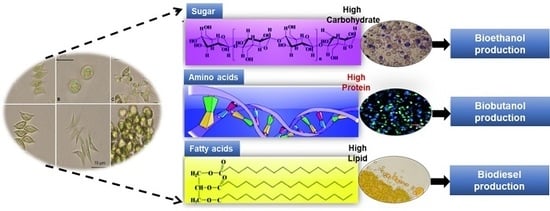
Utilization of Microalgal Biofractions for Bioethanol, Higher Alcohols, and Biodiesel Production: A Review
Abstract
:1. Introduction
Microalgal Biomass as a Potential Source for Biofuel Generation
2. Various Pretreatment Methods for the Extraction of Microalgal Biofractions
2.1. Physical Pretreatment
2.2. Chemical Pretreatment
2.3. Biological Pretreatment
Bio-Pretreatment: A New Approach to Economic Pretreatment for the Extraction of Microalgal Bioblocks
3. Fermentation of Carbohydrates from Microalgae for Production of Bioethanol
3.1. Metabolic Pathways
3.2. Saccharification and Fermentation Strategies
3.3. Long-Term and Continuous Production of Bioethanol through Repeated Batch Fermentation
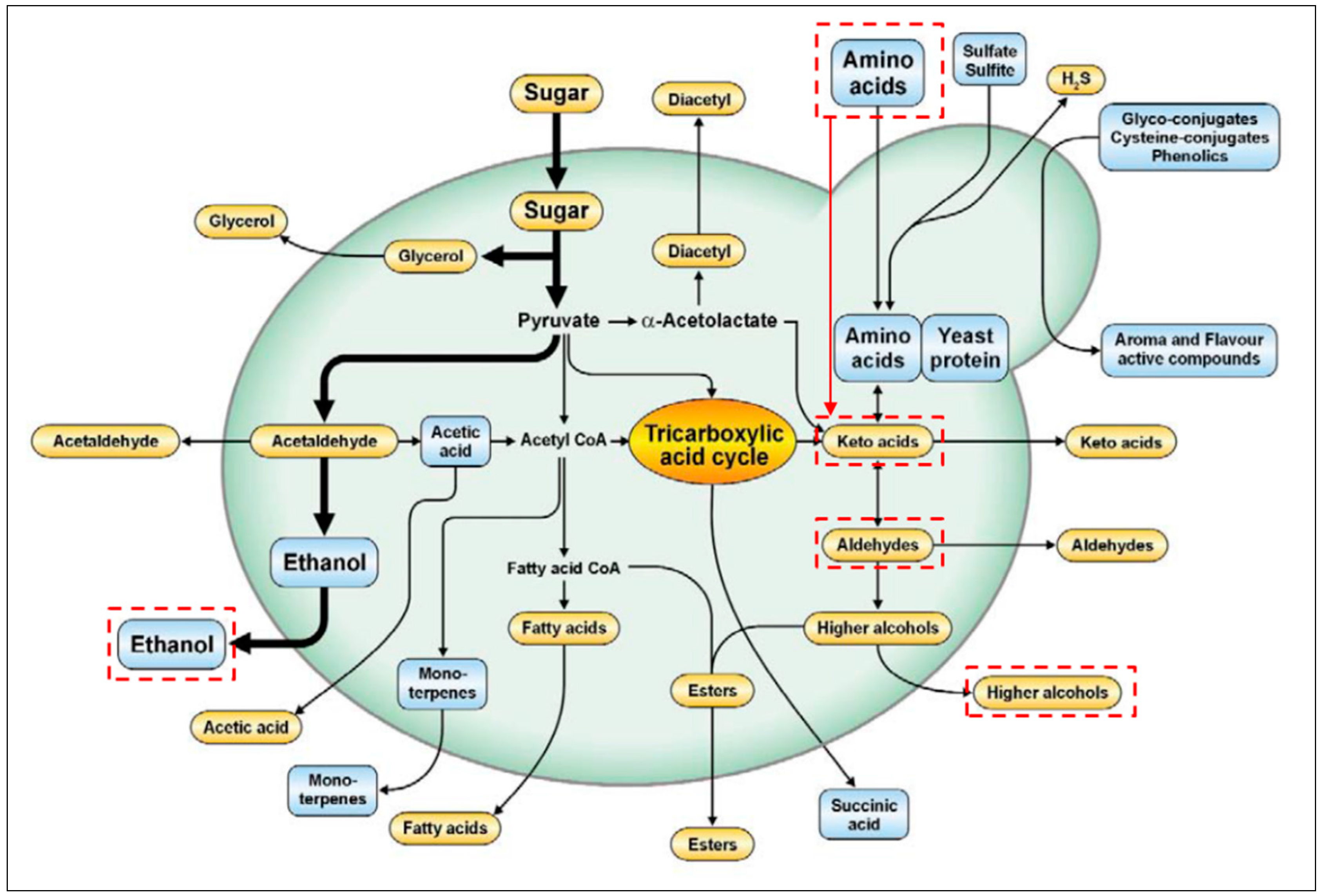
4. Conversions of Proteins into Higher Alcohol
5. Conversion of Microalgal Lipid Fraction into Biodiesel and Glycerol
6. Fermentation of by-Product Glycerol into Butanol
7. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rutz, D.; Janssen, R. Socio-Economic Impacts of Bioenergy Production; Springer: Cham, Switzerland; New York, NY, USA, 2014; pp. 26–297. [Google Scholar]
- Kruyt, B.; van Vuuren, D.P.; De Vries, H.; Groenenberg, H. Indicators for energy security. Energy Policy 2009, 37, 2166–2181. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, B.; Ding, T.; Zhang, M. Analysis of regional decoupling relationship between energy-related CO2 emission and economic growth in China. Nat. Hazards 2017, 87, 867–883. [Google Scholar] [CrossRef]
- O’Reilly, J.; Oreskes, N.; Oppenheimer, M. The rapid disintegration of projections: The West Antarctic Ice Sheet and the intergovernmental panel on climate change. Soc. Stud. Sci. 2012, 42, 709–731. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Higashide, W.; Liao, J.C. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol. 2009, 27, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Feng, Y.; Qu, Y.; Du, Y.; Zhou, X.; Liu, J. A combined system of microbial fuel cell and intermittently aerated biological filter for energy self-sufficient wastewater treatment. Sci. Rep. 2015, 5, 18070. [Google Scholar] [CrossRef] [PubMed]
- Nakata, T. Energy-economic models and the environment. Prog. Energy Combust. Sci. 2004, 30, 417–475. [Google Scholar] [CrossRef]
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Moser, D.; Pergl, J.; Pysek, P.; van Kleunen, M.; Weber, E.; Winter, M.; et al. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol. 2015, 21, 4128–4140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Obama, B. The irreversible momentum of clean energy. Science 2017, 355, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- DeLong, J.P.; Burger, O. Socio-Economic Instability and the Scaling of Energy Use with Population Size. PLoS ONE 2015, 10, e0130547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Vadenbo, C.; Tonini, D.; Astrup, T.F. Environmental Multiobjective Optimization of the Use of Biomass Resources for Energy. Environ. Sci. Technol. 2017, 51, 3575–3583. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.N.; Mathias, A.; Korth, K.; Betenbaugh, M.J.; Oyler, G.A. Microalgal biomass production and carbon dioxide sequestration from an integrated ethanol biorefinery in Iowa: A technical appraisal and economic feasibility evaluation. Biomass Bioenergy 2011, 35, 3865–3876. [Google Scholar] [CrossRef]
- Hariskos, I.; Posten, C. Biorefinery of microalgae—Opportunities and constraints for different production scenarios. Biotechnol. J. 2014, 9, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Lammers, P.J.; Huesemann, M.; Boeing, W.; Anderson, D.B.; Arnold, R.G.; Bai, X.; Bhole, M.; Brhanavan, Y.; Brown, L.; Brown, J. Review of the cultivation program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Res. 2017, 22, 166–186. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Rasoul-Amini, S.; Naseri, A.T.; Montazeri-Najafabady, N.; Mobasher, M.A.; Dabbagh, F. Microalgae biofuel potentials (Review). Appl. Biochem. Microbiol. 2012, 48, 126–144. [Google Scholar] [CrossRef]
- Kebelmann, K.; Hornung, A.; Karsten, U.; Griffiths, G. Intermediate pyrolysis and product identification by TGA and Py-GC/MS of green microalgae and their extracted protein and lipid components. Biomass Bioenergy 2013, 49, 38–48. [Google Scholar] [CrossRef]
- Sheehan, J.D.; Savage, P.E. Modeling the effects of microalga biochemical content on the kinetics and biocrude yields from hydrothermal liquefaction. Bioresour. Technol. 2017, 239, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Shakya, R.; Adhikari, S.; Mahadevan, R.; Shanmugam, S.R.; Nam, H.; Dempster, T.A. Influence of biochemical composition during hydrothermal liquefaction of algae on product yields and fuel properties. Bioresour. Technol. 2017, 243, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Biller, P.; Ross, A. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Eldalatony, M.M.; Kabra, A.N.; Hwang, J.H.; Govindwar, S.P.; Kim, K.H.; Kim, H.; Jeon, B.H. Pretreatment of microalgal biomass for enhanced recovery/extraction of reducing sugars and proteins. Bioprocess Biosyst. Eng. 2016, 39, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Georgianna, D.R.; Mayfield, S.P. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature 2012, 488, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Suganya, T.; Varman, M.; Masjuki, H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhao, X.-Q.; Yen, H.-W.; Ho, S.-H.; Cheng, C.-L.; Lee, D.-J.; Bai, F.-W.; Chang, J.-S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Tandon, P.; Jin, Q. Microalgae culture enhancement through key microbial approaches. Renew. Sustain. Energy Rev. 2017, 80, 1089–1099. [Google Scholar] [CrossRef]
- Gouveia, L.; Marques, A.E.; da Silva, T.L.; Reis, A. Neochloris oleabundans UTEX #1185: A suitable renewable lipid source for biofuel production. J. Ind. Microbiol. Biotechnol. 2009, 36, 821–826. [Google Scholar] [PubMed]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel production from oleaginous microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Wu, X.; Ruan, R.; Du, Z.; Liu, Y. Current status and prospects of biodiesel production from microalgae. Energies 2012, 5, 2667–2682. [Google Scholar] [CrossRef]
- Dragone, G.; Fernandes, B.D.; Abreu, A.P.; Vicente, A.A.; Teixeira, J.A. Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl. Energy 2011, 88, 3331–3335. [Google Scholar] [CrossRef] [Green Version]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae—Novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Sassano, C.; Gioielli, L.; Ferreira, L.; Rodrigues, M.; Sato, S.; Converti, A.; Carvalho, J. Evaluation of the composition of continuously-cultivated Arthrospira (Spirulina) platensis using ammonium chloride as nitrogen source. Biomass Bioenergy 2010, 34, 1732–1738. [Google Scholar] [CrossRef]
- Carrieri, D.; Momot, D.; Brasg, I.A.; Ananyev, G.; Lenz, O.; Bryant, D.A.; Dismukes, G.C. Boosting autofermentation rates and product yields with sodium stress cycling: Application to production of renewable fuels by cyanobacteria. Appl. Environ. Microbiol. 2010, 76, 6455–6462. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Simonciniand, E.; Pulselli, R. Bioethanol potentials from marine residual biomass: An energy evaluation. Energy Environ. 2009, 122, 379–387. [Google Scholar]
- Lai, Y.S.; Parameswaran, P.; Li, A.; Aguinaga, A.; Rittmann, B.E. Selective fermentation of carbohydrate and protein fractions of Scenedesmus, and biohydrogenation of its lipid fraction for enhanced recovery of saturated fatty acids. Biotechnol. Bioeng. 2016, 113, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; McCaw, A.; Ontiveros-Valencia, A.; Shi, Y.; Parameswaran, P.; Rittmann, B.E. Multiple synergistic benefits of selective fermentation of Scenedesmus biomass for fuel recovery via wet-biomass extraction. Algal Res. 2016, 17, 253–260. [Google Scholar] [CrossRef]
- Lee, O.K.; Seong, D.H.; Lee, C.G.; Lee, E.Y. Sustainable production of liquid biofuels from renewable microalgae biomass. J. Ind. Eng. Chem. 2015, 29, 24–31. [Google Scholar] [CrossRef]
- Huo, Y.X.; Cho, K.M.; Rivera, J.G.L.; Monte, E.; Shen, C.R.; Yan, Y.J.; Liao, J.C. Conversion of proteins into biofuels by engineering nitrogen flux. Nat. Biotechnol. 2011, 29, 346. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-A.; Hwang, J.-H.; Dempsey, B.A.; Abou-Shanab, R.A.I.; Min, B.; Song, H.; Lee, D.S.; Kim, J.R.; Cho, Y.; Hong, S.; et al. Enhancement of fermentative bioenergy (ethanol/hydrogen) production using ultrasonication of Scenedesmus obliquus YSW15 cultivated in swine wastewater effluent. Energy Environ. Sci. 2011, 4, 3513. [Google Scholar] [CrossRef]
- Williams, P.J.; Laurens, L.M. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar]
- Hwang, J.-H.; Kabra, A.N.; Ji, M.-K.; Choi, J.; El-Dalatony, M.M.; Jeon, B.-H. Enhancement of continuous fermentative bioethanol production using combined treatment of mixed microalgal biomass. Algal Res. 2016, 17, 14–20. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Hoang, A.L. A Review on Microalgae and Cyanobacteria in Biofuel Production; USTH: Hanoi, Vietnam, 2016. [Google Scholar]
- Millett, M.A.; Baker, A.J.; Satter, L.D. Physical and chemical pretreatments for enhancing cellulose saccharification. Biotechnol. Bioeng. Symp. 1976, 6, 125–153. [Google Scholar]
- Harun, R.; Danquah, M.K. Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem. 2011, 46, 304–309. [Google Scholar] [CrossRef]
- Jeon, B.-H.; Choi, J.A.; Kim, H.C.; Hwang, J.H.; Abou-Shanab, R.A.; Dempsey, B.A.; Regan, J.M.; Kim, J.R. Ultrasonic disintegration of microalgal biomass and consequent improvement of bioaccessibility/ bioavailability in microbial fermentation. Biotechnol. Biofuels 2013, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the structural and chemical changes of plant biomass following steam explosion pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, S.C.; Maciel Filho, R.; Costa, A.C. Lime pretreatment of sugarcane bagasse for bioethanol production. Appl. Biochem. Biotechnol. 2009, 153, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Choi, S.P.; Lee, J.; Lee, J.H.; Sim, S.J. Hydrothermal acid pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. J. Microbiol. Biotechnol. 2009, 19, 161–166. [Google Scholar] [PubMed]
- Salama, E.-S.; Kurade, M.B.; Abou-Shanab, R.A.; El-Dalatony, M.M.; Yang, I.-S.; Min, B.; Jeon, B.-H. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Saha, S.; Kurade, M.B.; El-Dalatony, M.M.; Chatterjee, P.K.; Lee, D.S.; Jeon, B.-H. Improving bioavailability of fruit wastes using organic acid: An exploratory study of biomass pretreatment for fermentation. Energy Convers. Manag. 2016, 127, 256–264. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Nguyen, T.N.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Park, Y.J.; Kim, J.C. Acid hydrolysis of Curcuma longa residue for ethanol and lactic acid fermentation. Bioresour. Technol. 2014, 151, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar] [CrossRef]
- Alzate, M.; Muñoz, R.; Rogalla, F.; Fdz-Polanco, F.; Pérez-Elvira, S. Biochemical methane potential of microalgae: Influence of substrate to inoculum ratio, biomass concentration and pretreatment. Bioresour. Technol. 2012, 123, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, D.; Tiwari, M.K.; Li, J.; Kim, S.C.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. A highly efficient recombinant laccase from the yeast Yarrowia lipolytica and its application in the hydrolysis of biomass. PLoS ONE 2015, 10, e0120156. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.P.; Wang, M.M.; Li, X.Z.; Li, H.X.; Zhao, J.; Qu, Y.B.; Choo, Y.M.; Loh, S.K. Fractionation of oil palm empty fruit bunch by bisulfite pretreatment for the production of bioethanol and high value products. Bioresour. Technol. 2016, 200, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Ehimen, E.A.; Holm-Nielsen, J.-B.; Poulsen, M.; Boelsmand, J. Influence of different pre-treatment routes on the anaerobic digestion of a filamentous algae. Renew. Energy 2013, 50, 476–480. [Google Scholar] [CrossRef]
- Jodayree, S.; Smith, J.C.; Tsopmo, A. Use of carbohydrase to enhance protein extraction efficiency and antioxidative properties of oat bran protein hydrolysates. Food Res. Int. 2012, 46, 69–75. [Google Scholar] [CrossRef]
- El-Dalatony, M.M.; Kurade, M.B.; Abou-Shanab, R.A.I.; Kim, H.; Salama, E.-S.; Jeon, B.-H. Long-term production of bioethanol in repeated-batch fermentation of microalgal biomass using immobilized Saccharomyces cerevisiae. Bioresour. Technol. 2016, 219, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.K.; Kim, A.L.; Seong, D.H.; Lee, C.G.; Jung, Y.T.; Lee, J.W.; Lee, E.Y. Chemo-enzymatic saccharification and bioethanol fermentation of lipid-extracted residual biomass of the microalga, Dunaliella tertiolecta. Bioresour. Technol. 2013, 132, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.P.; Nguyen, M.T.; Sim, S.J. Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef] [PubMed]
- Harun, R.; Jason, W.; Cherrington, T.; Danquah, M.K. Exploring alkaline pre-treatment of microalgal biomass for bioethanol production. Appl. Energy 2011, 88, 3464–3467. [Google Scholar] [CrossRef]
- Ostergaard, S.; Olsson, L.; Nielsen, J. Metabolic engineering of Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2000, 64, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Wakita, K.; Ogino, H. Global Metabolic Engineering of Glycolytic Pathway via Multicopy Integration in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Oh, E.J.; Million, G.; Cate, J.H.; Jin, Y.-S. Simultaneous utilization of cellobiose, xylose, and acetic acid from lignocellulosic biomass for biofuel production by an engineered yeast platform. ACS Synth. Biol. 2015, 4, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, K.; Sánchez, E.; El-Halwagi, M.; Kafarov, V. Exergy analysis and process integration of bioethanol production from acid pre-treated biomass: Comparison of SHF, SSF and SSCF pathways. Chem. Eng. J. 2011, 176–177, 195–201. [Google Scholar] [CrossRef]
- Gan, Q.; Allen, S.; Taylor, G. Kinetic dynamics in heterogeneous enzymatic hydrolysis of cellulose: An overview, an experimental study and mathematical modelling. Process Biochem. 2003, 38, 1003–1018. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K.; Thiruvenkadam, S. Particulate size of microalgal biomass affects hydrolysate properties and bioethanol concentration. Biomed. Res. Int. 2014, 2014, 435631. [Google Scholar] [CrossRef] [PubMed]
- Marwa, M.; Salama, E.-S.; Jeon, B.-H. Repeated-Batch Fermentation of Microalgal Biomass Utilizing Immobilized Yeast Cells for Bioethanol Production. In Proceedings of the 13th U.S.-Korea Forum on Nanotechnology, Seoul, Korea, 26–27 September 2016. [Google Scholar]
- Rattanapan, A.; Limtong, S.; Phisalaphong, M. Ethanol production by repeated batch and continuous fermentations of blackstrap molasses using immobilized yeast cells on thin-shell silk cocoons. Appl. Energy 2011, 88, 4400–4404. [Google Scholar] [CrossRef]
- Duarte, J.C.; Rodrigues, J.A.; Moran, P.J.; Valenca, G.P.; Nunhez, J.R. Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.X.; Wernick, D.G.; Liao, J.C. Toward nitrogen neutral biofuel production. Curr. Opin. Biotechnol. 2012, 23, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Mi, L.; Pontrelli, S.; Luo, S. Fuelling the future: Microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 2016, 14, 288. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, Y.; He, J. Enhanced direct fermentation of cassava to butanol by Clostridium species strain BOH3 in cofactor-mediated medium. Biotechnol. Biofuels 2015, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Chiappe, C.; Mezzetta, A.; Pomelli, C.S.; Masciocchi, B.; Gentile, A.; Iaquaniello, G. Development of cost-effective biodiesel from microalgae using protic ionic liquids. Green Chem. 2016, 18, 4982–4989. [Google Scholar] [CrossRef]
- Pate, R.; Wu, B.; Davis, R.; Land, T.; George, A.; Horvath, S.; Adey, W.; Calahan, D.; Zivojnovich, M.; Quinn, J. Algal Turf to Fuel (ATF): System overview and preliminary assessment of the production of biofuels from chemical, biochemical, and thermochemical processing and conversion of benthic polyculture biomass produced by algal turf cultivation. In Proceedings of the 2014 Algae Biomass Summit, San Diego, CA, USA, 29 September–2 October 2014. [Google Scholar]
- Huo, Y.X.; Cho, K.M.; Liao, J.C. Conversion of proteins into biofuels: Toward nitrogen neutral biofuel production. Abstr. Pap. Am. Chem. Soc. 2012, 243, 4712–4716. [Google Scholar]
- Wernick, D.G.; Liao, J.C. Protein-based biorefining: Metabolic engineering for production of chemicals and fuel with regeneration of nitrogen fertilizers. Appl. Microbiol. Biotechnol. 2013, 97, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.; Bartowsky, E.; Henschke, P.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Eden, A.; Van Nedervelde, L.; Drukker, M.; Benvenisty, N.; Debourg, A. Involvement of branched-chain amino acid aminotransferases in the production of fusel alcohols during fermentation in yeast. Appl. Microbiol. Biotechnol. 2001, 55, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Lan, E.I.; Liao, J.C. Microbial synthesis of n-butanol, isobutanol, and other higher alcohols from diverse resources. Bioresour. Technol. 2013, 135, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Si, T.; Liu, Z.; Zhang, H.; Ang, E.L.; Zhao, H. Metabolic engineering of a synergistic pathway for n-butanol production in Saccharomyces cerevisiae. Sci. Rep. 2016, 6, 25675. [Google Scholar] [CrossRef] [PubMed]
- Procopio, S.; Sprung, P.; Becker, T. Effect of amino acid supply on the transcription of flavour-related genes and aroma compound production during lager yeast fermentation. LWT Food Sci. Technol. 2015, 63, 289–297. [Google Scholar] [CrossRef]
- Fan, J.; Cui, Y.; Wan, M.; Wang, W.; Li, Y. Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnol. Biofuels 2014, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Huerlimann, R.; De Nys, R.; Heimann, K. Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol. Bioeng. 2010, 107, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Mechanism and challenges in commercialisation of algal biofuels. Bioresour. Technol. 2011, 102, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Dunahay, T.G.; Jarvis, E.E.; Dais, S.S.; Roessler, P.G. Manipulation of microalgal lipid production using genetic engineering. Appl. Biochem. Biotechnol. 1996, 57, 223. [Google Scholar] [CrossRef]
- Olofsson, M.; Lamela, T.; Nilsson, E.; Bergé, J.P.; Del Pino, V.; Uronen, P.; Legrand, C. Seasonal variation of lipids and fatty acids of the microalgae Nannochloropsis oculata grown in outdoor large-scale photobioreactors. Energies 2012, 5, 1577–1592. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High Lipid Induction in Microalgae for Biodiesel Production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Salama, E.-S.; Kim, H.C.; Abou-Shanab, R.A.; Ji, M.K.; Oh, Y.K.; Kim, S.H.; Jeon, B.-H. Biomass, lipid content, and fatty acid composition of freshwater Chlamydomonas mexicana and Scenedesmus obliquus grown under salt stress. Bioprocess Biosyst. Eng. 2013, 36, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G. Analysis of oxidized biodiesel by 1H-NMR and effect of contact area with air. Eur. J. Lipid Sci. Technol. 2006, 108, 493–500. [Google Scholar] [CrossRef]
- Guan, Q.; Li, Y.; Chen, Y.; Shi, Y.; Gu, J.; Li, B.; Miao, R.; Chen, Q.; Ning, P. Sulfonated multi-walled carbon nanotubes for biodiesel production through triglycerides transesterification. RSC Adv. 2017, 7, 7250–7258. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; Hernández, J.J.; Tsolakis, A. Potential for reducing emissions in a diesel engine by fuelling with conventional biodiesel and Fischer—Tropsch diesel. Fuel 2010, 89, 3106–3113. [Google Scholar] [CrossRef]
- Nakas, J.; Schaedle, M.; Parkinson, C.; Coonley, C.; Tanenbaum, S. System development for linked-fermentation production of solvents from algal biomass. Appl. Environ. Microbiol. 1983, 46, 1017–1023. [Google Scholar] [PubMed]
- Heyndrickx, M.; Vos, P.D.; Vancanneyt, M.; Ley, J.D. The fermentation of glycerol by Clostridium butyricum LMG 1212t2 and 1213t1 and C. pasteurianum LMG 3285. Appl. Microbiol. Biotechnol. 1991, 34, 637–642. [Google Scholar] [CrossRef]
- Biebl, H. Fermentation of glycerol by Clostridium pasteurianum—Batch and continuous culture studies. J. Ind. Microbiol. Biotechnol. 2001, 27, 18–26. [Google Scholar] [CrossRef] [PubMed]







| Microalgae strains | Carbohydrates | Proteins | Lipids |
|---|---|---|---|
| Scenedesmus (S-2) | 54.2 | 30.1 | 17.8 |
| Scenedesmus (S-1) | 50.4 | 7.15 | 35.7 |
| Chlamydomonas mexicana | 52.6 | 37.0 | 10.4 |
| Chlorella (C-2) | 49.7 | 14.6 | 30.3 |
| Nannochloropsis (N-4) | 8.92 | 62.8 | 18.1 |
| Nannochloropsis oculata | 8.00 | 57.0 | 32.0 |
| Nannochloropsis sp. | 21.0 | 56.0 | 9.00 |
| Chlorella vulgaris | 9.10 | 54.9 | 15.5 |
| Scenedesmus sp. | 31.0 | 50.0 | 8.00 |
| Chlamydomonas reinhardtii | 15.1 | 47.4 | 18.1 |
| Pavlova (P-1) | 28.0 | 46.9 | 13.9 |
| Chlorella (C-1) | 16.1 | 46.8 | 15.8 |
| Nannochloropsis (N-3) | 9.21 | 46.6 | 20.1 |
| Chlamydomonas reinhardtii CW15+ | 11.5 | 45.7 | 22.4 |
| Porphyridium cruentum | 40.0 | 43.0 | 8.00 |
| Aurantiochytrium sp. KRS101 | 5.80 | 30.0 | 57.5 |
| Nannochloropsis (N-1) | 12.9 | 12.9 | 55.4 |
| Chlorella protothecoides | 29.0 | 11.0 | 53.0 |
| Nannochloropsis (N-2) | 15.9 | 18.2 | 49.3 |
| Microalgae | Intrinsic Carbohydrate, % | Enhanced Carbohydrate, % | Carbohydrates Productivity, mg L−1 d−1 | Biomass Concentration, g L−1 | Technique | Reference |
|---|---|---|---|---|---|---|
| Chlorella vulgaris | 12–17 | 41 | 199 | - | Nitrogen starvation | [34] |
| 55 | - | ~0.6 | Phosphorus starvation | [35] | ||
| 38 | - | ~0.2 | Nitrogen starvation | |||
| 60 | - | ~1.0 | Sulfur starvation | |||
| 44 | 66–112 | - | Grow on glucose | [36] | ||
| 23 | 18–20 | - | Grow on acetate | |||
| 29–34 | 26–35 | - | Grow on glycerol | |||
| Spirulina platensis | 8–14 | 55–65 | - | 0.15–0.52 | Nitrogen starvation | [37] |
| 63 | 170 | 0.94 | Phosphorus starvation | |||
| 50 | 290 | 1.6 | Light intensity and nitrate supply | |||
| Spirulina maxima | 13–16 | 34 | - | ~1.3 | Light intensity | [38] |
| 60–70 | - | ~1.3 | Nitrogen starvation | |||
| 50 | - | ~1.2 | Salt stress |
| Enzyme | pH | Temp., °C | Substrate | Total Reducing Sugar Yield | Ethanol Concentration | Reference |
|---|---|---|---|---|---|---|
| Cellulase (≥1 U mg−1) | 5 | 50 | Chlamydomonas mexicana | 445.5 mg (g biomass)−1 | 10.5 g L−1 | [64] |
| (Celluclast 1.5L, Novoprime B957), + Amyloglucosidase (300 L), | 5.5 | 55 | Dunaliella tertiolecta LB999 | 42.0% (w w−1) | 14% | [65] |
| α-amylase 0.2 (%, v w−1) | 4.5 | 55 | Chlamydomonas reinhardtii UTEX 90 | 43.6% (w w−1) | 11.73 g L−1 | [66] |
| NaOH (0.75 w v−1) | - | 120 | Chlorococcum infusionum | 350.13 mg (g biomass)−1 | 26.13% | [67] |
| Sulfuric acid (3% v v−1) | - | 160 | Chlorococcum humicola | 43.6% (w w−1) | 6.47 g L−1 | [50] |
| Property | Diesel Fuel | Biodiesel | Blend a |
|---|---|---|---|
| Cetane number | 53 | 70–90 | 57.8 |
| Sulfur content (mg L−1) | <10 | <1 | 4.7 |
| Distillation (°C) | 180–360 | 265–320 | 249–341 |
| Lower heating value (MJ kg−1) | 35.7 | 44 | 36.5 |
| Cloud point (°C) | −5 | −20 | −4.1 |
| Stability | Baseline | Baseline | Baseline |
| Specific gravity (kg m−3) | 835 | 780 | 827 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Dalatony, M.M.; Salama, E.-S.; Kurade, M.B.; Hassan, S.H.A.; Oh, S.-E.; Kim, S.; Jeon, B.-H. Utilization of Microalgal Biofractions for Bioethanol, Higher Alcohols, and Biodiesel Production: A Review. Energies 2017, 10, 2110. https://doi.org/10.3390/en10122110
El-Dalatony MM, Salama E-S, Kurade MB, Hassan SHA, Oh S-E, Kim S, Jeon B-H. Utilization of Microalgal Biofractions for Bioethanol, Higher Alcohols, and Biodiesel Production: A Review. Energies. 2017; 10(12):2110. https://doi.org/10.3390/en10122110
Chicago/Turabian StyleEl-Dalatony, Marwa M., El-Sayed Salama, Mayur B. Kurade, Sedky H. A. Hassan, Sang-Eun Oh, Sunjoon Kim, and Byong-Hun Jeon. 2017. "Utilization of Microalgal Biofractions for Bioethanol, Higher Alcohols, and Biodiesel Production: A Review" Energies 10, no. 12: 2110. https://doi.org/10.3390/en10122110