., Anna M. Stewart-Ibarra, Eunice Ordóñez-Enireb, Winnie Chu, Julia L. Finkelstein, Christine A. King, Luis E. Escobar, Christina Lupone, Froilan Heras, Erica Tauzer, and et al. 2018. "Spatiotemporal Variation in Environmental Vibrio cholerae in an Estuary in Southern Coastal Ecuador" International Journal of Environmental Research and Public Health 15, no. 3: 486. https://doi.org/10.3390/ijerph15030486





