Pyrolysis of Polyolefins Using Rotating Arc Plasma Technology for Production of Acetylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Feedstock and Injection
2.2. Thermodynamic Simulation
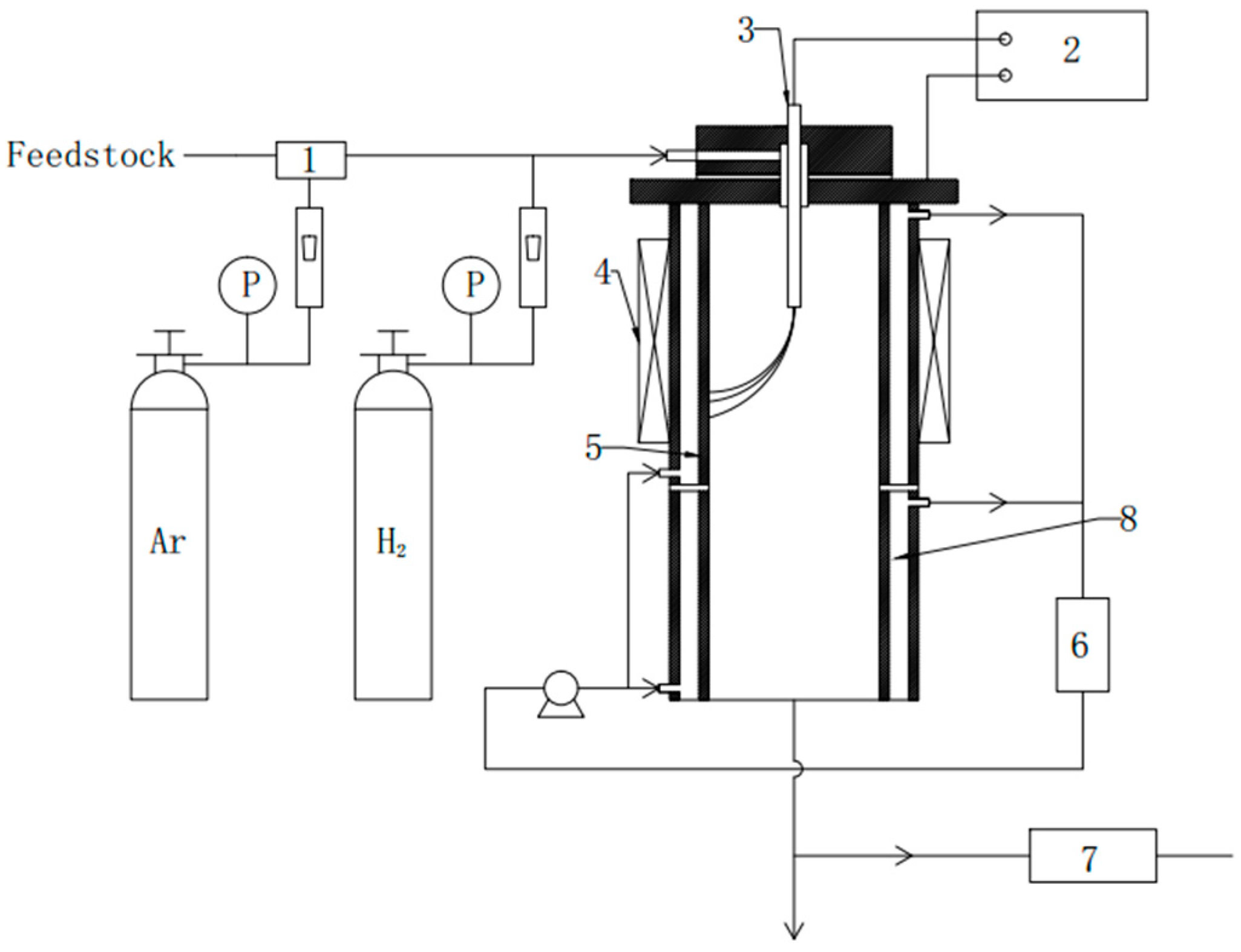
2.3. Reactor Set-Up
2.4. Gas Analysis
2.5. Evaluation of Pyrolysis
3. Results and Discussion
3.1. Thermodynamic Simulation
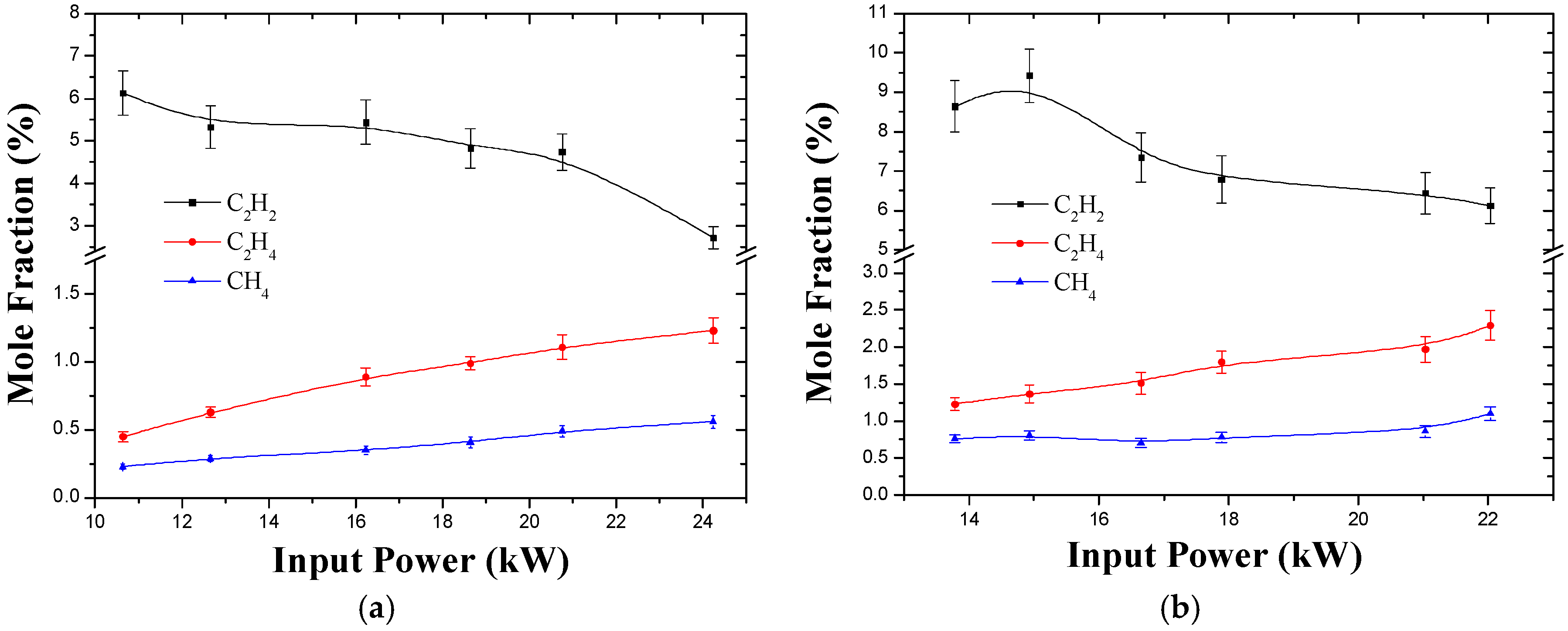
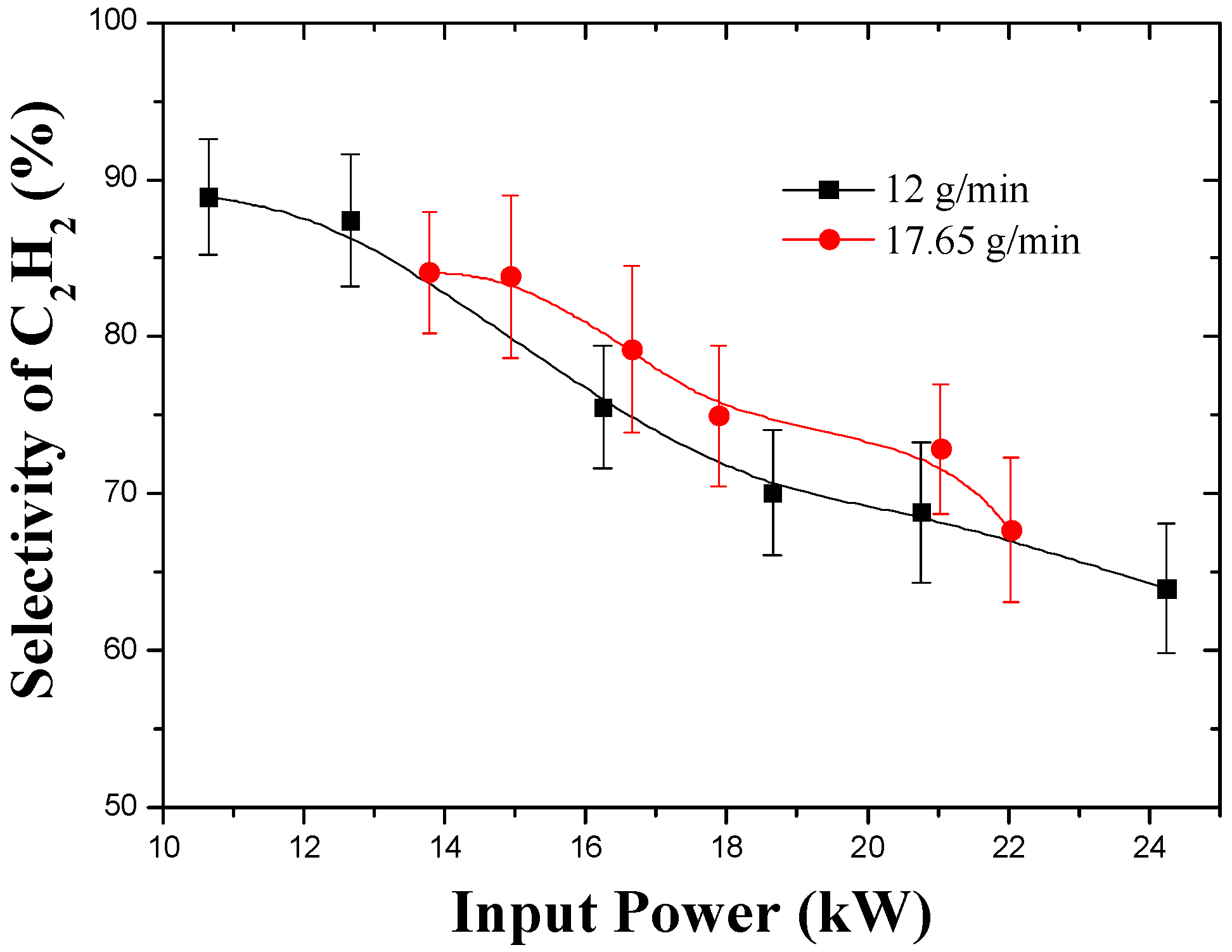
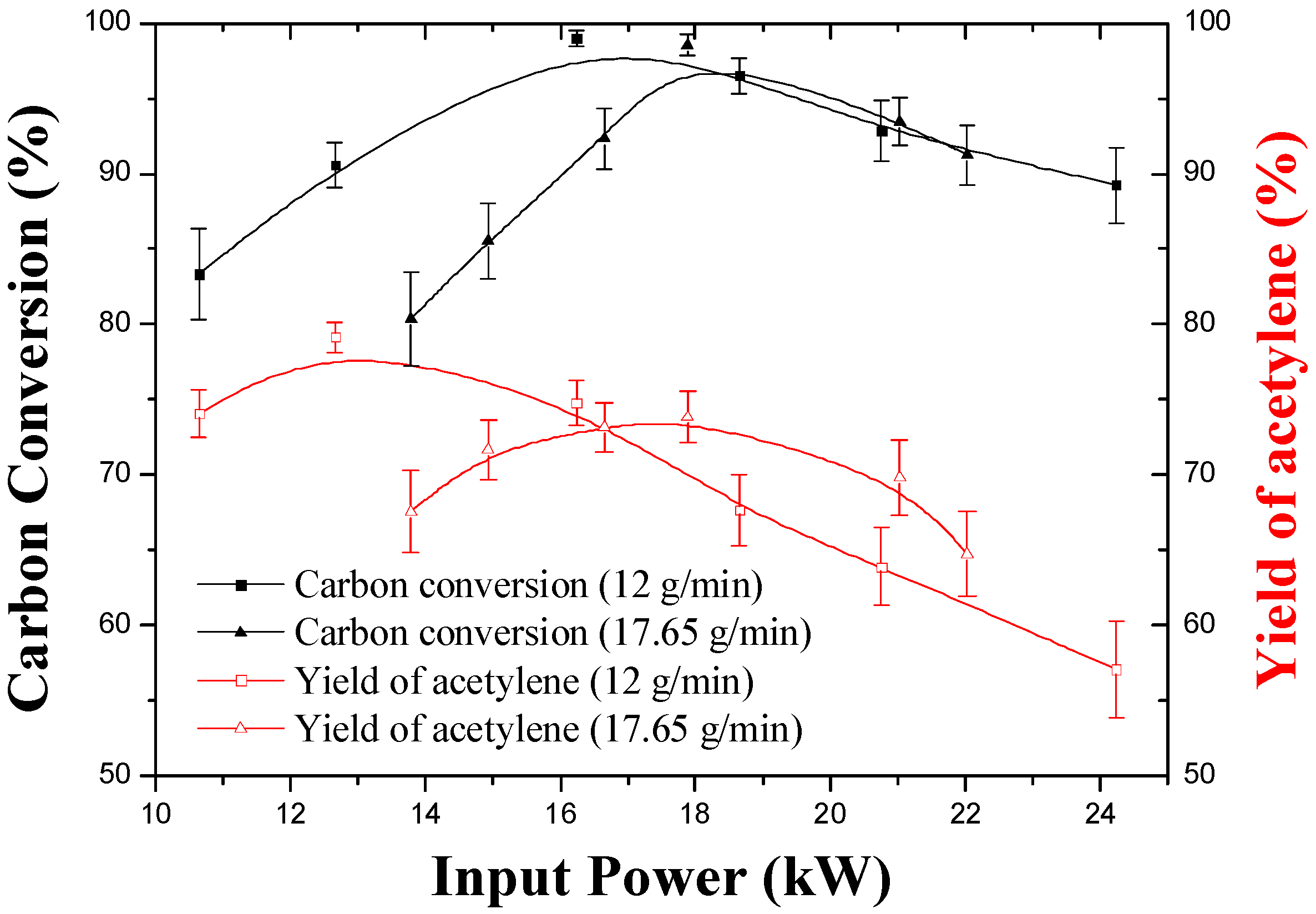
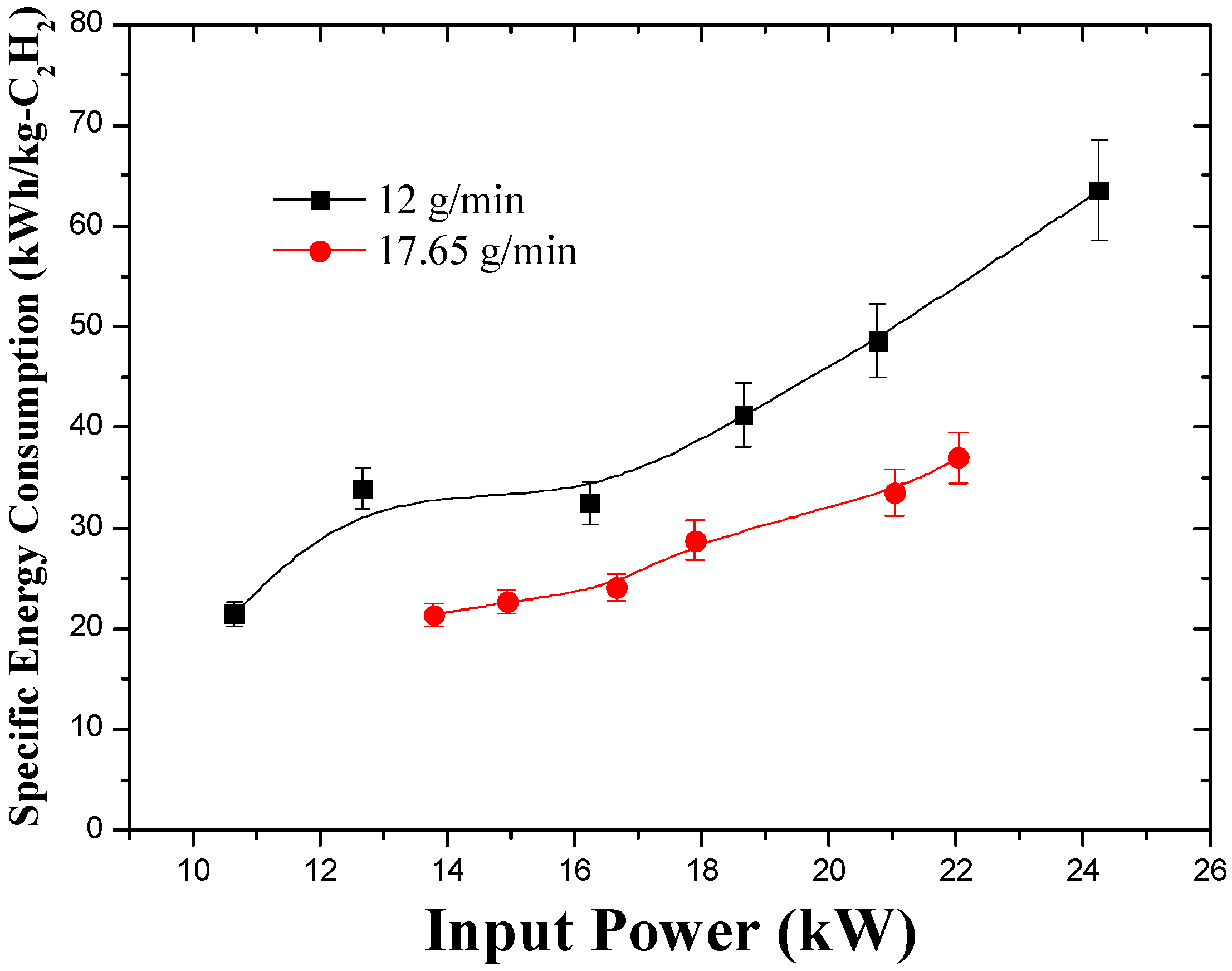
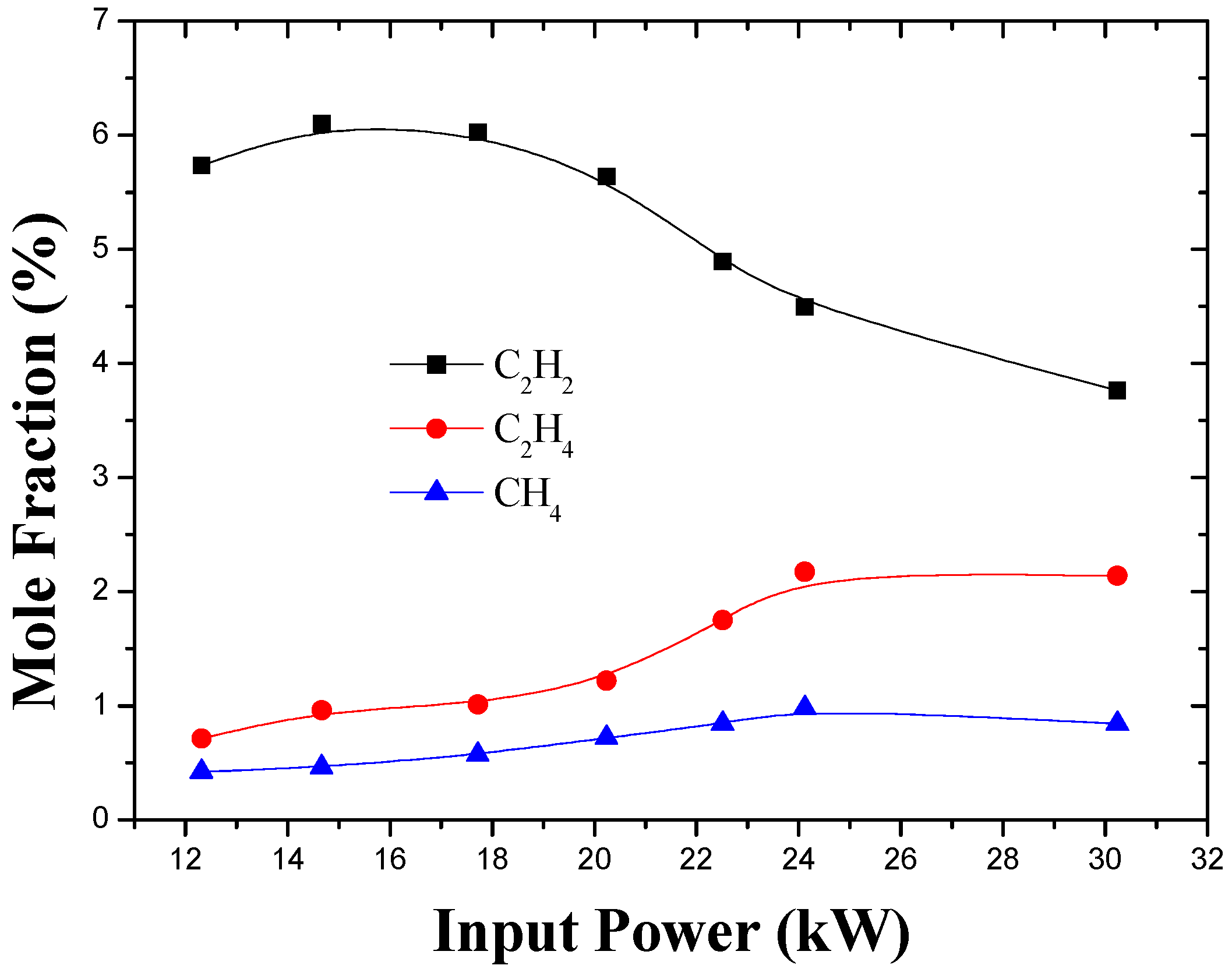
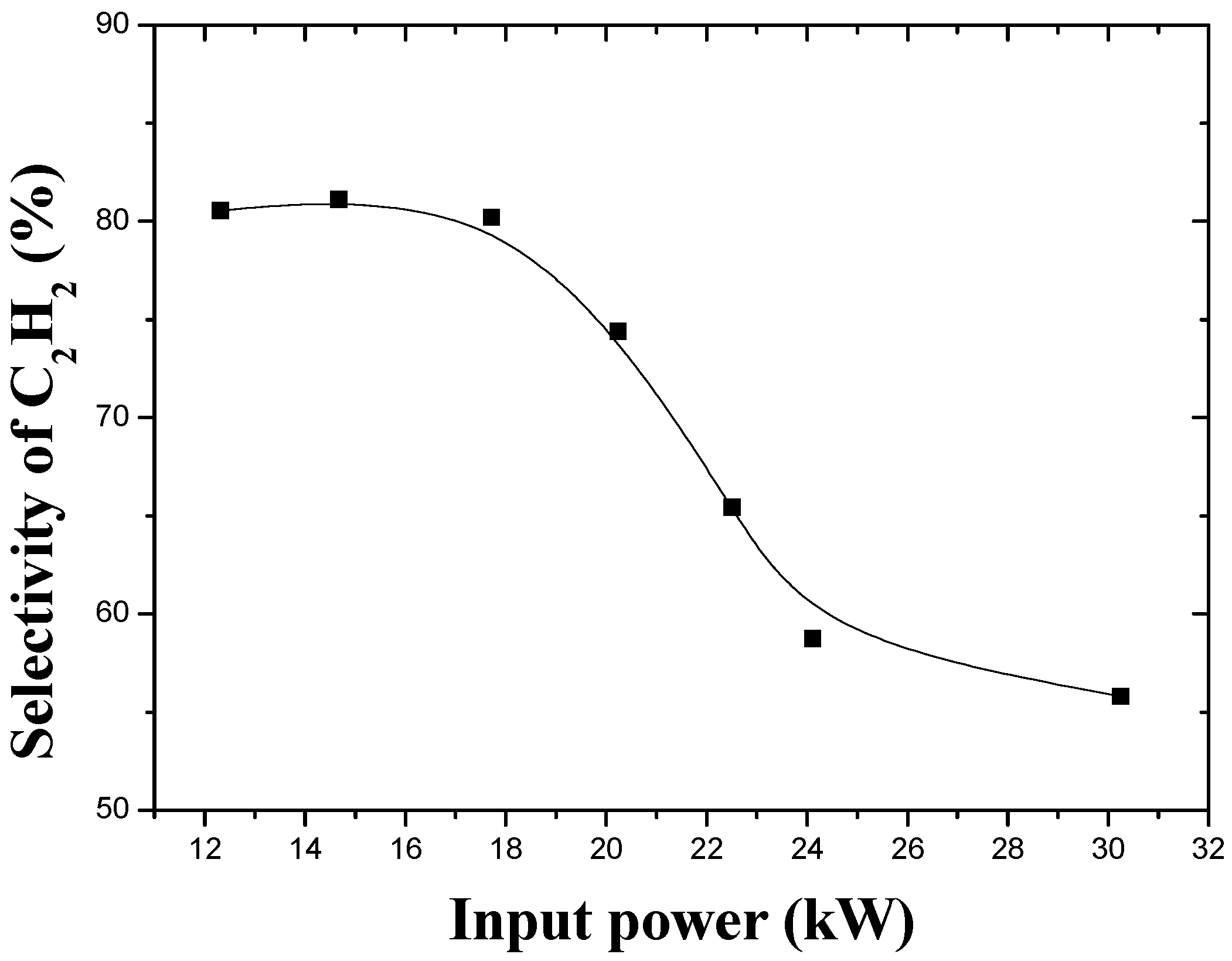
3.2. Effect of Input Power and Feed Rate
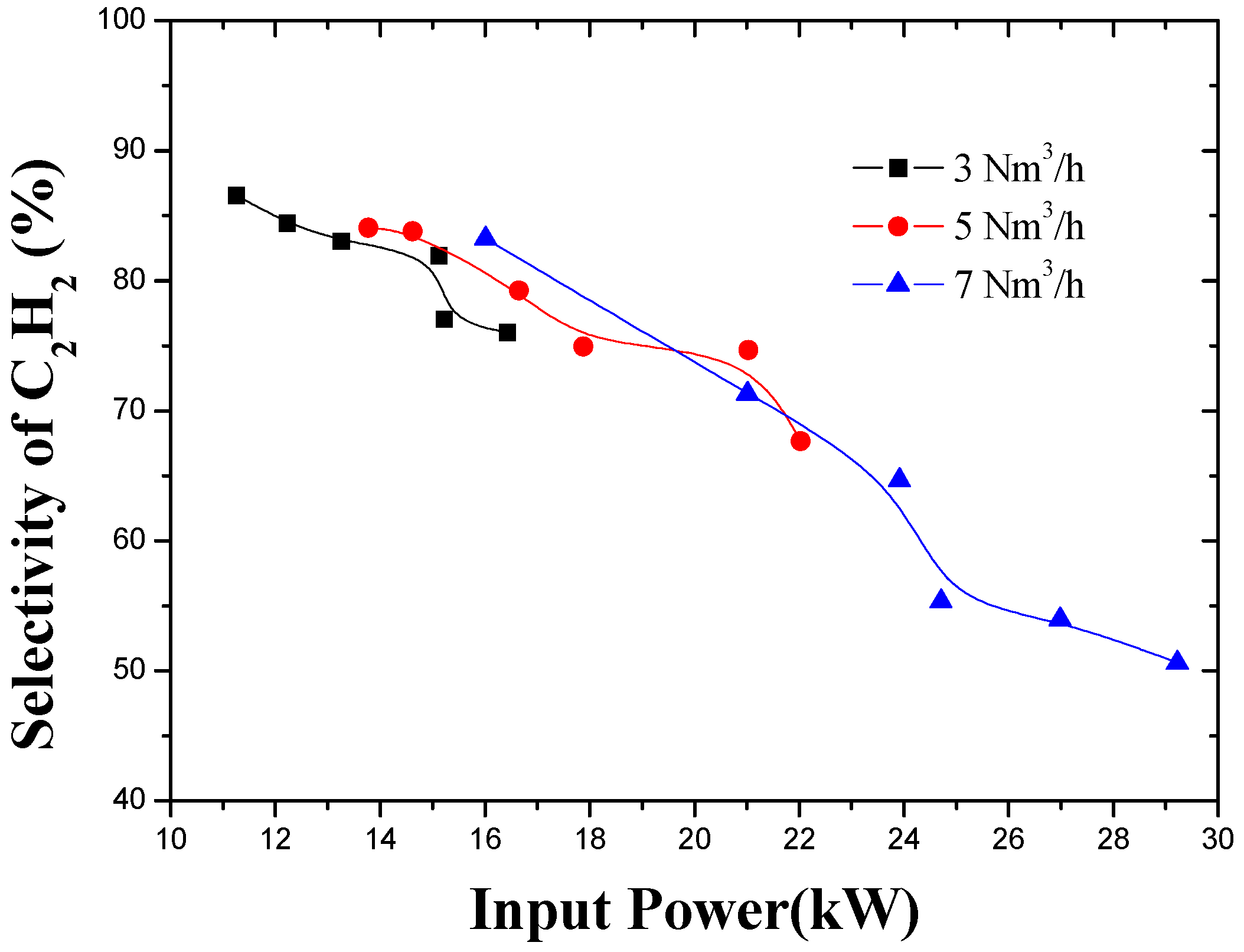
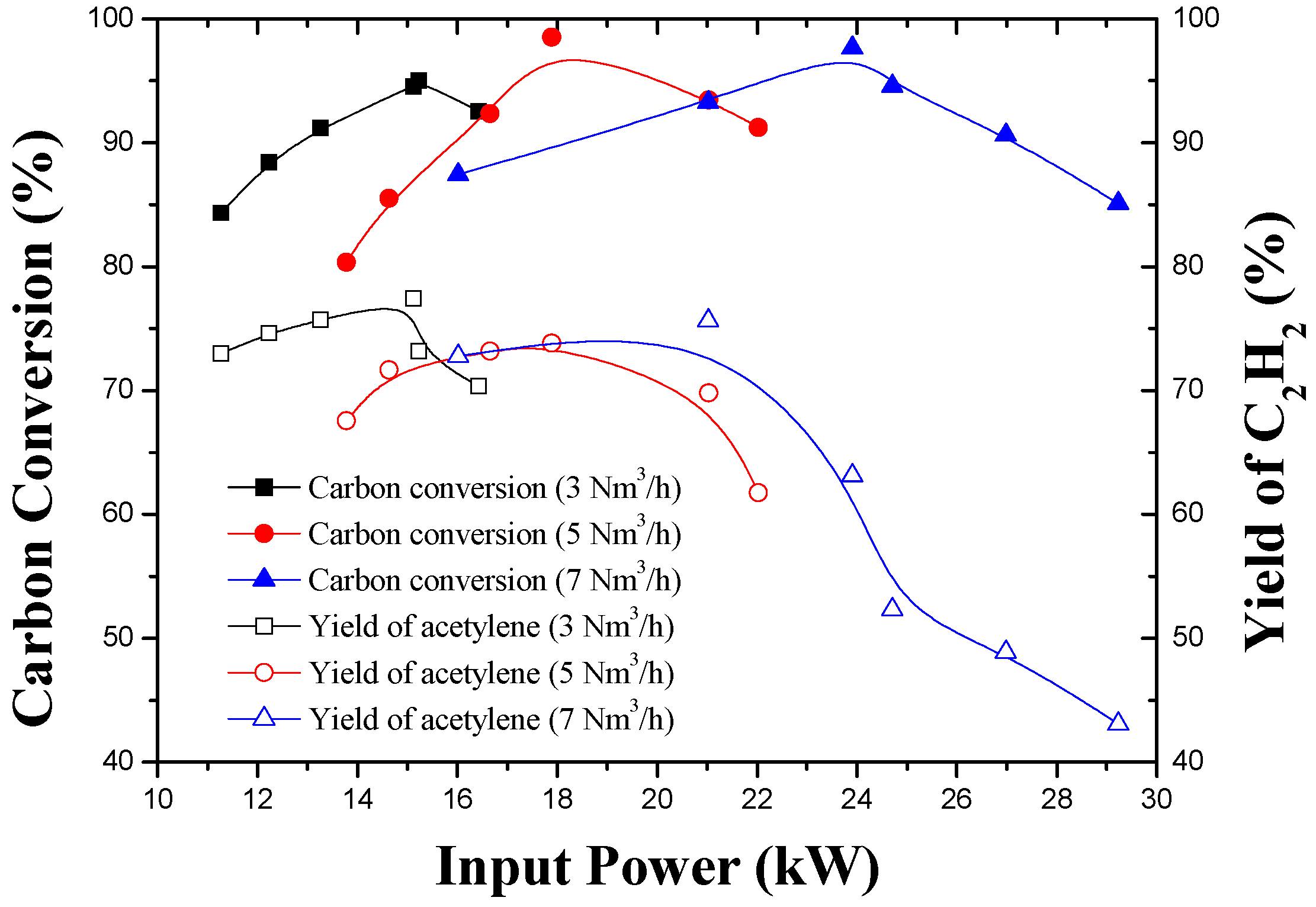
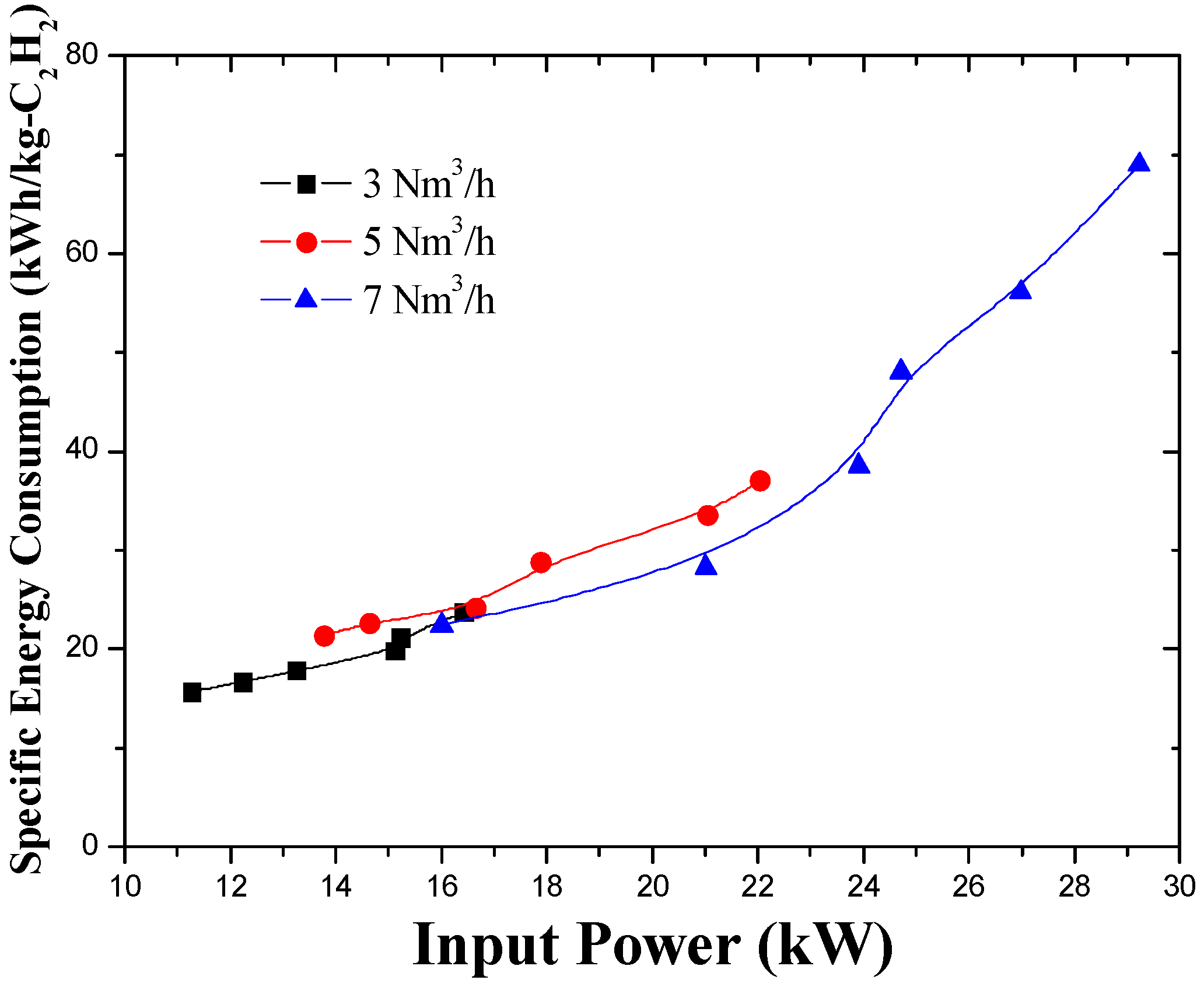
3.3. Effect of Working Gas Flow Rate
3.4. Polypropylene Pyrolysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| mi | Flow rate of component i (g/min) |
| Ci% | Mass fraction of C in component i (%) |
| mfs | Feed rate (g/min) |
| Cfs% | Mass fraction of C in feedstock (%) |
| U | Arc voltage (V) |
| I | Arc current (A) |
| X | Carbon conversion to gaseous product (%) |
| Se | Selectivity of acetylene (%) |
| Y | Yield of acetylene (%) |
| SEC | Specific energy consumption of acetylene (%) |
References
- Moulay, S. Chemical modification of poly(vinyl chloride)-still on the run. Prog. Polym. Sci. 2010, 35, 303–331. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degrad. Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Achiliasa, D.S.; Roupakiasa, C.; Megalokonomosa, P.; Lappasb, A.A.; Antonakoub, E.V. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Abreu, D.A.P.; Losada, P.P.; Angulo, I.; Cruz, J.M. Development of new polyolefin films with nanoclays for application in food packaging. Eur. Polym. J. 2007, 43, 2229–2243. [Google Scholar] [CrossRef]
- Pasti, L.; Melucci, D.; Contado, C.; Dondi, F.; Mingozzi, I. Calibration in thermal field flow fractionation with polydisperse standards: Application to polyolefin characterization. J. Sep. Sci. 2015, 25, 691–702. [Google Scholar] [CrossRef]
- Huang, S.-J. Polymer Waste management–biodegradation, incineration, and recycling. J. Macromol. Sci. A 1995, 32, 593–597. [Google Scholar] [CrossRef]
- He, M.; Xiao, B.; Hu, Z.; Liu, S.; Guo, X.; Luo, S. Syngas production from catalytic gasification of waste polyethylene: Influence of temperature on gas yield and composition. Int. J. Hydrogen Energy 2009, 34, 1342–1348. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Conesa, J.A.; Font, R.; Marcilla, A.; Garcia, A.N. Pyrolysis of polyethylene in a fluidized bed reactor. Energy Fuels 1994, 8, 1238–1246. [Google Scholar] [CrossRef]
- Westerhout, R.W.J.; Waanders, J.; Kuipers, J.A.M.; Swaaij, W.P.M. Kinetics of the low-temperature pyrolysis of polyethene, polypropene, and polystyrene modeling, experimental determination, and comparison with literature models and data. Ind. Eng. Chem. Res. 1997, 36, 1955–1964. [Google Scholar] [CrossRef]
- Hınıslıoğlu, S.; Ağar, E. Use of waste high density polyethylene as bitumen modifier in asphalt concrete mix. Mater. Lett. 2004, 58, 267–271. [Google Scholar] [CrossRef]
- Ciliz, N.K.; Ekinci, E.; Snape, C.E. Pyrolysis of virgin and waste polypropylene and its mixtures with waste polyethylene and polystyrene. Waste Manag. 2004, 24, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Christmann, W.; Kasiske, D.; Klöppel, K.D.; Partscht, H.; Rotard, W. Combustion of polyvinylchloride: An important source for the formation of PCDD/PCDF. Chemosphere 1989, 19, 387–392. [Google Scholar] [CrossRef]
- Ma, J.; Su, B.; Wen, G.; Ren, Q.; Yang, Y.; Yang, Q.; Xing, H. Kinetic modeling and experimental validation of the pyrolysis of propane in hydrogen plasma. Int. J. Hydrogen Energy 2016, 41, 22689–22697. [Google Scholar] [CrossRef]
- Yan, B.; Cheng, Y.; Cheng, Y. Particle-scale modeling of coal devolatilization behaviors for coal pyrolysis in thermal plasma reactors. AIChE J. 2014, 61, 913–921. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, W.; Su, B.; Bao, Z.; Wen, G.; Xing, H.; Ren, Q. Conversion of glycerol into syngas by rotating DC arc plasma. Energy 2017, 123, 1–8. [Google Scholar] [CrossRef]
- Xu; Yang, X.; Sun, Y.; Zhang, J.; Song, J. MW-DC hybrid plasma conversion of natural gas to acetylene. Acta Chim. Sin. 2005, 63, 625–630. [Google Scholar]
- Gehrmann, K.; Schmidt, H. Pyrolysis of hydrocarbons using a hydrogen plasma. In Proceedings of the 8th World Petroleum Congress, Moscow, Russia, 13–18 June 1971. [Google Scholar]
- Beiers, H.G.; Baumann, H.; Bittner, D.; Klein, J.; Jüntgen, H. Pyrolysis of some gaseous and liquid hydrocarbons in hydrogen plasma. Fuel 1988, 67, 1012–1016. [Google Scholar] [CrossRef]
- Tao, X.; Dai, W.; Chen, Q.; Yin, Y.; Dai, X. Experimental study on production of acetylene from natural gas by plasma jet. Nat. Gas Ind. 2006, 26, 131–134. [Google Scholar]
- Chen, H.; Xie, K. Thermodynamic analysis of the hydrogen-carbon system for acetylene production by plasma. Chin. J. Process Eng. 2002, 2, 112–117. [Google Scholar]
- Arena, U. Process and technological aspects of municipal solid waste gasification: A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Hiraoka, M. Municipal solid waste incinerator residue recycling by thermal processes. Waste Manag. 2000, 20, 249–258. [Google Scholar] [CrossRef]
- Zhang, Q.; Dor, L.; Fenigshtein, D.; Yang, W.; Blasiak, W. Gasification of municipal solid waste in the Plasma Gasification Melting process. Appl. Energy 2012, 90, 106–112. [Google Scholar] [CrossRef]
- Kerzelis, R.; Mecius, V.; Valinciute, V.; Valincius, V. Waste and biomass treatment employing plasma technology. High Temp. Mater. Processes 2004, 8, 273–282. [Google Scholar] [CrossRef]
- Guddeti, R.R.; Knight, R.; Grossmann, E.D. Depolymerization of polyethylene using induction-coupled plasma technology. Plasma Chem. Plasma Process. 2000, 20, 37–64. [Google Scholar] [CrossRef]
- Guddeti, R.R.; Knight, R.; Grossmann, E.D. Depolymerization of polypropylene in an induction-coupled plasma (ICP) reactor. Ind. Eng. Chem. Res. 2000, 39, 1171–1176. [Google Scholar] [CrossRef]
- Chang, J.-S.; Gu, B.-W.; Looy, P.C.; Chu, F.-Y.; Simpson, C.J. Thermal plasma pyrolysis of used old tires for production of syngas. J. Environ. Sci. Health A 1996, 31, 1781–1799. [Google Scholar] [CrossRef]
- Baumann, H.; Bitter, D.; Beiers, H.G.; Klein, J.; Jüntgen, H. Pyrolysis of coal in hydrogen and helium plasmas. Fuel 1988, 67, 1120–1123. [Google Scholar] [CrossRef]



| Process Parameters | Range | Optimum |
|---|---|---|
| Current (A) | 60–200 | 80–100 |
| Voltage (V) | 180–270 | 200 |
| Input power (kW) | 10–30 | 12–15 |
| Magnetic flux intensity (T) | 0.020–0.096 | 0.096 |
| Feed rate (g/min) | 10–20 | / |
| Carrier gas (Ar) (Nm3/h) | 1 | 1 |
| Working gas (H2) (Nm3/h) | 3–7 | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Ma, J.; Su, B.; Wen, G.; Yang, Q.; Ren, Q. Pyrolysis of Polyolefins Using Rotating Arc Plasma Technology for Production of Acetylene. Energies 2017, 10, 513. https://doi.org/10.3390/en10040513
Zhang M, Ma J, Su B, Wen G, Yang Q, Ren Q. Pyrolysis of Polyolefins Using Rotating Arc Plasma Technology for Production of Acetylene. Energies. 2017; 10(4):513. https://doi.org/10.3390/en10040513
Chicago/Turabian StyleZhang, Ming, Jie Ma, Baogen Su, Guangdong Wen, Qiwei Yang, and Qilong Ren. 2017. "Pyrolysis of Polyolefins Using Rotating Arc Plasma Technology for Production of Acetylene" Energies 10, no. 4: 513. https://doi.org/10.3390/en10040513






