CO2 Foam Stability Improvement Using Polyelectrolyte Complex Nanoparticles Prepared in Produced Water
Abstract
:1. Introduction
2. Materials
2.1. Polyethylenimine
2.2. Dextran Sulfate
2.3. Surfactant
2.4. Mississippian Brine
2.5. Crude Oil
3. Sample Preparation
3.1. Surfactant Solutions
3.2. Polyethylenimine Solution
3.3. Dextran Sulfate Solution
3.4. Nanoparticle Solutions
3.5. PEI-Surfactant and PECNP-Surfactant Systems
4. Methods
4.1. Zeta Potential and DLS Particle Size Measurements
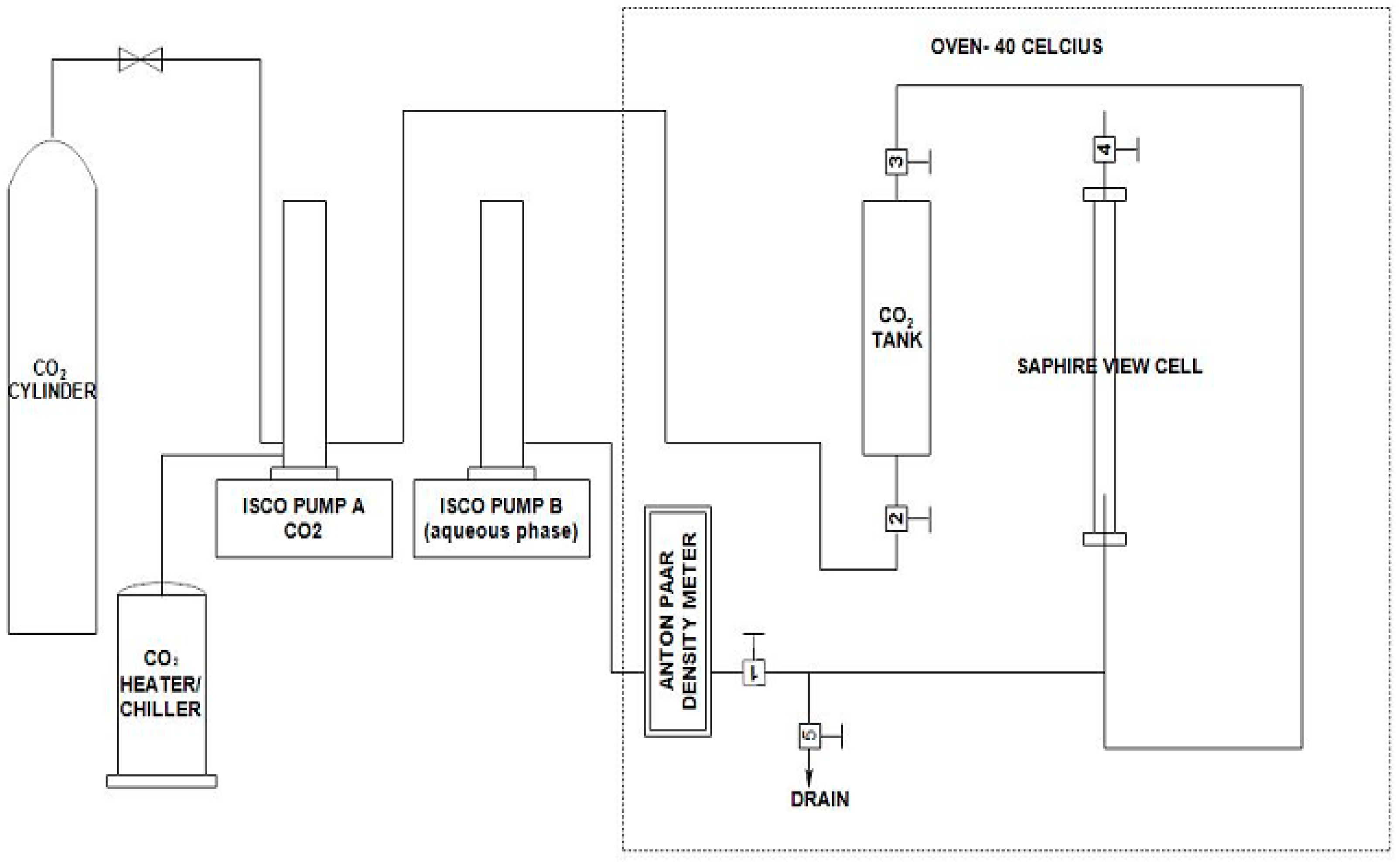
4.2. Interfacial Tension Measurements
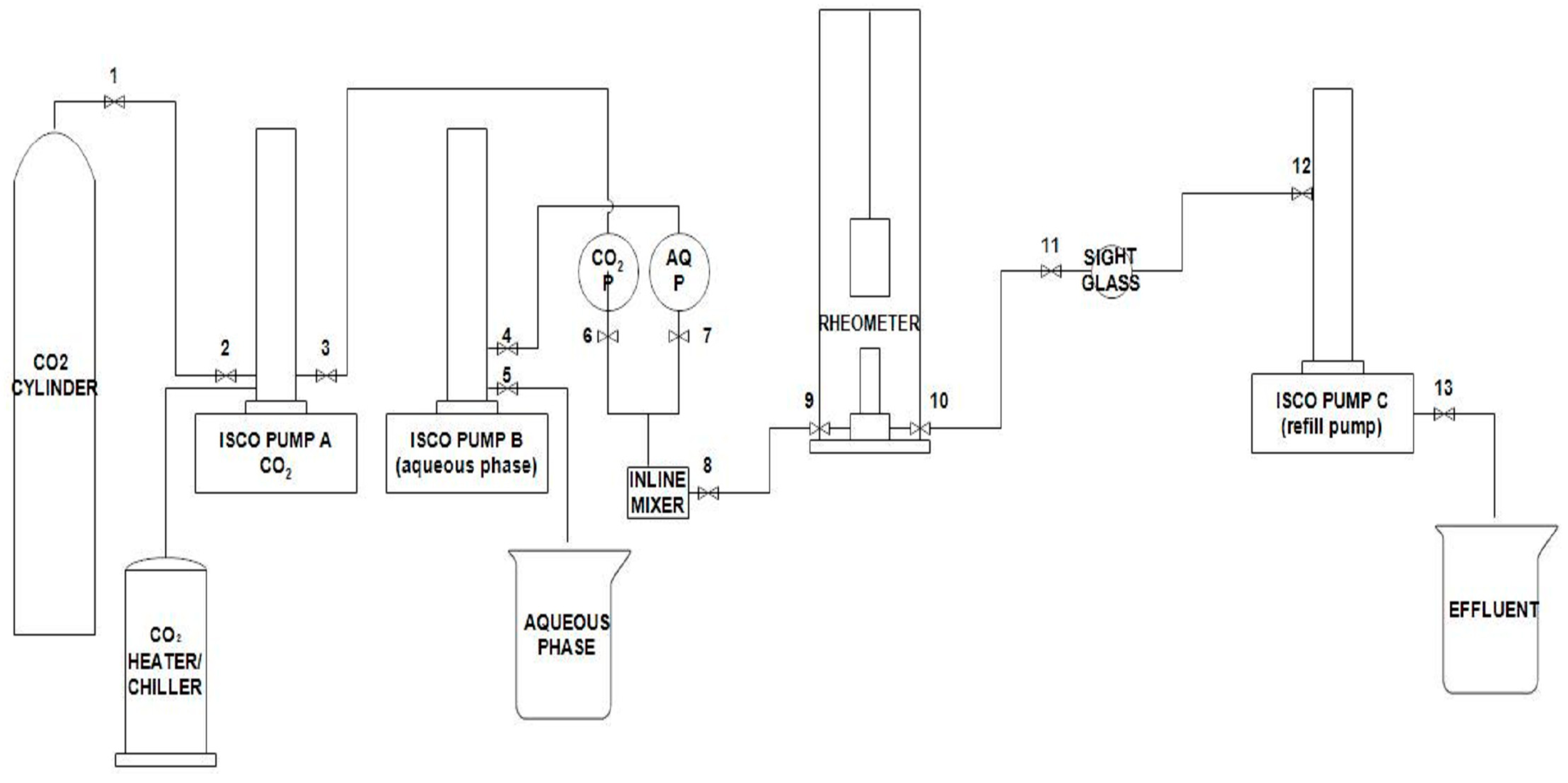
4.3. Rheology Measurements
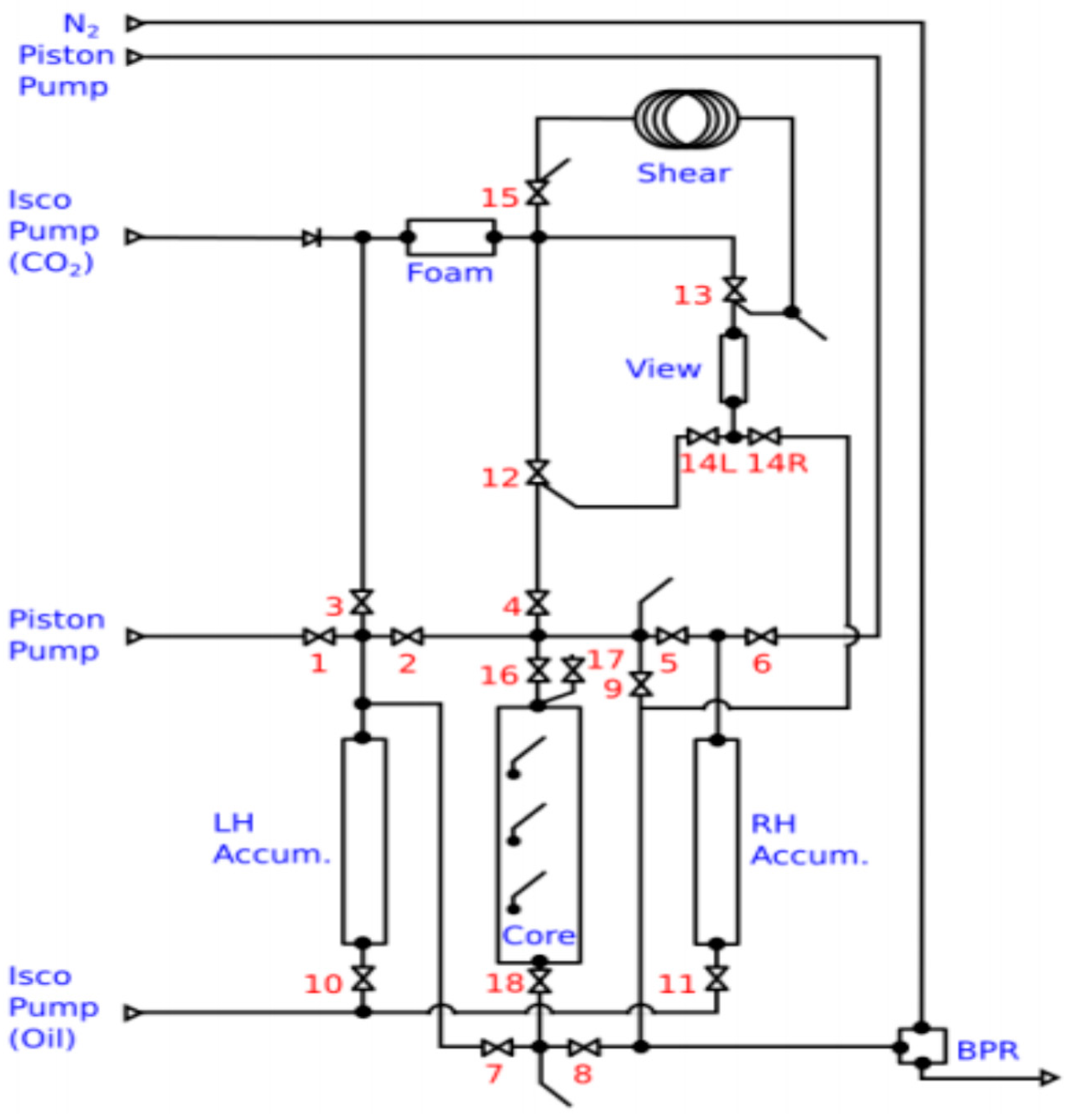
4.4. View Cell Testing
5. Results and Discussion
5.1. Particle Size and Zeta Potential Results
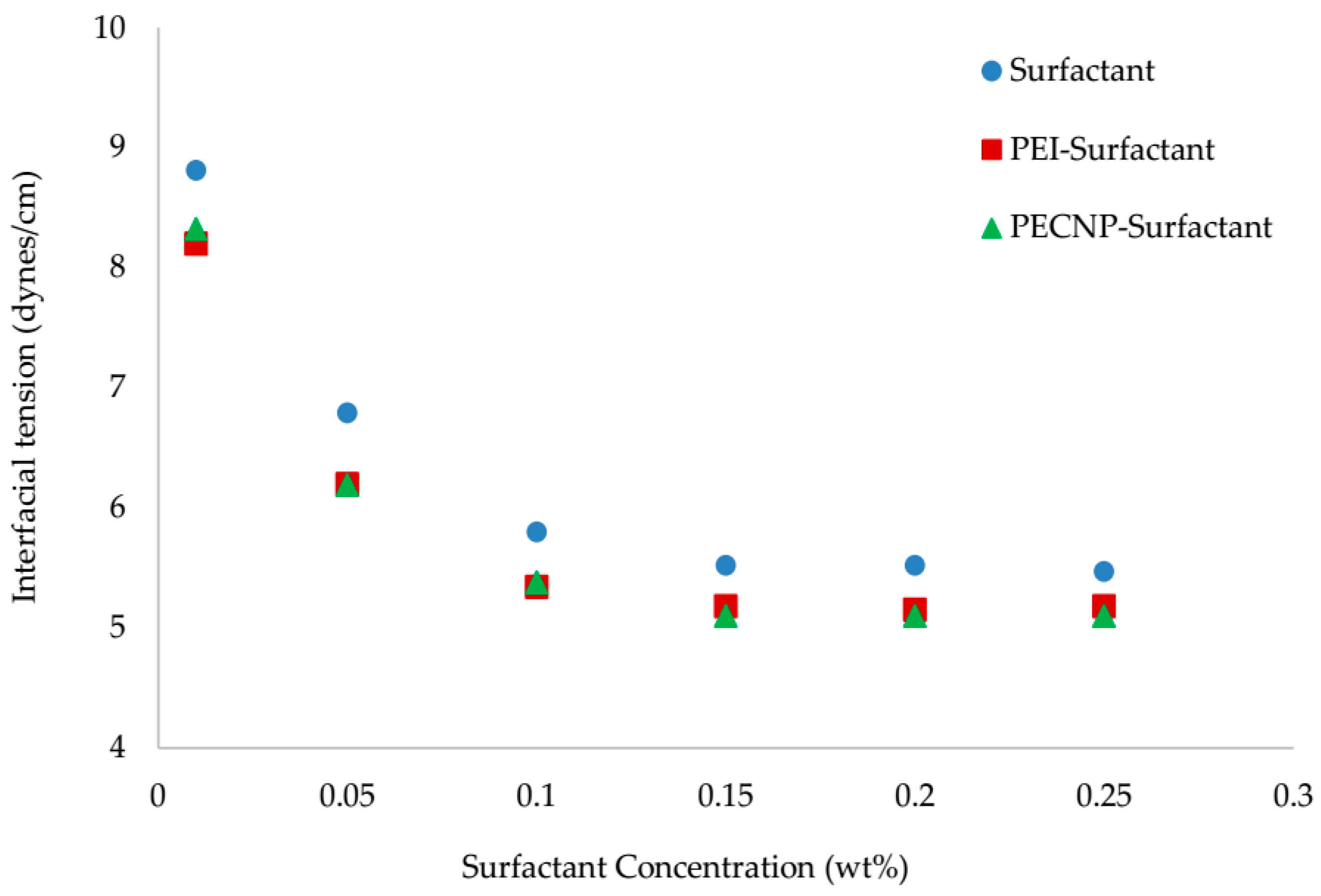
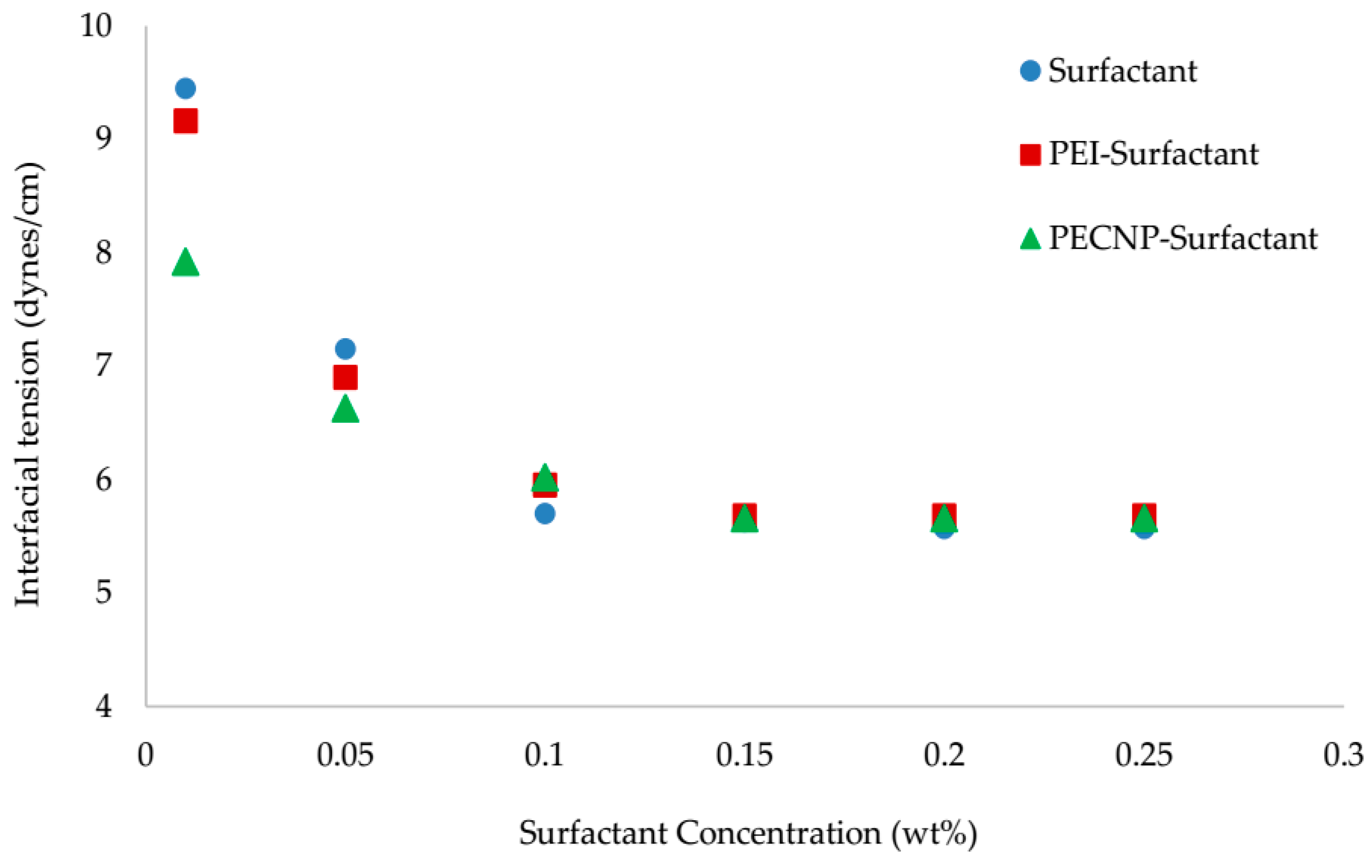
5.2. Interfacial Tension Measurement Results
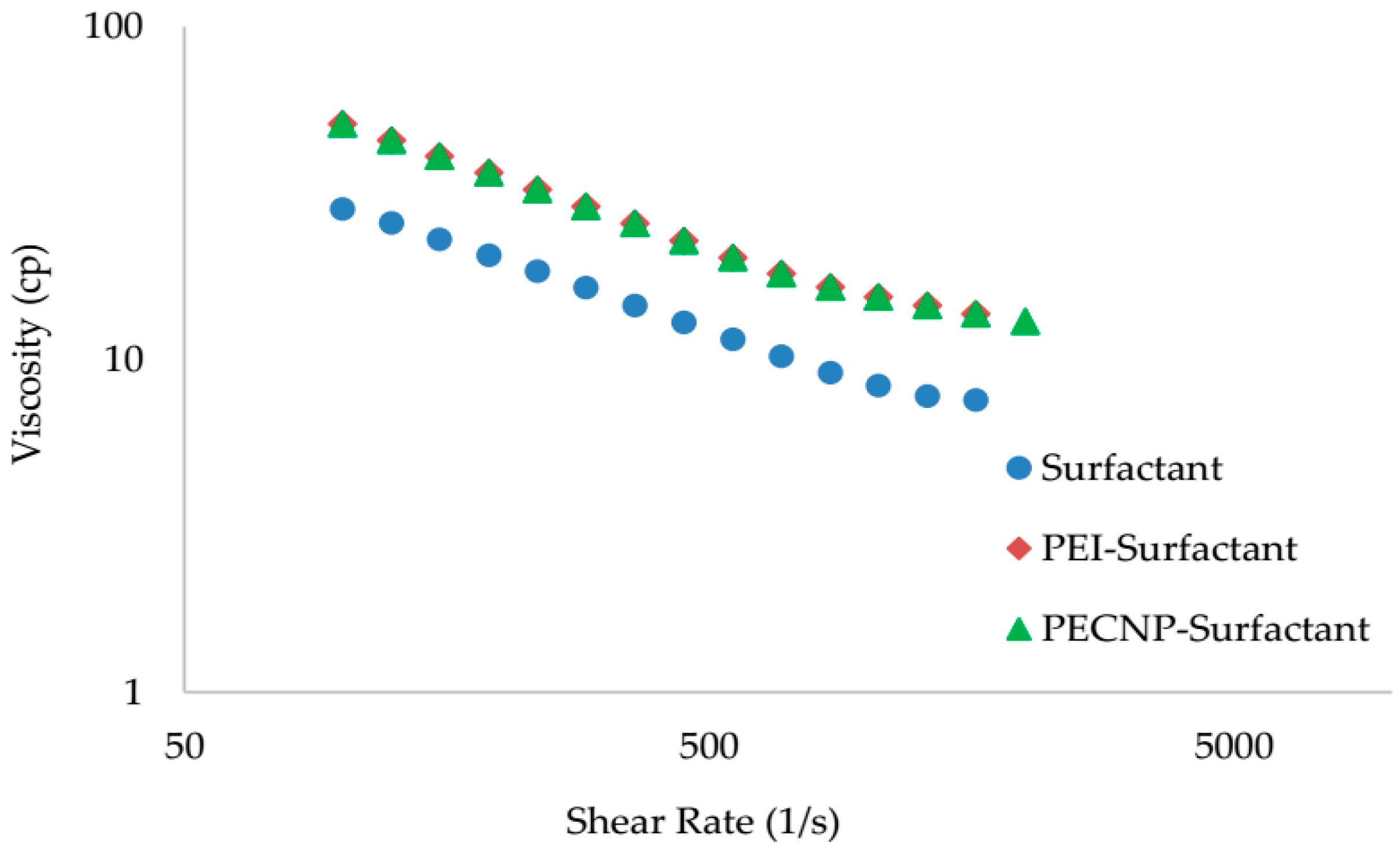
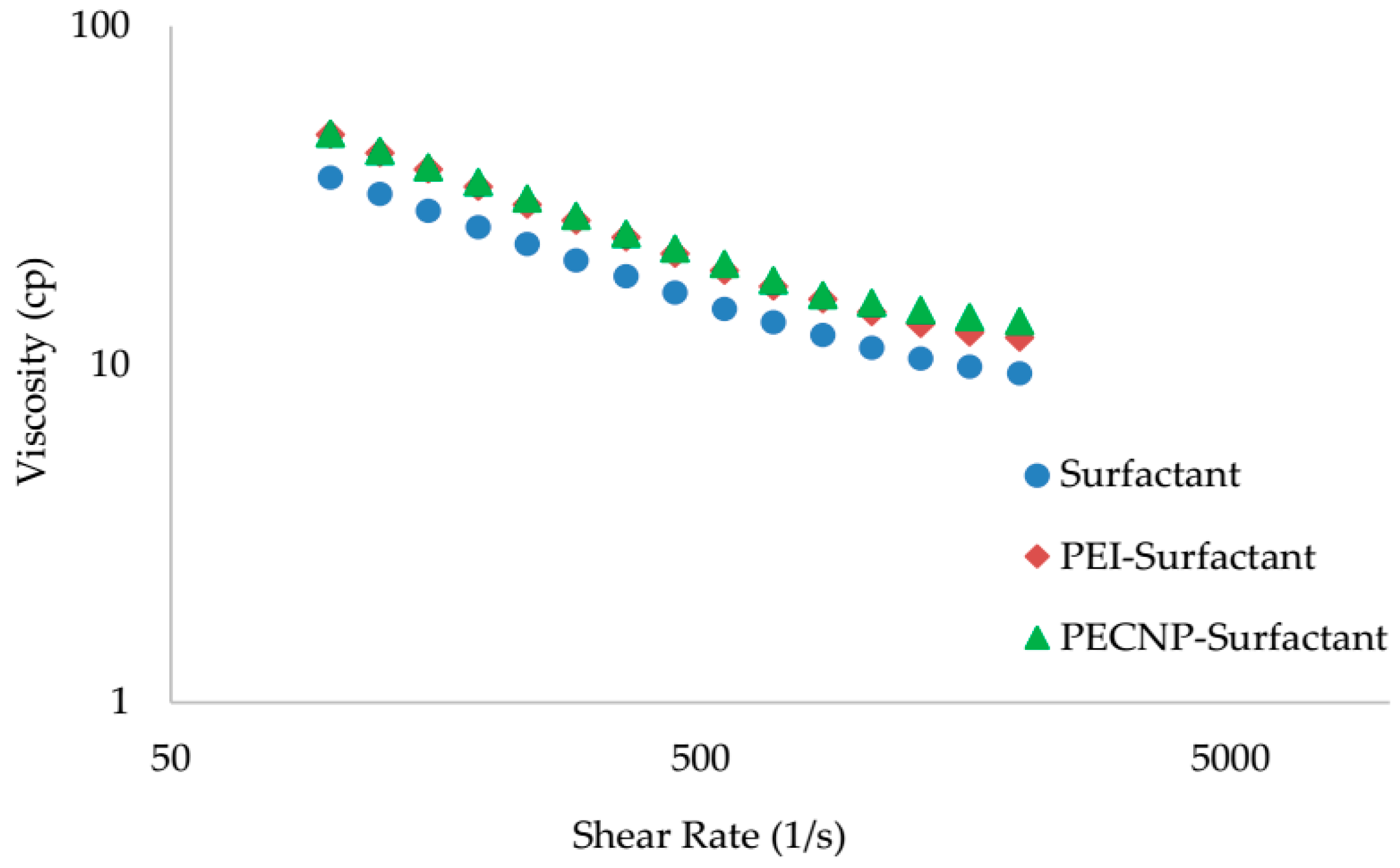
5.3. Rheology Measurement Results
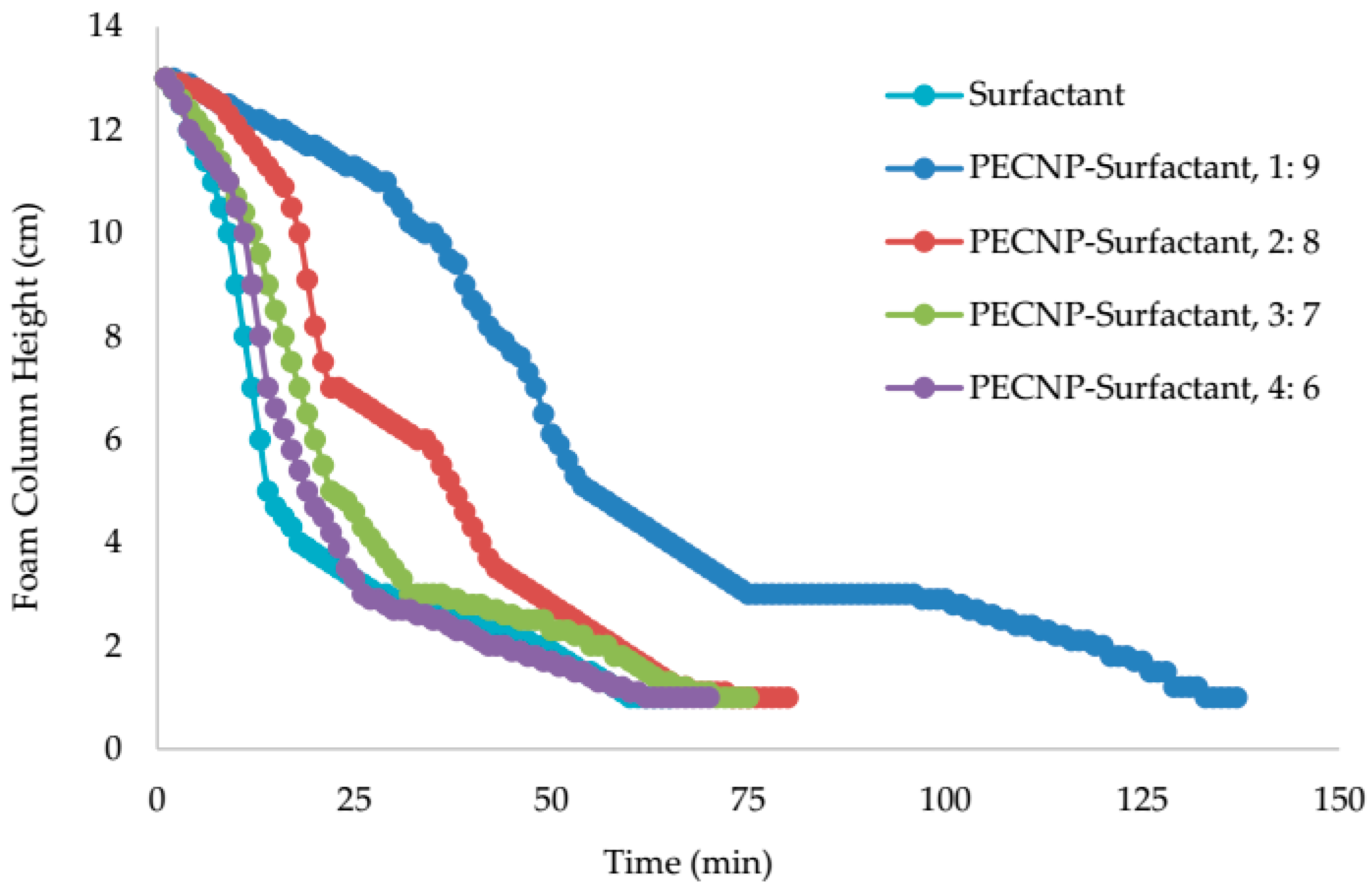
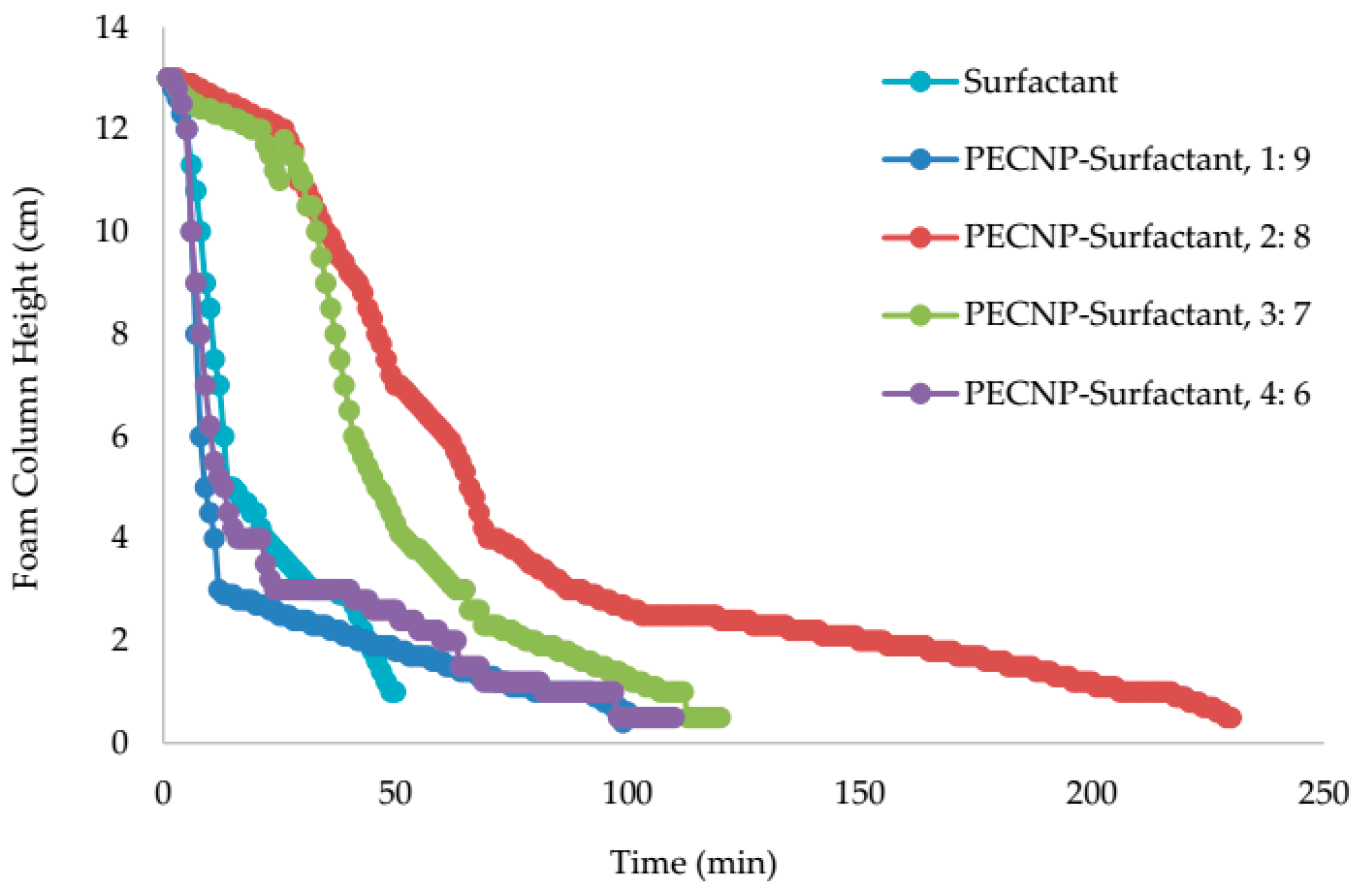
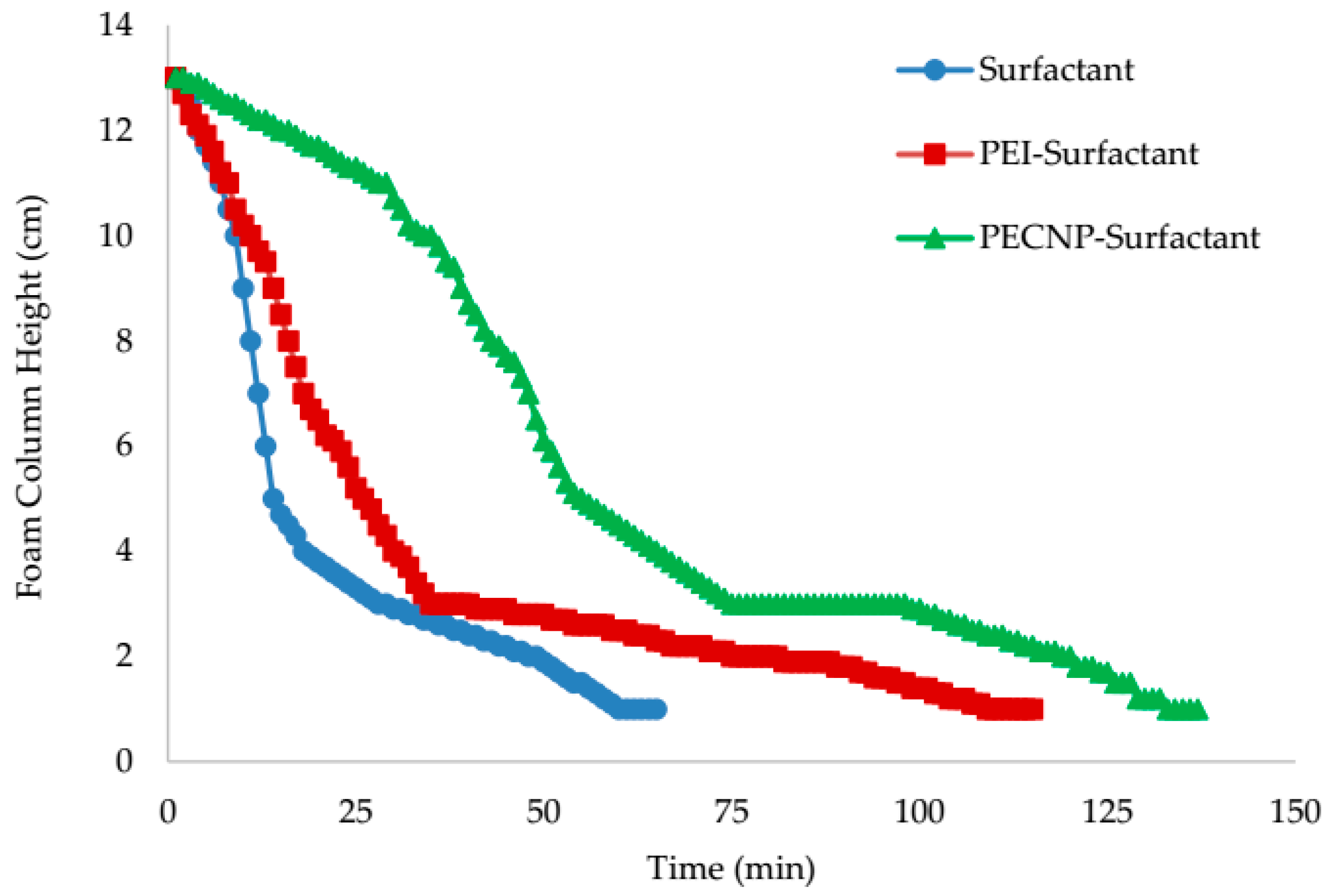
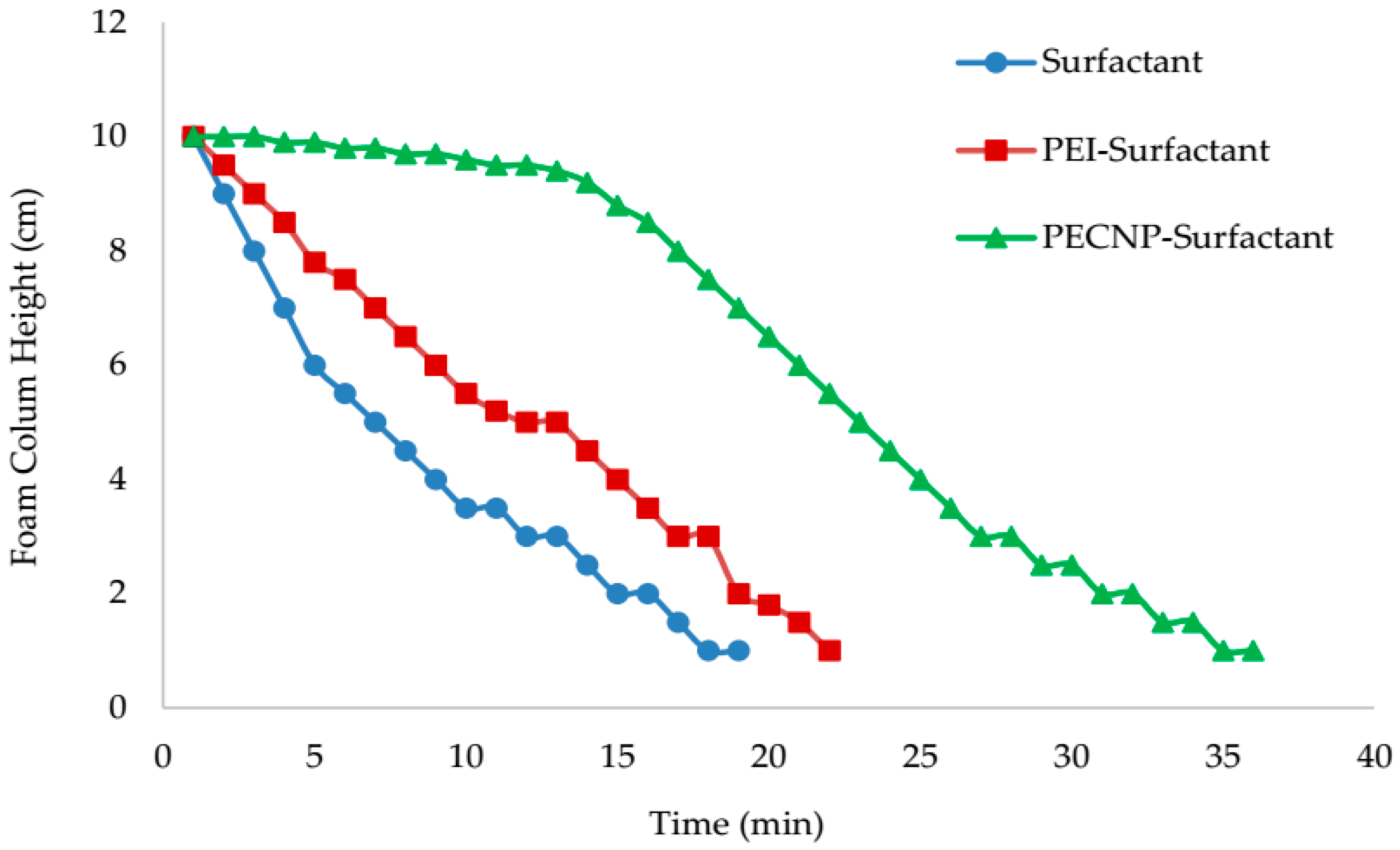
5.4. View Cell Testing without Crude Oil
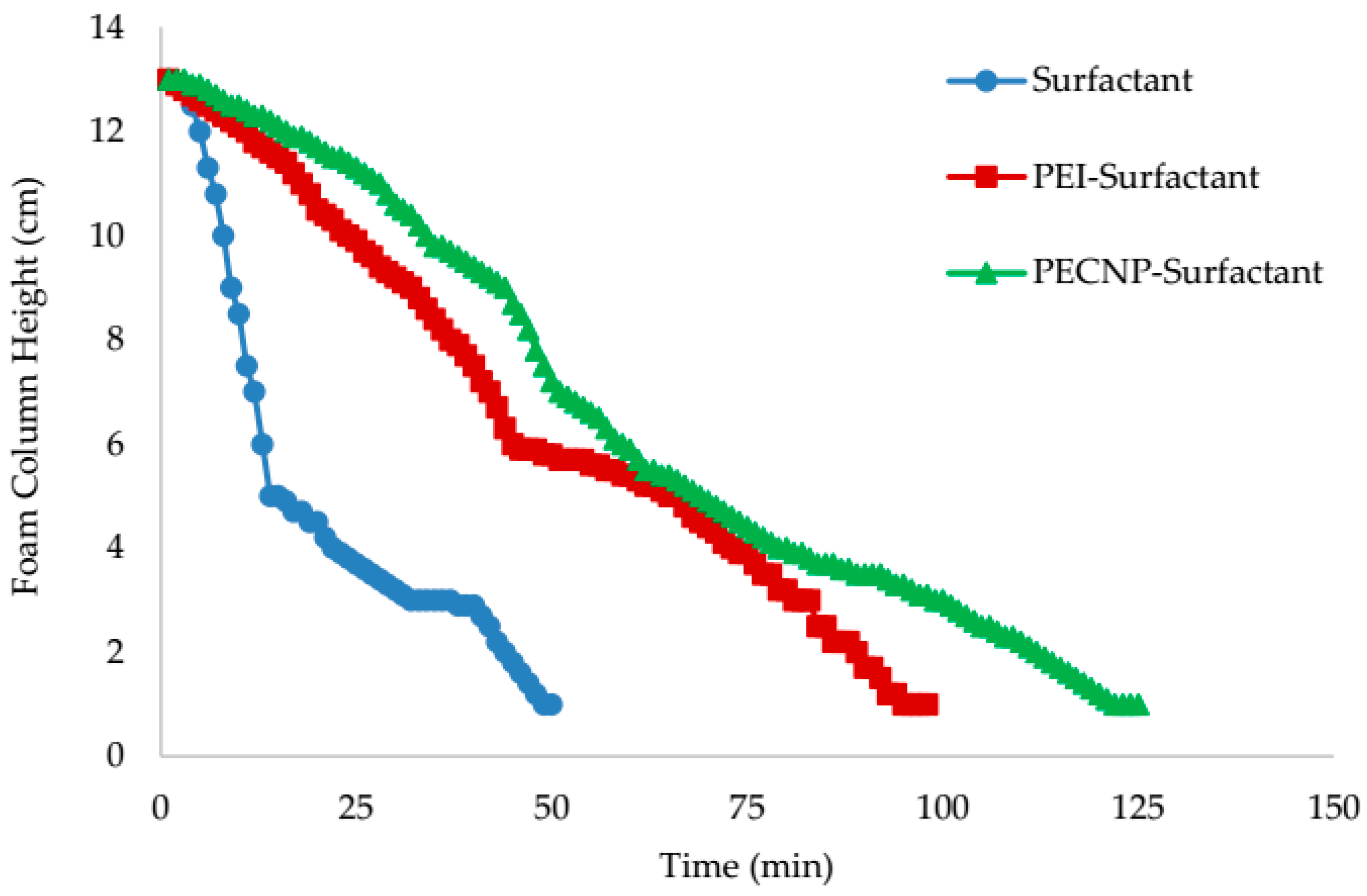
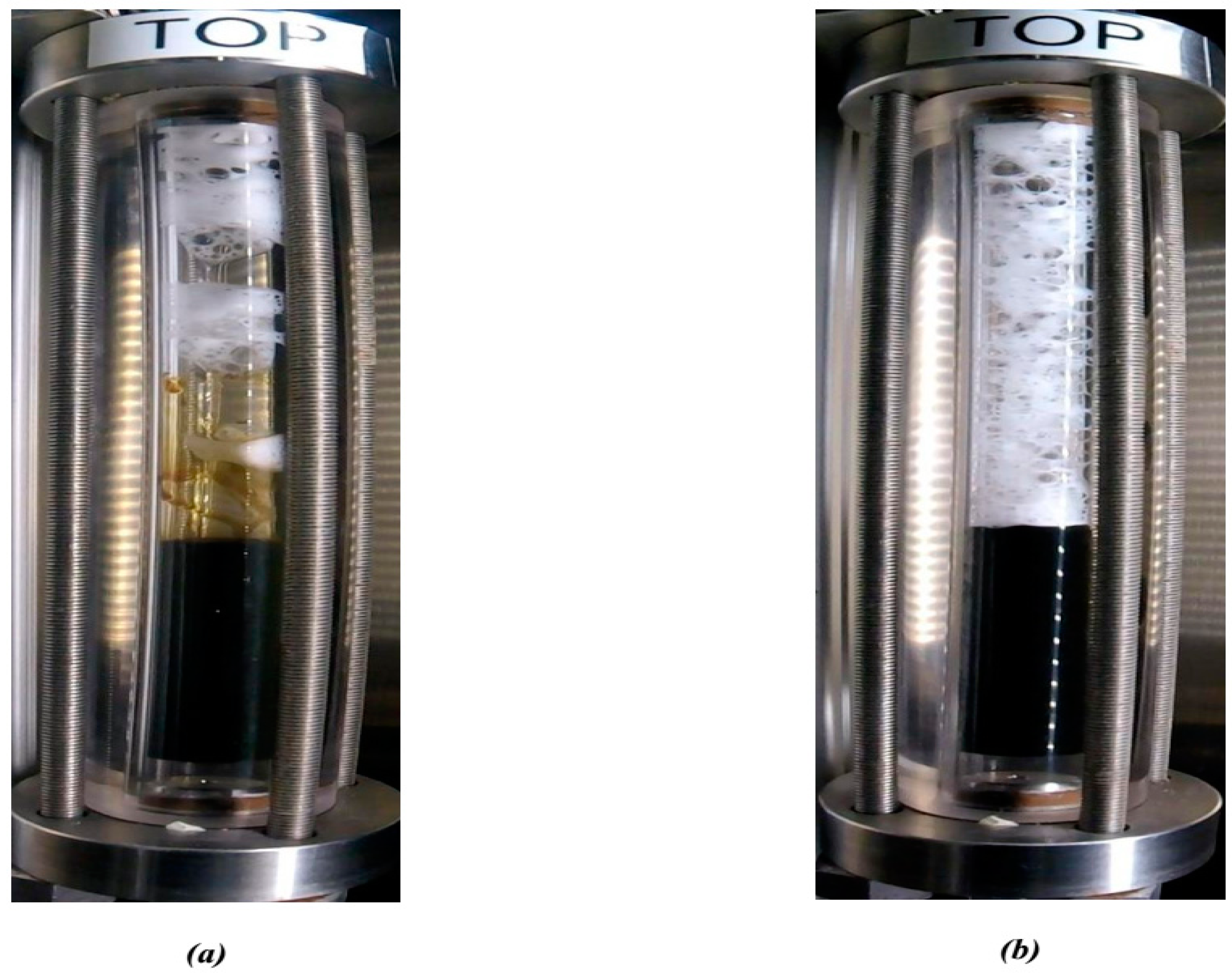
5.5. View Cell Testing with the Mississippian Crude Oil
6. Conclusions
- The rheology tests indicated that adding PEI and PECNP to the surfactant solution helps to improve the rheological properties of the foam. Under the ramp test, adding PEI and PECNP helped to improve both the flow consistency and behavior indices.
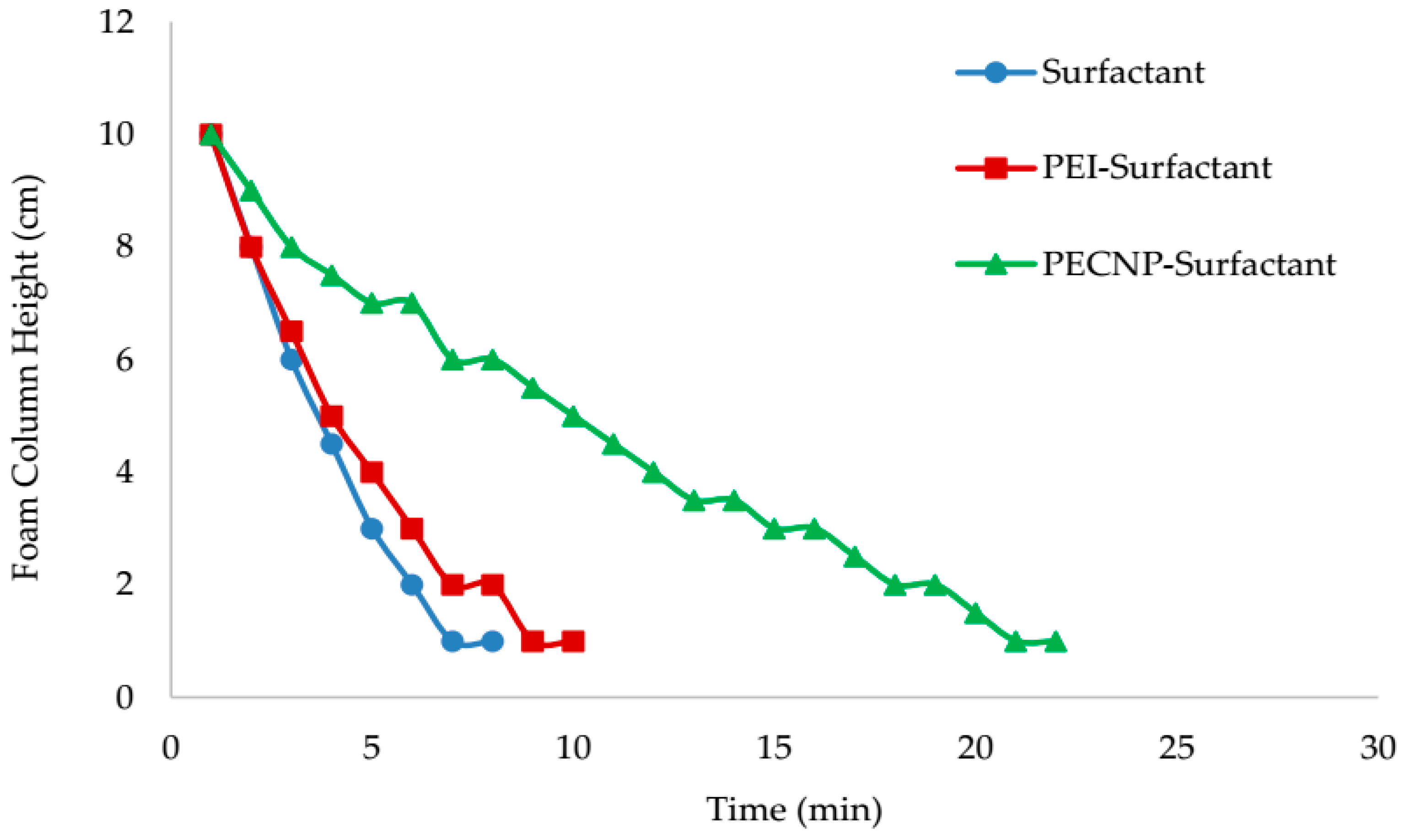
- The view cell test results without the crude oil showed that adding PEI and PECNP, in any ratio of PEI:surfactant and PECNP:surfactant to the surfactant solutions, improves the foam durability. Furthermore, the PECNP-surfactant generated CO2 foam showed the highest durability compared to the surfactant generated foam and the PEI-surfactant generated CO2 foam systems.
- From the view cell test results in the presence of the crude oil, it can be concluded that the PECNP-surfactant generated CO2 foam is more durable compared to the other two systems of the PEI-surfactant and surfactant generated CO2 foam. Additionally, the PECNP-surfactant generated CO2 foam stabilizes the interface of the foam when it reaches to the crude oil.
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclatures
| Shear stress, lb/ft2 | |
| Flow consistency index | |
| Flow behavior index | |
| Viscosity, cp | |
| Effective viscosity, cp | |
| γ | Shear rate, s−1 |
References
- Langston, M.; Hoadley, S.; Young, D. Definitive CO2 Flooding Response in the SACROC Unit. In Proceedings of the Enhancced Oil Recovery Symposium, Tulsa, OK, USA, 16–21 April 1988. [Google Scholar]
- Holm, W.L. CO2 Flooding: Its Time Has Coming. J. Pet. Technol. 1982, 34, 2739. [Google Scholar] [CrossRef]
- Stalkup, F.I. Miscible Displacement. In Monograph; Society of Petroleum Engineers: Dallas, TX, USA, 1983; Volume 8. [Google Scholar]
- Green, D.; Willhite, P. Enhanced Oil Recovery; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 1998. [Google Scholar]
- Barati, R.; Pennel, S.; Matson, M.; Linroth, M. Overview of CO2 Injection and WAG Sensitivity in SACROC. In Proceedings of the Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Bernard, G.G.; Holm, L.W. Method for Recovering Oil from Subterranean Formations. U.S. Patent 3,342,256, 19 September 1967. [Google Scholar]
- JPT Staff. CO2 Foam Floods: Foam Properties and Mobility Reduction Effectiveness. J. Pet. Technol. 1998. [Google Scholar] [CrossRef]
- Fried, A. The Foam-Drive Process for Increasing the Recovery of Oil; Report of Investigation 5866; USBM: Washington, DC, USA, 1961. [Google Scholar]
- Lord, D.L. Analysis of Dynamic and Static Foam Behavior. J. Pet. Technol. 1981, 33, 39–45. [Google Scholar] [CrossRef]
- Tsau, J.S.; Heller, J.P. Evaluation of Surfactants for CO2 Foam Mobility Control. In Proceedings of the Permian Basin Oil and Gas Recovery Conference, Midland, TX, USA, 18–20 March 1992. [Google Scholar]
- Farajzadeh, R.; Andrianov, A.; Krastev, R.; Hirasaki, G.J.; Rossen, W.R. Foam-Oil Interaction in Porous Media: Implications for Foam Assisted Enhanced Oil Recovery. Adv. Colloid Interface Sci. 2012. [CrossRef]
- Schramm, L.L. Foam Sensitivity to Crude Oil in Porous Media; Foams, Fundamentals and Applications in the Petroleum Industry, American Chemical Society: Washington, DC, USA, 1994. [Google Scholar]
- Kalyanaraman, N.; Arnold, C.; Gupta, A.; Tsau, J.S.; Barati, R. Stability Improvement of CO2 Foam for Enhanced Oil Recovery Applications Using Polyelectrolytes and Polyelectrolyte Complex Nanoparticles. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015. [Google Scholar]
- Kristen-Hochrein, N.; Laschewsky, A.; Miller, R.; Klitzing, R.V. Stability of Foam Films of Oppositely Charged Polyelectrolyte/Surfactant Mixtures: Effect of Isoelectric Point. J. Phys. Chem. 2011, 115, 14475–14483. [Google Scholar] [CrossRef] [PubMed]
- Kristen, N.; Klitzing, R.V. Effect of Polyelectrolyte/Surfactant Combinations on the Stability of Foam Films. Soft Matter 2009, 6, 849–861. [Google Scholar] [CrossRef]
- Patel, C.; Barrufet, M.A.; Petriciolet, A.B. Effective Resource Management of Produced Water in Oil and Gas Operations. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 8–10 June 2004. [Google Scholar]
- Evans, P.; Robinson, K. Produced Water Management-Reservoir and Facilities Engineering Aspects. In Proceedings of the Middle East Oil Show and Conference, Bahrain, 20–23 February 1999. [Google Scholar]
- Caudle, D.D. Produced Water Regulations in United States: Then, Now and in the Future. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 29 September–2 October 2002. [Google Scholar]
- International Union of Pure and Applied Chemistry (IUPAC). Compendium of Chemical Terminology (The Gold Book); Blackwell Scientific: Oxford, UK, 1997. [Google Scholar]
- Schramm, L.L.; Marangoni, G. Surfactants and Their Solutions: Basic Principles. In Surfactants: Fundamentals and Applications in the Petroleum Industry; Cambridge University Press: Cambridge, UK, 2000; pp. 9–10. [Google Scholar]
- Guerra, K.; Dahm, K.; Dundorf, S. Oil and Gas Produced Water Management and Beneficial Use in the Western United States; Science and Technology Program Report; U.S. Department of the Interior Bureau of Reclamation: Denver, CO, USA, 2011. [Google Scholar]
- Folarin, Y.; An, D.; Caffrey, S.; Soh, J.; Sensen, C.W.; Voordouw, J.; Jack, T.; Voordouw, G. Contribution of Make-up Water to the Microbial Community in an Oilfield from Which Oil is Produced by Produced Water Re-injection. Int. Biodeterior. Biodegrad. 2013, 81, 44–50. [Google Scholar] [CrossRef]

| Brine Composition | Concentration (mg/L) | Provider | Lot# | CAS# | Location |
|---|---|---|---|---|---|
| NaCl | 163,661.82 | Fisher Chemical | 166221 | 7647-14-5 | Lenexa, KS |
| Na2SO4 | 1224.30 | Fisher Chemical | 134830A | 7757-82-6 | |
| KCl | 714.93 | AMRESCO | 1015C371 | 7447-40-7 | Solon, OH |
| MgCl2·6H2O | 21,759.36 | Fisher Chemical | 134660 | 7791-18-6 | |
| CaCl2·2H2O | 46,886.13 | Fisher Chemical | 159018 | 10035-04-8 | |
| SrCl2·6H2O | 1535.5989 | Fisher Chemical | 4AD13081428A | 10025-70-4 | |
| Total | 235,782.11 |
| Batch# | PEI:DS:Brine | PECNP:Surfactant | Final pH | Effective Diameter (nm) | Polydispersity | Zeta Potential (mV) | Mobility (μ/s)/(V/cm) |
|---|---|---|---|---|---|---|---|
| 1 | 3:1:0.1 | 1:9 | 7.91 | 108.93 ± 0.40 | 0.27 | 21.18 ± 1.41 | 1.66 |
| 2 | 3:1:0.1 | 2:8 | 7.93 | 106.57 ± 1.50 | 0.24 | 13.79 ± 2.99 | 1.08 |
| 3 | 3:1:0.1 | 3:7 | 7.94 | 102.74 ± 1.18 | 0.25 | - | - |
| 4 | 3:1:0.1 | 4:6 | 7.94 | 105.59 ± 1.87 | 0.25 | 15.30 ± 1.68 | 1.20 |
| 5 | 3:1:0.1 | 5:5 | 7.90 | 106.99 ± 2.29 | 0.25 | 5.97 ± 5.04 | 0.47 |
| 6 | 4:1:0.1 | 1:9 | 7.85 | 116.52 ± 2.14 | 0.26 | 11.00 ± 0.29 | 0.86 |
| 7 | 4:1:0.1 | 2:8 | 7.92 | 110.32 ± 1.05 | 0.26 | 4.73 ± 1.04 | 0.65 |
| 8 | 4:1:0.1 | 3:7 | 7.96 | 103.96 ± 1.42 | 0.25 | 14.93 ± 1.53 | 1.17 |
| 9 | 4:1:0.1 | 4:6 | 7.97 | 107.94 ± 0.89 | 0.25 | - | - |
| 10 | 4:1:0.1 | 5:5 | 8.00 | 108.66 ± 4.76 | 0.24 | - | - |
| Batch# | PEI:DS:Brine | PECNP:Surfactant | Final pH | Effective Diameter (nm) | Polydispersity | Zeta Potential (mV) | Mobility (μ/s)/(V/cm) |
|---|---|---|---|---|---|---|---|
| 1 | 3:1:0.1 | 1:9 | 8.08 | 114.00 ± 2.00 | 0.268 | 9.81 ± 3.99 | 0.77 |
| 2 | 3:1:0.1 | 2:8 | 8.18 | 128.08 ± 1.26 | 0.300 | 19.27 ± 0.63 | 1.51 |
| 3 | 3:1:0.1 | 3:7 | 8.12 | 128.98 ± 4.20 | 0.319 | 13.49 ± 2.44 | 1.05 |
| 4 | 3:1:0.1 | 4:6 | 8.15 | 132.71 ± 3.13 | 0.311 | 22.10 ± 0.34 | 1.84 |
| 5 | 3:1:0.1 | 5:5 | 8.25 | 120.71 ± 0.75 | 0.283 | 17.66 ± 0.67 | 1.38 |
| 6 | 4:1:0.1 | 1:9 | 8.01 | 114.38 ± 3.15 | 0.264 | 14.42 ± 2.66 | 1.13 |
| 7 | 4:1:0.1 | 2:8 | 8.21 | 103.84 ± 1.43 | 0.265 | 11.82 ± 2.10 | 0.92 |
| 8 | 4:1:0.1 | 3:7 | 8.15 | 99.71 ± 1.11 | 0.261 | 12.25 ± 3.15 | 0.96 |
| 9 | 4:1:0.1 | 4:6 | 8.15 | 107.10 ± 0.92 | 0.273 | 16.31 ± 3.57 | 1.27 |
| 10 | 4:1:0.1 | 5:5 | 8.27 | 111.88 ± 6.47 | 0.286 | 14.25 ± 4.36 | 1.11 |
| System | K | n | |
|---|---|---|---|
| Surfactant | 0.250 | 0.520 | 0.99 |
| PEI-Surfactant, 1:9 | 0.436 | 0.520 | 0.99 |
| PECNP-Surfactant, 1:9 | 0.440 | 0.520 | 0.99 |
| System | K | n | |
|---|---|---|---|
| Surfactant | 0.284 | 0.540 | 0.99 |
| PEI-Surfactant, 2:8 | 0.333 | 0.570 | 0.99 |
| PECNP-Surfactant, 2:8 | 0.360 | 0.550 | 0.99 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazari, N.; Tsau, J.-S.; Barati, R. CO2 Foam Stability Improvement Using Polyelectrolyte Complex Nanoparticles Prepared in Produced Water. Energies 2017, 10, 516. https://doi.org/10.3390/en10040516
Nazari N, Tsau J-S, Barati R. CO2 Foam Stability Improvement Using Polyelectrolyte Complex Nanoparticles Prepared in Produced Water. Energies. 2017; 10(4):516. https://doi.org/10.3390/en10040516
Chicago/Turabian StyleNazari, Negar, Jyun-Syung Tsau, and Reza Barati. 2017. "CO2 Foam Stability Improvement Using Polyelectrolyte Complex Nanoparticles Prepared in Produced Water" Energies 10, no. 4: 516. https://doi.org/10.3390/en10040516







