Mechanisms of Geomagnetic Field Influence on Gene Expression Using Influenza as a Model System: Basics of Physical Epidemiology
Abstract
:1. Introduction
- virus evolution dynamics;
- host’s sensitivity;
- conditions for viral transmission, etc.
- To bring attention to periodicity as a common feature of numerous biological processes and to discuss the nature of corresponding regulatory influences;
- To show theoretical possibility of bio-regulatory effects of magnetic fields;
- To outline some signaling pathways capable of implementing bio-regulatory (including genome-regulatory) functions of electromagnetic fields;
- To summarize our knowledge about Geomagnetic field, its principle parameters and sources of variation;
- To review possible evidences of regulatory influence of Solar cycles and corresponding Geomagnetic field perturbations on flu epidemic process;
- To describe probable mechanisms of Solar cycles and Geomagnetic field regulatory influences on virus-host interactions and other biological processes.
2. Periodicity as a Common Feature of Numerous Biological Processes and Nature of Corresponding Regulatory Influences
- - pacemaker;
- - regulatory signals emitted by pacemaker;
- - receptors of the regulatory signals in the controlled constituent.
- - in most evident cases it is solar light, which represent electromagnetic waves of definite wavelength, and has numerous biological effects, including regulation of circadian rhythms in living beings.
- - in the case of cycles of Solar activity it is alterations of the Earth’s Magnetic field, caused by fluctuations in levels of solar ionizing radiations and solar energy output (Figure 1).
- - it is also worthy to mention a slowly-varying Microwave emission from the solar corona.
3. Theoretical Possibility and Evidences of Bio-Regulatory Effects of Weak Magnetic Fields
- - electrical fields are able to interact with charged molecules, surfaces and electric dipoles of biomolecules, and
- - magnetic fields can interact with magnetic dipoles of electron spins, whose carriers are paramagnetic molecules, metal ions and ion-radicals [35].
- Mechanisms following the “plasma membrane hypothesis”, which proposes that the cell membrane is a primary biological receiver of magnetic signals, in that it responds to magnetic field influences by changes of its potential, and modulates the distribution and activity of integral membrane proteins and ion channels (e.g., Ca2+ channels). However, the primary molecular interaction remains unclear.
- Free radical mechanisms. The basis of these mechanisms is the phenomenon that magnetic fields can increase the lifetime of free radicals, i.e., stabilize them for longer. This results in an increase in free radical concentration in cell compartments, and hence biological changes including activation of signaling cascades (reviewed in [33]).
3.1. Radical Pairs and Pair Radical Reactions
3.2. Key Features of a Radical Pair Magnetoreceptor
4. Signaling Pathways Capable of Implementing Bio-Regulatory (Including Genome-Regulatory) Functions of Magnetic Fields
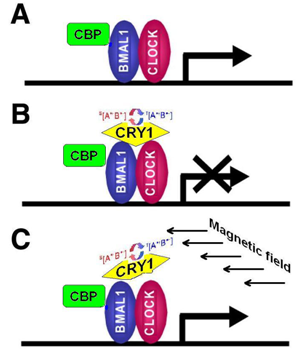
4.1. Cryptochromes: Ancient Regulatory Proteins Sensitive to Electromagnetic Radiation and Magnetic Fields
4.2. Cryptochrome-Mediated Pathways and Biological Effects
4.3. Ca2+-Operated Pathways and Ion Resonance Effects
5. Brief Summary of Our Knowledge about Geomagnetic Field, its Principle Parameters and Sources of Variation
6. Possible Evidence of Regulatory Influence of Solar Cycles and Corresponding Geomagnetic Field Perturbations on Flu Epidemic Process
- Occurrence of major influenza epidemics shows definite periodicity with an average period of 11, 3 years, equal to the period of Solar activity fluctuations;
- As a rule, significant influenza epidemics do not occur in years of minimum solar activity;
- Most major influenza epidemics occurred in time interval starting 2–3 years before and ending 2–3 years after solar activity maxima.
7. Probable Mechanisms of the Geomagnetic Field and Solar Cycles Influences on Biological Functions and Virus-Host Interactions
- - decrease influenza virus production in infected cells and viral gene expression;
- - block early stages of the influenza virus life cycle;
- - specifically decrease the vRNA level during influenza virus infection [98].
8. Conclusions
- Perturbations of cosmic plasma flow (such called star or solar “wind”) caused by Solar activity alterations induce periodic changes in the geomagnetic field that is among immediate regulatory signals for Solar-correlated cycles in Biosphere, including cyclic modulation in gene expression patterns of living beings. This solar activity-dependent regulation of gene expression can clearly lead to immunological, epidemiological and other consequences.
- Among the universal biological “antennae” of the magnetic regulatory signals, it is important to include proteins of the Cryptochrome family and Ca2+ signaling pathways. Cryptochromes can function as “epigenetic sensors” of the geomagnetic field fluctuations, the magnetic field-sensitive part of the epigenetic regulatory mechanism.
- The radical pair mechanism of magnetoreception (effects related to spin chemistry) can account for high magnetic responsiveness of Cryptochromes, which incorporates the radical pairs in their functionally active sites.
- CRY are transcriptional repressors of the major circadian complex CLOCK/BMAL1, therefore magnetic fields via modulation of CRY function can influence circadian gene expression, modify activity of NF-κB- and glucocorticoids-dependent signaling pathways.
- The pattern of stress-induced gene expression and organismal response to stress will vary depending on the functional activity of Cryptochromes, which in turn may be regulated by magnetic fields and, correspondingly—by solar activity cycles.
- We hypothesize that solar cycles are able to both regulate, entrain processes of biological microevolution and to tune biological rhythms (bio-clocks) in living beings implementing mechanisms stated above.
Abbreviations:
| EM | electromagnetic |
| EMF— | electromagnetic fields |
| ELF EMF— | extremely low frequency EMF |
| CRY— | Cryptochromes |
| CBP— | CREB binding protein |
Acknowledgments
References and Notes
- Chizhevsky, AL. The Terrestrial Echo of Solar Storms; Mysl: Moscow, Russia, 1976; p. 366. [Google Scholar]
- Lev Bar-Or, R; Maya, R; Segel, LA; Alon, U; Levine, AJ; Oren, M. Generation of oscillations by the p53-Mdm2 feedback loop: a theoretical and experimental study. Proc. Natl. Acad. Sci. USA 2000, 97, 11250–11255. [Google Scholar]
- Hoffmann, A; Levchenko, A; Scott, ML; Baltimore, D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science 2002, 298, 1241–1245. [Google Scholar]
- Pourquie, O. The segmentation clock: converting embryonic time into spatial pattern. Science 2003, 301, 328–330. [Google Scholar]
- Paranjpe, DA; Sharma, VK. Evolution of temporal order in living organisms. J. Circadian Rhythms 2005, 3, 7. [Google Scholar]
- Goldbeter, A. Biological rhythms: clocks for all times. Curr. Biol 2008, 18, R751–R753. [Google Scholar]
- Tromp, SW. Possible causes of short and long periodical changes in blood sedimentation rate patterns in the Netherlands, and their possible significance for the prediction of influenza epidemics. Biol. Rhythm Res 1971, 2, 313–314. [Google Scholar]
- Lucke, C; Hehrmann, R; von Mayersbach, K; von zur Muhlen, A. Studies on circadian variations of plasma TSH, thyroxine and triiodothyronine in man. Acta Endocrinol. (Copenh.) 1977, 86, 81–88. [Google Scholar]
- Li, Y; Lu, D; Ge, J; Zhuo, Y; Sears, ML. Identified circadian rhythm genes of ciliary epithelium with differential display. Yan Ke Xue Bao 2001, 17, 133–137. [Google Scholar]
- Zhadin, MN. Review of russian literature on biological action of DC and low-frequency AC magnetic fields. Bioelectromagnetics 2001, 22, 27–45. [Google Scholar]
- Neronov, VV; Malkhazova, SM; Ponirovskii, EN; Charyev, B. The multiyear changes in the epidemic activity of the foci of zoonotic cutaneous leishmaniasis at the Murgab oasis. I. An analysis of the relations of morbidity to heliogeophysical factors. Med. Parazitol. (Mosk.) 1996, 3, 3–7. [Google Scholar]
- Kamo, M; Sasaki, A. Evolution toward multi-year periodicity in epidemics. Ecol. Lett 2005, 8, 378–385. [Google Scholar]
- Kilbourne, ED. An explanation of the interpandemic antigenic mutability of influenza viruses. J. Infect. Dis 1973, 128, 668–670. [Google Scholar]
- Hope-Simpson, RE. Sunspots and flu: a correlation. Nature 1978, 275, 86. [Google Scholar]
- Ertel, S. Influenza pandemics and sunspots—easing the controversy. Naturwissenschaften 1994, 81, 308–311. [Google Scholar]
- Yeung, JW. A hypothesis: Sunspot cycles may detect pandemic influenza A in 1700–2000 A.D. Med. Hypotheses 2006, 67, 1016–1022. [Google Scholar]
- Vaquero, JM; Gallego, MC. Sunspot numbers can detect pandemic influenza A: the use of different sunspot numbers. Med. Hypotheses 2007, 68, 1189–1190. [Google Scholar]
- Ventura, C; Maioli, M; Asara, Y; Santoni, D; Mesirca, P; Remondini, D; Bersani, F. Turning on stem cell cardiogenesis with extremely low frequency magnetic fields. FASEB J 2005, 19, 155–157. [Google Scholar]
- McCaig, CD; Rajnicek, AM; Song, B; Zhao, M. Controlling cell behavior electrically: current views and future potential. Physiol. Rev 2005, 85, 943–978. [Google Scholar]
- Sage, C; Carpenter, D (Eds.) A Rationale for a Biologically-based Public Exposure Standard for Electromagnetic Fields (ELF and RF). BioInitiative Report.
- Carpenter, DO; Sage, C. Setting prudent public health policy for electromagnetic field exposures. Rev. Environ. Health 2008, 23, 91–117. [Google Scholar]
- Mycielska, ME; Djamgoz, MB. Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J. Cell Sci 2004, 117, 1631–1639. [Google Scholar]
- Slack, JM. The spark of life: electricity and regeneration. Sci STKE 2007, 405, pe54. [Google Scholar]
- Zaporozhan, VN; Ponomarenko, AI. Evidences of regulatory and signaling role of electromagnetic fields in biological objects (review of literature and own studies).
- McCaig, CD; Song, B; Rajnicek, AM. Electrical dimensions in cell science. J. Cell Sci 2009, 122, 4267–4276. [Google Scholar]
- Pulkkinen, T. “Space Weather: Terrestrial Perspective”. Living Rev. Solar Phys 2007, 4, 1. [Google Scholar]
- .
- Lupke, M; Frahm, J; Lantow, M; Maercker, C; Remondini, D; Bersani, F; Simko, M. Gene expression analysis of ELF-MF exposed human monocytes indicating the involvement of the alternative activation pathway. Biochim. Biophys. Acta 2006, 1763, 402–412. [Google Scholar]
- Maercker, C. In In vitro Gene Expression Studies and Their Impact on High Content Screening Assays in EMF Research, Application of Proteomics and Transcriptomics in EMF Research, Helsinki, Finland, Oct 30–Nov 1, 2005; Available online: www.cost281.org/download.php?fid=792 (accessed on 5 October 2009).
- Goldberg, RB; Creasey, WA. A review of cancer induction by extremely low frequency electromagnetic fields. Is there a plausible mechanism? Med. Hypotheses 1991, 35, 265–274. [Google Scholar]
- Wertheimer, N; Leeper, E. Adult cancer related to electrical wires near the home. Int. J. Epidemiol 1982, 11, 345–355. [Google Scholar]
- Ritz, T; Adem, S; Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J 2000, 78, 707–718. [Google Scholar]
- Simko, M. Cell type specific redox status is responsible for diverse electromagnetic field effects. Curr. Med. Chem 2007, 14, 1141–1152. [Google Scholar]
- Fursa, EY. Magnetic resonance as a channel of directed transmission of electromagnetic energy in animate nature.
- Buchachenko, AL; Kuznetsov, DA; Berdinskii, VL. New mechanisms of biological effects of electromagnetic fields. Biofizika 2006, 51, 545–552. [Google Scholar]
- Nagakura, SO; Hayashi, H; Azumi, T. Dynamic Spin Chemistry: Magnetic Controls and Spin Dynamics of Chemical Reactions; Wiley: New York, NY, USA, 1998; p. 297. [Google Scholar]
- Lednev, VV. Possible mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics 1991, 12, 71–75. [Google Scholar]
- Liboff, AR. Electric-field ion cyclotron resonance. Bioelectromagnetics 1997, 18, 85–87. [Google Scholar]
- Schulten, K; Swenberg, CE; Weller, A. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z. Phys. Chem 1978, 111, 1–5. [Google Scholar]
- Rodgers, CT; Hore, PJ. Chemical magnetoreception in birds: the radical pair mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 353–360. [Google Scholar]
- Jonah, CD; Madhava Rao, BS. Radiation Chemistry: Present Status and Future Trends, 1st ed; Elsevier: Amsterdam, The Netherland, 2001; p. 755. [Google Scholar]
- Hayashi, H; ebrary Inc. Introduction to dynamic spin chemistry magnetic field effects on chemical and biochemical reactions. In World Scientific Lecture and Course Notes in Chemistry; World Scientific: River Edge, NJ, USA, 2004; Volume 8. [Google Scholar]
- Eichwald, C; Walleczek, J. Model for magnetic field effects on radical pair recombination in enzyme kinetics. Biophys. J 1996, 71, 623–631. [Google Scholar]
- Izmaylov, AF; Tully, JC; Frisch, MJ. Relativistic interactions in the radical pair model of magnetic field sense in CRY-1 protein of Arabidopsis thaliana. J. Phys. Chem. A 2009, 113, 12276–12284. [Google Scholar]
- Brocklehurst, B. Magnetic fields and radical reactions: recent developments and their role in nature. Chem. Soc. Rev 2002, 31, 301–311. [Google Scholar]
- Ahmad, M; Galland, P; Ritz, T; Wiltschko, R; Wiltschko, W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta 2007, 225, 615–624. [Google Scholar]
- Harris, SR; Henbest, KB; Maeda, K; Pannell, JR; Timmel, CR; Hore, PJ; Okamoto, H. Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J. R. Soc. Interface 2009, 6, 1193–1205. [Google Scholar]
- Lin, C; Todo, T. The cryptochromes. Genome Biol 2005, 6, 220. [Google Scholar]
- Brudler, R; Hitomi, K; Daiyasu, H; Toh, H; Kucho, K; Ishiura, M; Kanehisa, M; Roberts, VA; Todo, T; Tainer, JA; Getzoff, ED. Identification of a new cryptochrome class. Structure, function, and evolution. Mol. Cell 2003, 11, 59–67. [Google Scholar]
- Partch, CL; Sancar, A. Photochemistry and photobiology of cryptochrome blue-light photopigments: the search for a photocycle. Photochem. Photobiol 2005, 81, 1291–1304. [Google Scholar]
- Solov’yov, IA; Chandler, DE; Schulten, K. Magnetic field effects inArabidopsis thaliana Cryptochrome-1. Biophys. J 2007, 92, 2711–2726. [Google Scholar]
- Zhu, H; Conte, F; Green, CB. Nuclear localization and transcriptional repression are confined to separable domains in the circadian protein Cryptochrome. Curr. Biol 2003, 13, 1653–1658. [Google Scholar]
- Chaves, I; Yagita, K; Barnhoorn, S; Okamura, H; van der Horst, GT; Tamanini, F. Functional evolution of the photolyase/cryptochrome protein family: importance of the C terminus of mammalian CRY1 for circadian core oscillator performance. Mol. Cell. Biol 2006, 26, 1743–1753. [Google Scholar]
- Allada, R; Emery, P; Takahashi, JS; Rosbash, M. Stopping time: the genetics of fly and mouse circadian clocks. Annu. Rev. Neurosci 2001, 24, 1091–1119. [Google Scholar]
- Cashmore, AR. Cryptochromes: enabling plants and animals to determine circadian time. Cell 2003, 114, 537–543. [Google Scholar]
- Kaushik, R; Nawathean, P; Busza, A; Murad, A; Emery, P; Rosbash, M. PER-TIM interactions with the photoreceptor cryptochrome mediate circadian temperature responses in Drosophila. PLoS Biol 2007, 5, e146. [Google Scholar]
- Panda, S; Hogenesch, JB. It’s all in the timing: many clocks, many outputs. J. Biol. Rhythms 2004, 19, 374–387. [Google Scholar]
- Langmesser, S; Tallone, T; Bordon, A; Rusconi, S; Albrecht, U. Interaction of circadian clock proteins PER2 and CRY with BMAL1 and CLOCK. BMC Mol. Biol 2008, 9, 41. [Google Scholar]
- Etchegaray, JP; Lee, C; Wade, PA; Reppert, SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 2003, 421, 177–182. [Google Scholar]
- Kondratov, RV; Shamanna, RK; Kondratova, AA; Gorbacheva, VY; Antoch, MP. Dual role of the CLOCK/BMAL1 circadian complex in transcriptional regulation. FASEB J 2006, 20, 530–532. [Google Scholar]
- Reiter, RJ. Static and extremely low frequency electromagnetic field exposure: reported effects on the circadian production of melatonin. J. Cell. Biochem 1993, 51, 394–403. [Google Scholar]
- Choi, YM; Jeong, JH; Kim, JS; Lee, BC; Je, HD; Sohn, UD. Extremely low frequency magnetic field exposure modulates the diurnal rhythm of the pain threshold in mice. Bioelectromagnetics 2003, 24, 206–210. [Google Scholar]
- Goodman, R; Weisbrot, D; Uluc, A; Henderson, A. Transcription in Drosophila melanogaster salivary gland cells is altered following exposure to low-frequency electromagnetic fields: analysis of chromosome 3R. Bioelectromagnetics 1992, 13, 111–118. [Google Scholar]
- Litovitz, TA; Montrose, CJ; Goodman, R; Elson, EC. Amplitude windows and transiently augmented transcription from exposure to electromagnetic fields. Bioelectromagnetics 1990, 11, 297–312. [Google Scholar]
- Wei, LX; Goodman, R; Henderson, A. Changes in levels of c-myc and histone H2B following exposure of cells to low-frequency sinusoidal electromagnetic fields: evidence for a window effect. Bioelectromagnetics 1990, 11, 269–272. [Google Scholar]
- Hirai, T; Yoneda, Y. Transcriptional regulation of neuronal genes and its effect on neural functions: gene expression in response to static magnetism in cultured rat hippocampal neurons. J. Pharmacol. Sci 2005, 98, 219–224. [Google Scholar]
- Barnes, FS; Greenebaum, B. Handbook of Biological Effects of Electromagnetic Fields Bioengineering and Biophysical Aspects of Electromagnetic Fields, 3rd ed; CRC/Taylor & Francis: Boca Raton, FL, USA, 2007; p. 440. [Google Scholar]
- Mellstrom, B; Savignac, M; Gomez-Villafuertes, R; Naranjo, JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol. Rev 2008, 88, 421–449. [Google Scholar]
- Bootman, MD; Fearnley, C; Smyrnias, I; MacDonald, F; Roderick, HL. An update on nuclear calcium signalling. J. Cell. Sci 2009, 122, 2337–2350. [Google Scholar]
- Mellstrom, B; Naranjo, JR. Mechanisms of Ca(2+)-dependent transcription. Curr. Opin. Neurobiol 2001, 11, 312–319. [Google Scholar]
- Savignac, M; Mellstrom, B; Naranjo, JR. Calcium-dependent transcription of cytokine genes in T lymphocytes. Pflugers Arch 2007, 454, 523–533. [Google Scholar]
- Nuccitelli, S; Cerella, C; Cordisco, S; Albertini, MC; Accorsi, A; de Nicola, M; D’Alessio, M; Radogna, F; Magrini, A; Bergamaschi, A; Ghibelli, L. Hyperpolarization of plasma membrane of tumor cells sensitive to antiapoptotic effects of magnetic fields. Ann. N. Y. Acad. Sci 2006, 1090, 217–225. [Google Scholar]
- Cho, MR; Thatte, HS; Silvia, MT; Golan, DE. Transmembrane calcium influx induced by ac electric fields. FASEB J 1999, 13, 677–683. [Google Scholar]
- Blackman, CF; Benane, SG; House, DE; Joines, WT. Effects of ELF (1–120 Hz) and modulated (50 Hz) RF fields on the efflux of calcium ions from brain tissuein vitro. Bioelectromagnetics 1985, 6, 1–11. [Google Scholar]
- Smith, SD; McLeod, BR; Liboff, AR; Cooksey, K. Calcium cyclotron resonance and diatom mobility. Bioelectromagnetics 1987, 8, 215–227. [Google Scholar]
- Liboff, AR. Electric polarization and the viability of living systems: ion cyclotron resonance-like interactions. Electromagn. Biol. Med 2009, 28, 124–134. [Google Scholar]
- Maercker, C.
- Walleczek, J. Electromagnetic field effects on cells of the immune system: the role of calcium signaling. FASEB J 1992, 6, 3177–3185. [Google Scholar]
- Conti, P; Gigante, GE; Alesse, E; Cifone, MG; Fieschi, C; Reale, M; Angeletti, PU. A role for Ca2+ in the effect of very low frequency electromagnetic field on the blastogenesis of human lymphocytes. FEBS Lett 1985, 181, 28–32. [Google Scholar]
- Flipo, D; Fournier, M; Benquet, C; Roux, P; Le Boulaire, C; Pinsky, C; LaBella, FS; Krzystyniak, K. Increased apoptosis, changes in intracellular Ca2+, and functional alterations in lymphocytes and macrophages after in vitro exposure to static magnetic field. J. Toxicol. Environ. Health A 1998, 54, 63–76. [Google Scholar]
- Tapping, KF. Recent solar radio astronomy at centimeter wavelengths: the temporal variability of the 10.7 cm flux. J. Geophys. Res 1987, 92, 829. [Google Scholar]
- Tapping, KF; DeTracey, B. The origin of the 10.7 cm flux. Solar Physics 1990, 127, 321–332. [Google Scholar]
- Nishida, A. Geomagnetic Diagnosis of the Magnetosphere; Springer-Verlag: New York, NY, USA, 1978; p. 256. [Google Scholar]
- Ptitsyna, NG; Villoresi, G; Kopytenko, YA; Kudrin, VA; Tyasto, MI; Kopytenko, EA; Iucci, N; Voronov, PM; Zaitsev, DB. Coronary heart diseases: assessment of risk associated with work exposure to ultralow-frequency magnetic fields. Bioelectromagnetics 1996, 17, 436–444. [Google Scholar]
- Ptitsyna, NG; Kopytenko, YA; Villoresi, G; Pfluger, DH; Ismaguilov, V; Iucci, N; Kopytenko, EA; Zaitzev, DB; Voronov, PM; Tyasto, MI. Waveform magnetic field survey in Russian DC and Swiss AC powered trains: a basis for biologically relevant exposure assessment. Bioelectromagnetics 2003, 24, 546–556. [Google Scholar]
- Tapping, KF; Mathias, RG; Surkan, DL. Pandemics and solar activity.
- Patterson, KD. Pandemic Influenza, 1700–1900: A Study in Historical Epidemiology; Rowman & Littlefield: Totowa, NJ, USA, 1986; p. 118. [Google Scholar]
- Beveridge, WI. The chronicle of influenza epidemics. Hist. Philos. Life Sci 1991, 13, 223–234. [Google Scholar]
- Potter, CW. A history of influenza. J. Appl. Microbiol 2001, 91, 572–579. [Google Scholar]
- Dowdle, WR. Influenza pandemic periodicity, virus recycling, and the art of risk assessment. Emerg. Infect. Dis 2006, 12, 34–39. [Google Scholar]
- Gurfinkel’ Iu, I; Kuleshova, VP; Oraevskii, VN. Assessment of the effect of a geomagnetic storm on the frequency of appearance of acute cardiovascular pathology. Biofizika 1998, 43, 654–658. [Google Scholar]
- Oraevskii, VN; Breus, TK; Baevskii, RM; Rapoport, SI; Petrov, VM; Barsukova Zh, V; Gurfinkel’ Iu, I; Rogoza, AT. Effect of geomagnetic activity on the functional status of the body. Biofizika 1998, 43, 819–826. [Google Scholar]
- Watanabe, Y; Hillman, DC; Otsuka, K; Bingham, C; Breus, TK; Cornelissen, G; Halberg, F. Cross-spectral coherence between geomagnetic disturbance and human cardiovascular variables at non-societal frequencies. Chronobiologia 1994, 21, 265–272. [Google Scholar]
- Halberg, F; Cornelissen, G; Otsuka, K; Watanabe, Y; Katinas, GS; Burioka, N; Delyukov, A; Gorgo, Y; Zhao, Z; Weydahl, A; Sothern, RB; Siegelova, J; Fiser, B; Dusek, J; Syutkina, EV; Perfetto, F; Tarquini, R; Singh, RB; Rhees, B; Lofstrom, D; Lofstrom, P; Johnson, PW; Schwartzkopff, O; the International, B.S.G. Cross-spectrally coherent ~10.5- and 21-year biological and physical cycles, magnetic storms and myocardial infarctions. Neuro Endocrinol. Lett 2000, 21, 233–258. [Google Scholar]
- Breus, TK; Pimenov, KY; Cornelissen, G; Halberg, E; Syutkina, EV; Baevsky, RM; Petrov, VM; Orth-Gomer, K; Akerstedt, T; Otsuka, K; Watanabe, Y; Chibisov, SM. The biological effects of solar activity. Biomed. Pharmacother 2002, 56, 273s–283s. [Google Scholar]
- Bonizzi, G; Karin, M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 2004, 25, 280–288. [Google Scholar]
- Hayden, MS; Ghosh, S. Signaling to NF-kappaB. Genes Dev 2004, 18, 2195–2224. [Google Scholar]
- Kumar, N; Xin, ZT; Liang, Y; Ly, H. NF-kappaB signaling differentially regulates influenza virus RNA synthesis. J. Virol 2008, 82, 9880–9889. [Google Scholar]
- Goodman, EM; Greenebaum, B; Marron, MT. Magnetic fields alter translation in Escherichia coli. Bioelectromagnetics 1994, 15, 77–83. [Google Scholar]
- Gold, S; Goodman, R; Shirley-Henderson, A. Exposure of simian virus-40-transformed human cells to magnetic fields results in increased levels of T-antigen mRNA and protein. Bioelectromagnetics 1994, 15, 329–336. [Google Scholar]
- Bozek, K; Kielbasa, SM; Kramer, A; Herzel, H. Promoter analysis of Mammalian clock controlled genes. Genome Inform 2007, 18, 65–74. [Google Scholar]
- Nader, N; Chrousos, GP; Kino, T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J 2009, 23, 1572–1583. [Google Scholar]
- McKay, LI; Cidlowski, JA. CBP (CREB binding protein) integrates NF-kappaB (nuclear factor-kappaB) and glucocorticoid receptor physical interactions and antagonism. Mol. Endocrinol 2000, 14, 1222–1234. [Google Scholar]
- Nikolova, T; Czyz, J; Rolletschek, A; Blyszczuk, P; Fuchs, J; Jovtchev, G; Schuderer, J; Kuster, N; Wobus, AM. Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cells. FASEB J 2005, 19, 1686–1688. [Google Scholar]
- Delle Monache, S; Alessandro, R; Iorio, R; Gualtieri, G; Colonna, R. Extremely low frequency electromagnetic fields (ELF-EMFs) inducein vitro angiogenesis process in human endothelial cells. Bioelectromagnetics 2008, 29, 640–648. [Google Scholar]
- Goodman, R; Lin-Ye, A; Geddis, MS; Wickramaratne, PJ; Hodge, SE; Pantazatos, S; Blank, M; Ambron, RT. Extremely low frequency electromagnetic fields activate the ERK cascade, increase hsp70 protein levels and promote regeneration in Planaria. Int. J. Radiat. Biol 2009, 7, 1–9. [Google Scholar]
- Simko, M; Mattsson, MO. Extremely low frequency electromagnetic fields as effectors of cellular responsesin vitro: possible immune cell activation. J. Cell. Biochem 2004, 93, 83–92. [Google Scholar]
- Bonhomme-Faivre, L; Mace, A; Bezie, Y; Marion, S; Bindoula, G; Szekely, AM; Frenois, N; Auclair, H; Orbach-Arbouys, S; Bizi, E. Alterations of biological parameters in mice chronically exposed to low-frequency (50 Hz) electromagnetic fields. Life Sci 1998, 62, 1271–1280. [Google Scholar]
- Bonhomme-Faivre, L; Marion, S; Bezie, Y; Auclair, H; Fredj, G; Hommeau, C. Study of human neurovegetative and hematologic effects of environmental low-frequency (50-Hz) electromagnetic fields produced by transformers. Arch. Environ. Health 1998, 53, 87–92. [Google Scholar]
- Frahm, J; Lantow, M; Lupke, M; Weiss, DG; Simko, M. Alteration in cellular functions in mouse macrophages after exposure to 50 Hz magnetic fields. J. Cell. Biochem 2006, 99, 168–177. [Google Scholar]
- Hiscott, J; Kwon, H; Genin, P. Hostile takeovers: viral appropriation of the NF-kappaB pathway. J. Clin. Invest 2001, 107, 143–151. [Google Scholar]
- Pikarsky, E; Ben-Neriah, Y. NF-kappaB inhibition: a double-edged sword in cancer? Eur. J. Cancer 2006, 42, 779–784. [Google Scholar]
- Li, Q; Withoff, S; Verma, IM. Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends Immunol 2005, 26, 318–325. [Google Scholar]
- Solar Cycle Progression.








© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zaporozhan, V.; Ponomarenko, A. Mechanisms of Geomagnetic Field Influence on Gene Expression Using Influenza as a Model System: Basics of Physical Epidemiology. Int. J. Environ. Res. Public Health 2010, 7, 938-965. https://doi.org/10.3390/ijerph7030938
Zaporozhan V, Ponomarenko A. Mechanisms of Geomagnetic Field Influence on Gene Expression Using Influenza as a Model System: Basics of Physical Epidemiology. International Journal of Environmental Research and Public Health. 2010; 7(3):938-965. https://doi.org/10.3390/ijerph7030938
Chicago/Turabian StyleZaporozhan, Valeriy, and Andriy Ponomarenko. 2010. "Mechanisms of Geomagnetic Field Influence on Gene Expression Using Influenza as a Model System: Basics of Physical Epidemiology" International Journal of Environmental Research and Public Health 7, no. 3: 938-965. https://doi.org/10.3390/ijerph7030938