Using SF6 Decomposed Component Analysis for the Diagnosis of Partial Discharge Severity Initiated by Free Metal Particle Defect
Abstract
:1. Introduction
2. Experiment
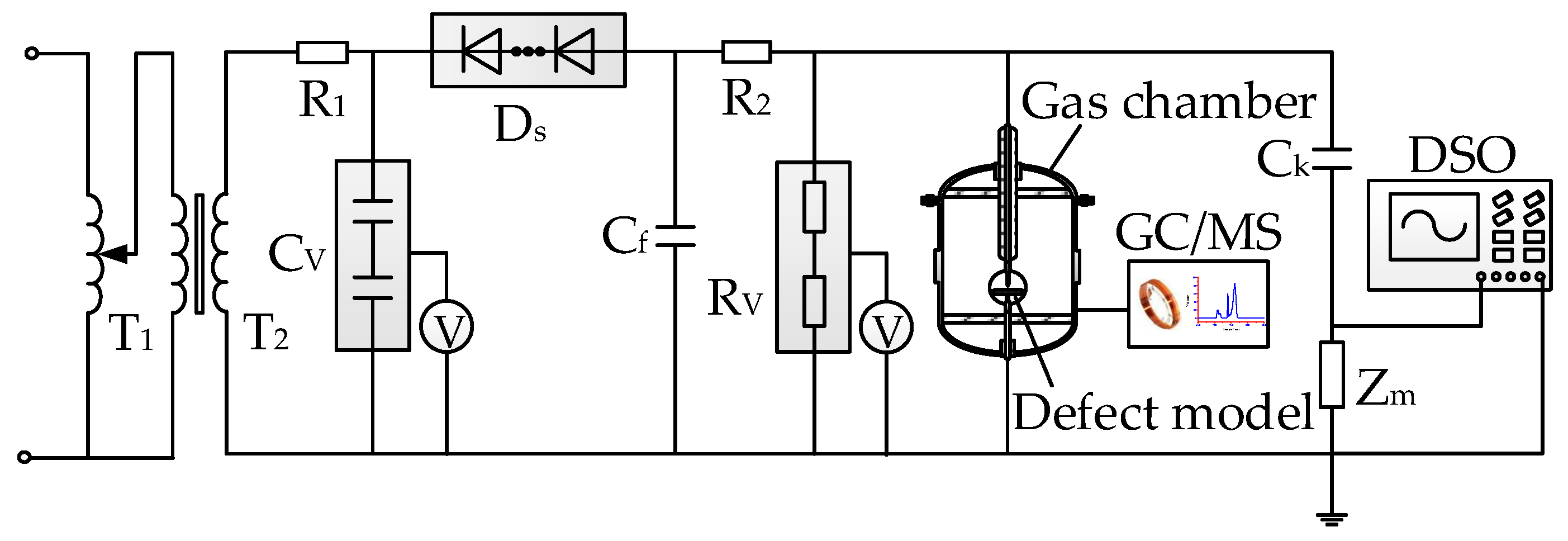
2.1. Experimental Platform
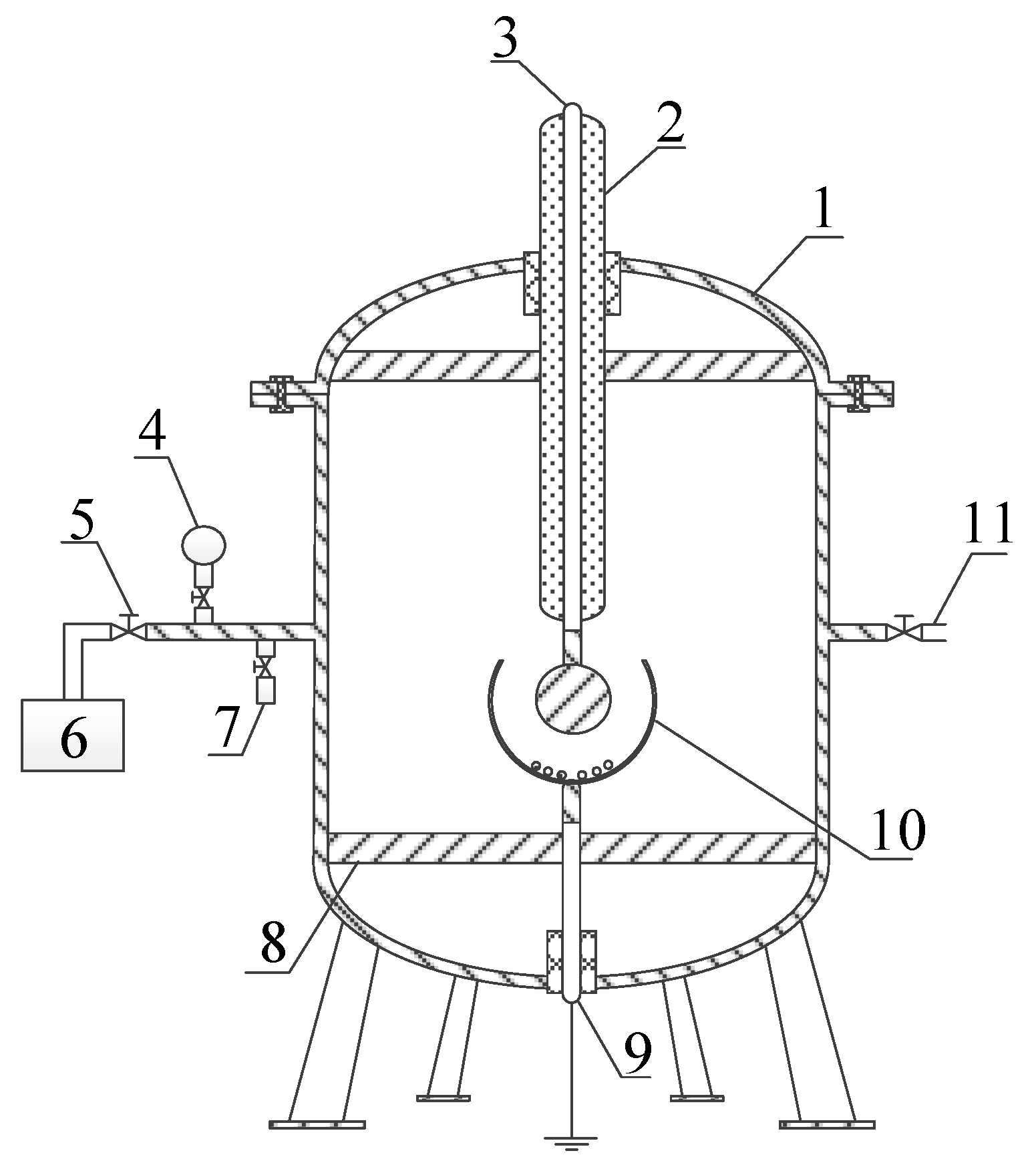
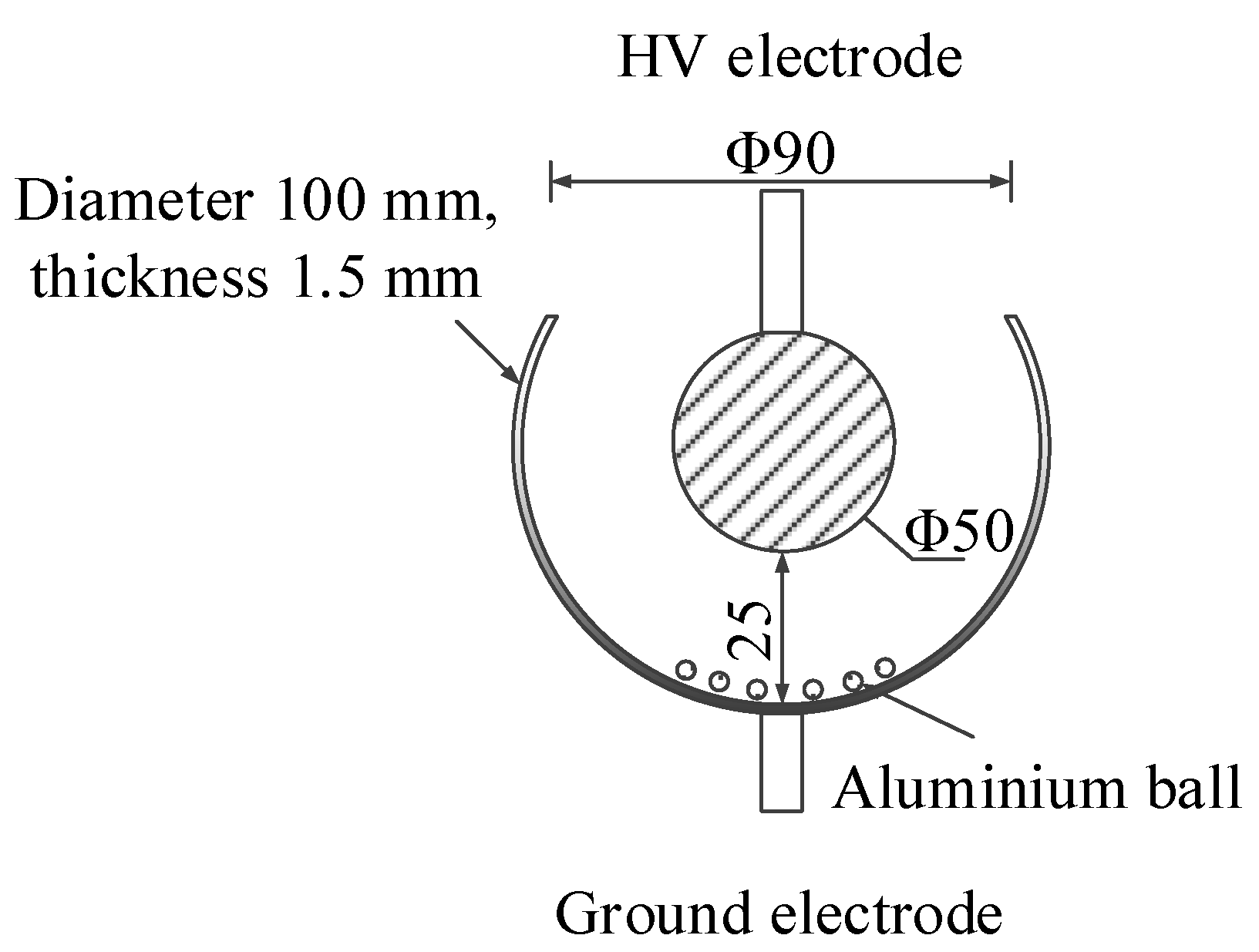
2.2. Insulation Defect Model
2.3. Experimental Method
- (1)
- The experimental platform is connected as shown in Figure 1. The absolute alcohol is used to scrub the insulation defect model and the inner wall of the PD gas chamber. The chamber is set aside for 1 h to completely volatize the alcohol. The defect model is placed in the gas chamber.
- (2)
- The PD gas chamber is vacuumed and then filled with 0.3 MPa new SF6 gas. This process is repeated several times for purification. A GE600 mirror dew-point meter is used to measure the H2O concentration, and a GPR-1200 ppm portable oxygen analyzer is used to measure the O2 concentration in the chamber. The concentrations of H2O and O2 are ensured to satisfy the industrial standard of DL/T 596-1996 [49].
- (3)
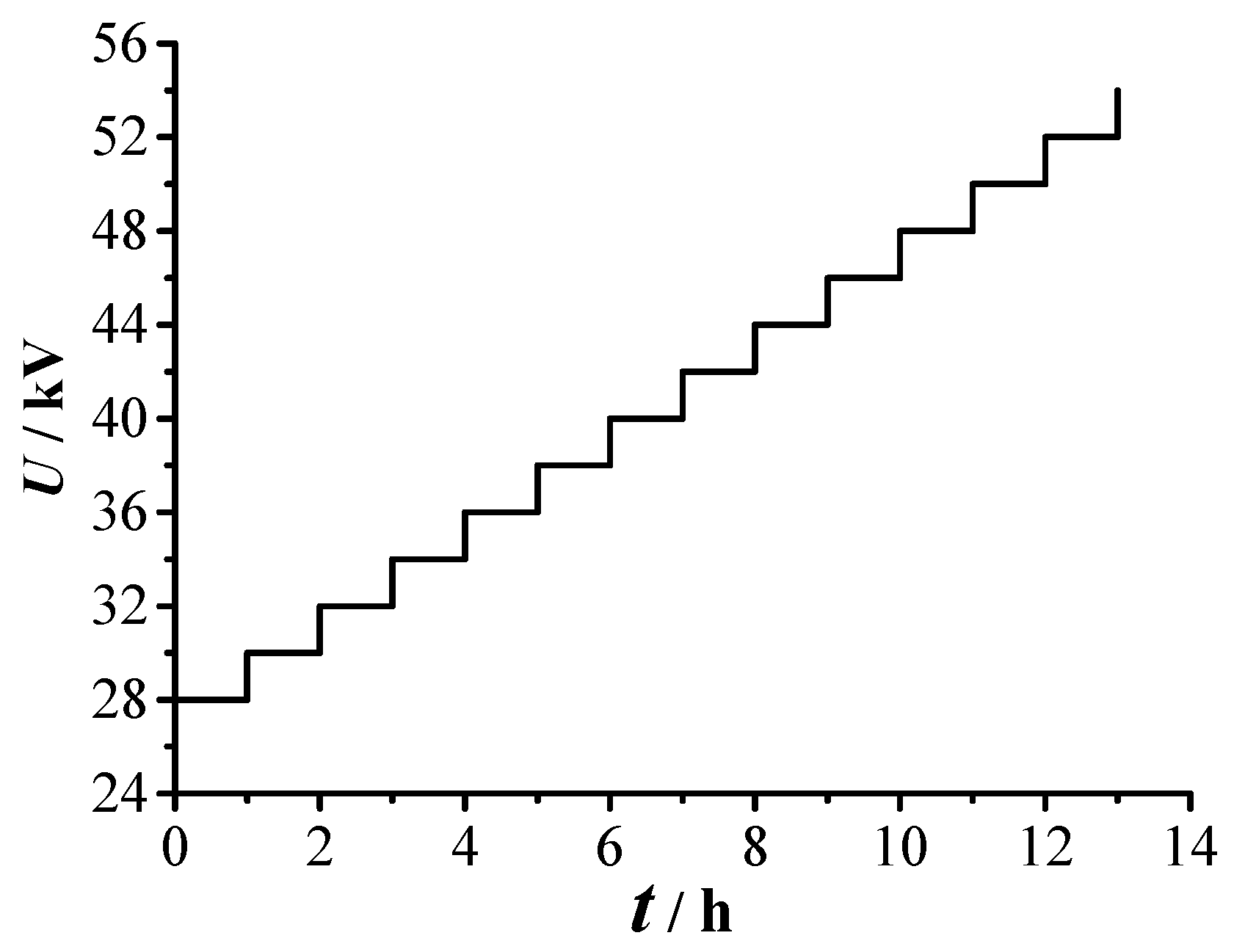
- The voltage applied is gradually increased to the required experimental voltage. The decomposition experiment of SF6 is conducted for 96 h under each voltage value. The SF6 decomposed components are collected every 12 h. GC/MS is used to measure the concentrations of the decomposed components, and DSO is used to display and store the PD signals.
3. Experimental Results
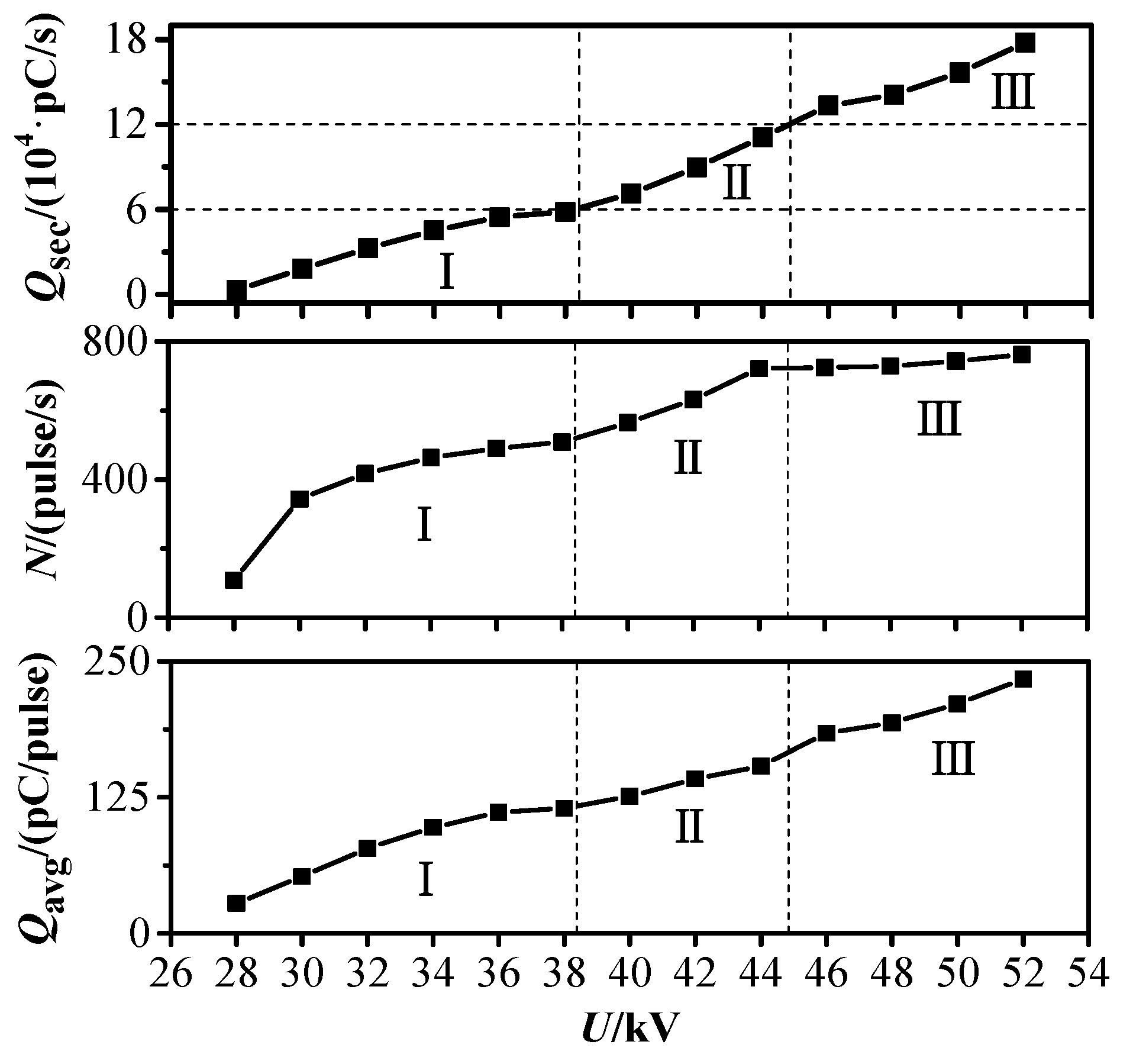
3.1. PD Severity Division
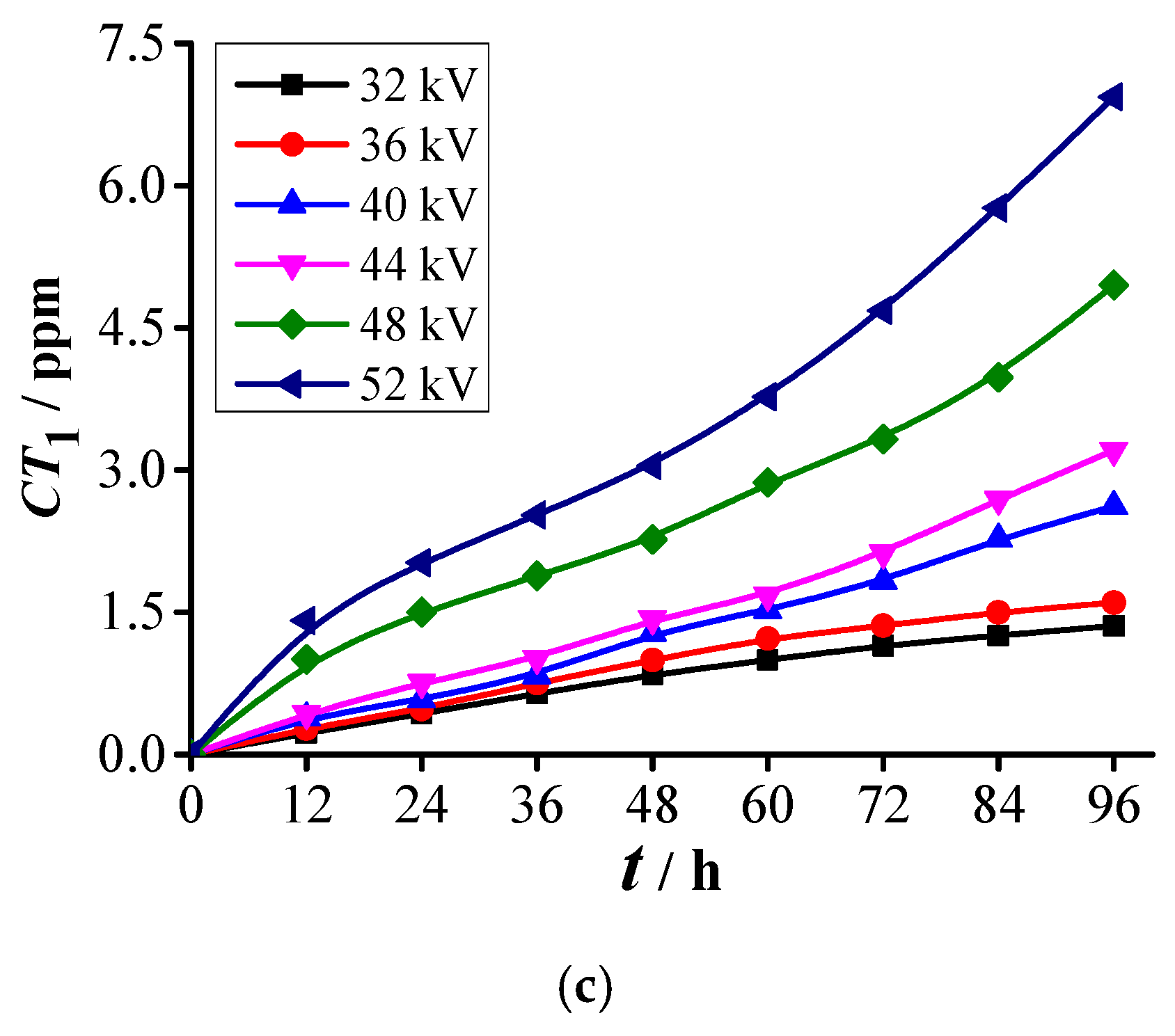
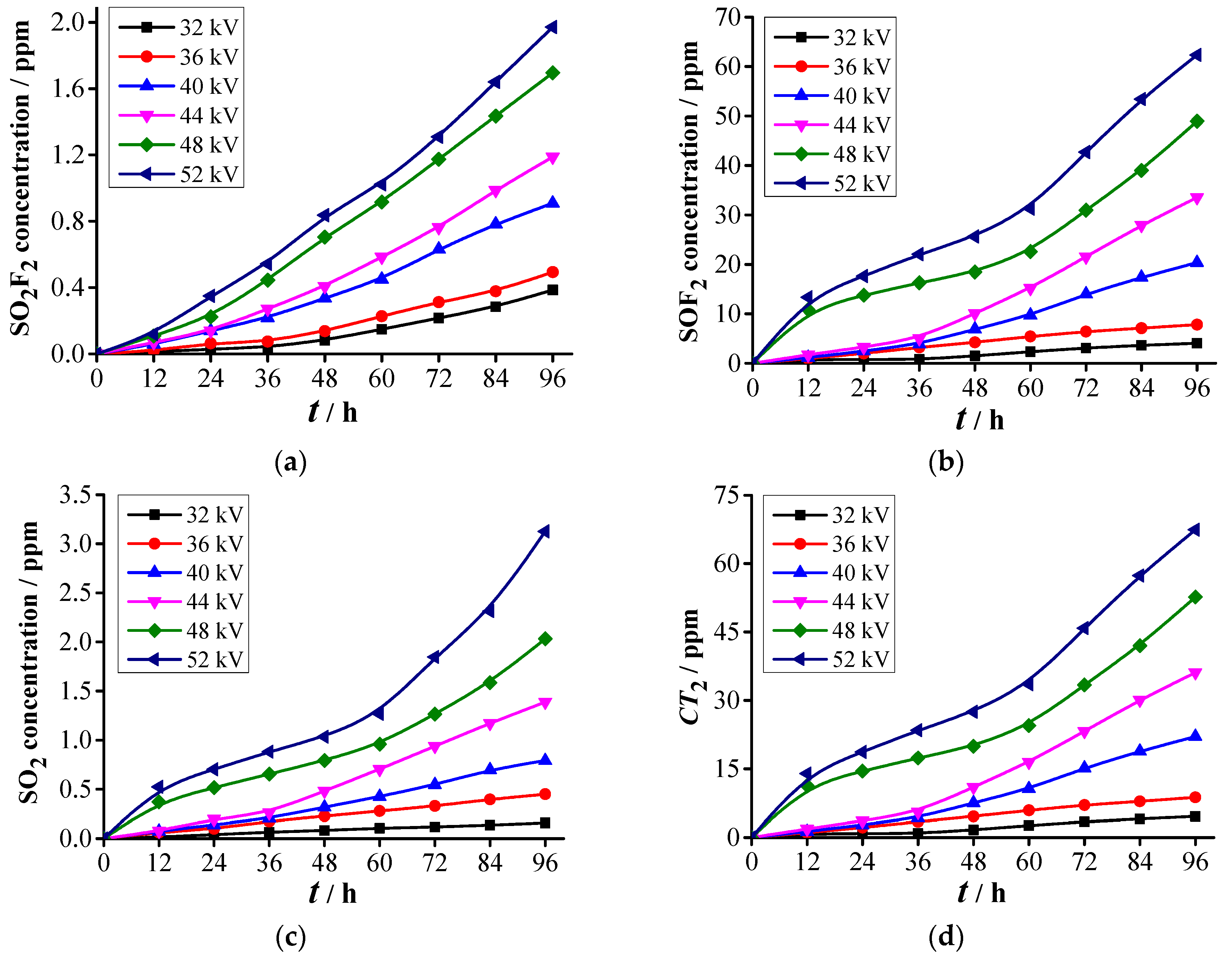
3.2. Analysis of SF6 Decomposed Characteristics
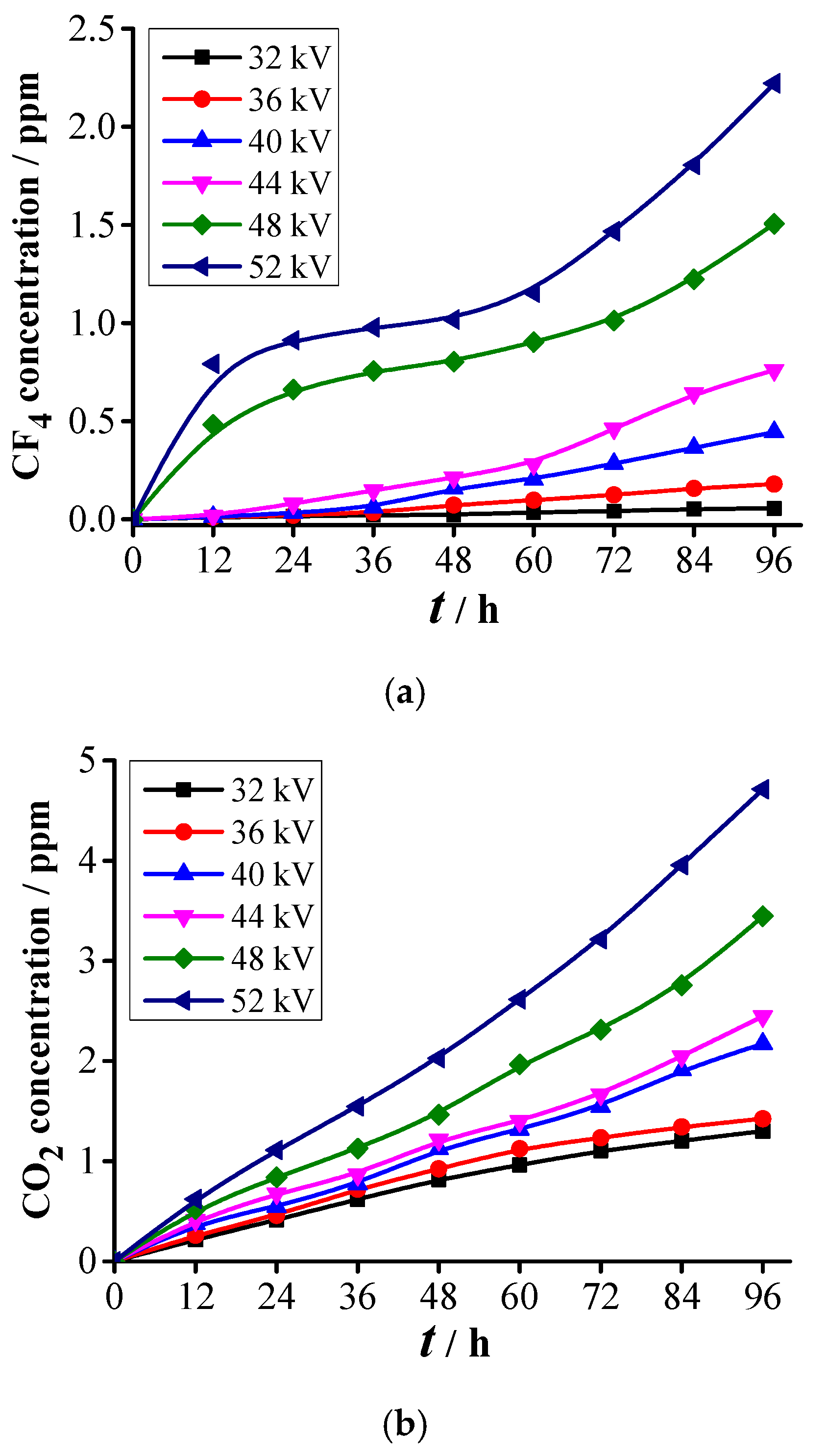
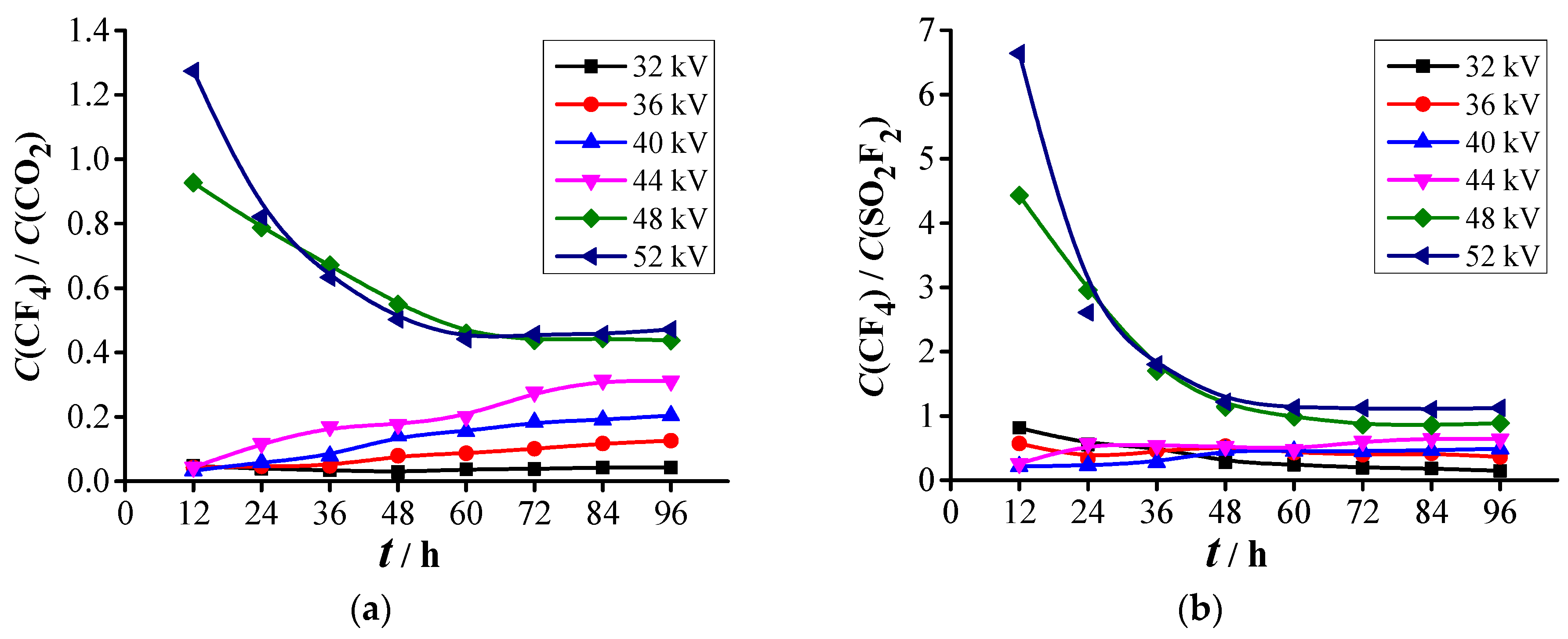
3.2.1. Formation Characteristics of Carbon-Containing Components
3.2.2. Formation Characteristics of Sulfur-Containing Components
4. PD Severity Diagnosis
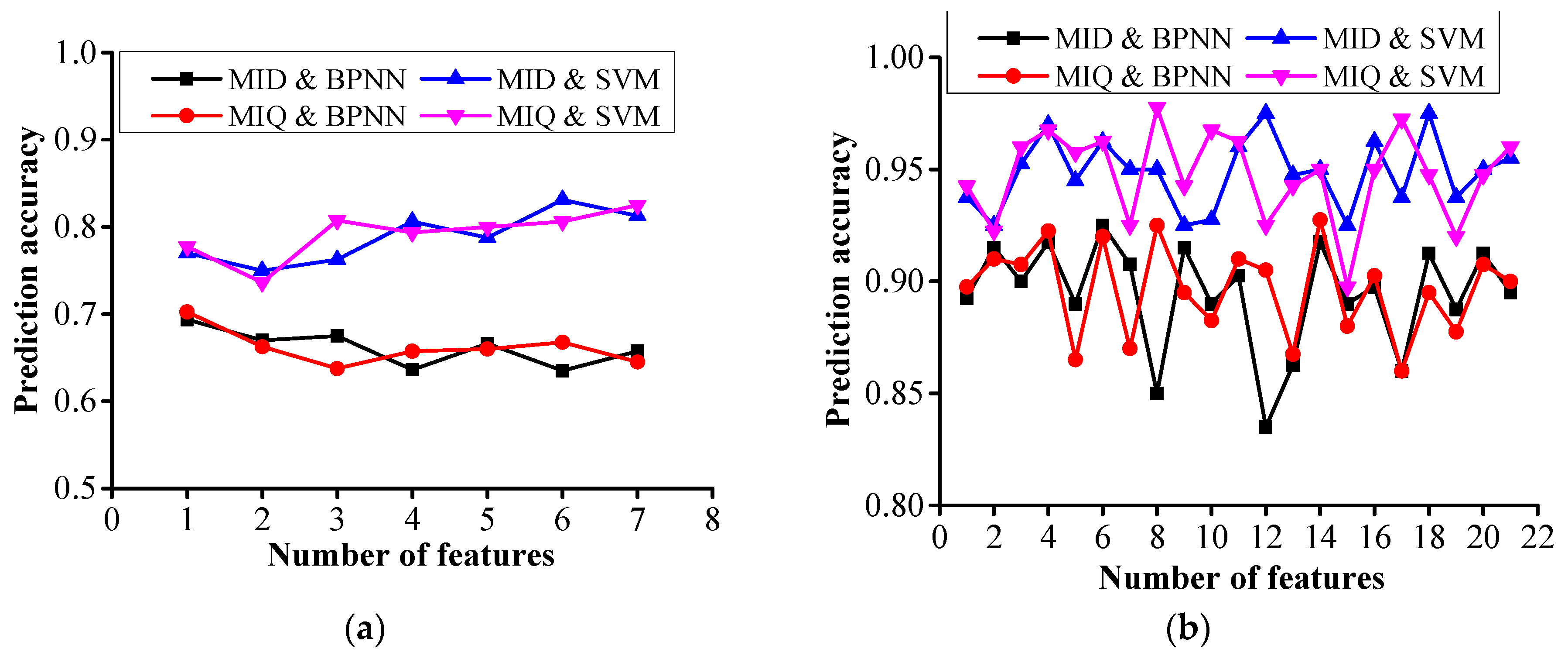
4.1. mRMR Principle
4.2. Feature Selection
4.3. PD Severity Diagnosis
5. Discussion
6. Conclusions
- The PD characteristics of the whole process from the initial PD to the breakdown initiated by free metal particle defect in DC-GIE are studied. The average discharge magnitude in a second Qsec is proposed to be used to characterize the PD severity. Based on Qsec, the PD is divided into three levels: mild PD, medium PD, and dangerous PD.
- Two kinds of voltage in each abovementioned PD level are selected for the decomposition experiment of SF6. Experimental results show that five stable decomposed components, namely, CF4, CO2, SO2F2, SOF2, and SO2, are detected under each voltage. The PD severity has close relationships with C(CF4), C(CO2), C(SO2F2), C(SOF2), C(SO2), CT1, and CT2. The concentrations and concentration ratios of SF6 decomposed components can be used to diagnose the PD severity.
- mRMR-based feature selection algorithm is used to sort the concentrations and concentration ratios of SF6 decomposed components, and BPNN and SVM algorithms are used to diagnose the PD severity. The prediction accuracy of PD severity obtained by using the concentration ratios is higher than that obtained by using the concentrations, and the use of C(CO2)/CT1, C(CF4)/C(SO2), C(CO2)/C(SOF2), and C(CF4)/C(CO2) shows good performance in diagnosing the PD severity.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Benato, R.; Dughiero, F.; Forzan, M.; Paolucci, A. Proximity effect and magnetic field calculation in GIL and in isolated phase bus ducts. IEEE Trans. Magn. 2002, 38, 781–784. [Google Scholar] [CrossRef]
- Benato, R.; Mario, C.D.; Koch, H. High-capability applications of long gas-insulated lines in structures. IEEE Trans. Power Del. 2007, 22, 619–626. [Google Scholar] [CrossRef]
- Benato, R.; Brunello, P.; Fellin, L. Thermal behavior of EHV gas-insulated lines in Brenner pass pilot tunnel. IEEE Trans. Power Del. 2010, 25, 2717–2725. [Google Scholar] [CrossRef]
- Benato, R.; Dughiero, F. Solution of coupled electromagnetic and thermal problems in gas-insulated transmission lines. IEEE Trans. Magn. 2003, 39, 1741–1744. [Google Scholar] [CrossRef]
- Benato, R.; Napolitano, D. Reliability assessment of EHV gas-insulated transmission lines: Effect of redundancies. IEEE Trans. Power Del. 2008, 23, 2174–2181. [Google Scholar] [CrossRef]
- Tenzer, M.; Koch, H.; Imamovic, D. Underground transmission lines for high power AC and DC transmission. In Proceedings of the 2016 IEEE/PES Transmission and Distribution Conference and Exposition, Dallas, TX, USA, 3–5 May 2016. [Google Scholar]
- Magier, T.; Tenzer, M.; Koch, H. Direct current gas-insulated transmission lines. IEEE Trans. Power Del. 2017. [Google Scholar] [CrossRef]
- Mendik, M.; Lowder, S.M.; Elliott, F. Long term performance verification of high voltage DC GIS. In Proceedings of the 1999 IEEE Transmission and Distribution Conference, New Orleans, LA, USA, 11–16 April 1999; pp. 484–488. [Google Scholar]
- Ohki, Y. Thyristor valves and GIS in Kii channel HVDC link. IEEE Electr. Insul. Mag. 2001, 17, 78–79. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamaji, K.; Hatano, M. Development of insulation structure and enhancement of insulation reliability of 500 kV DC GIS. IEEE Trans. Power Del. 1997, 12, 192–202. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamaji, K.; Hatano, M. DC dielectric characteristics and conception of insulation design for DC GIS. IEEE Trans. Power Del. 1996, 11, 1776–1782. [Google Scholar] [CrossRef]
- Beyer, C.; Jenett, H.; Kfockow, D. Influence of reactive SFx gases on electrode surfaces after electrical discharges under SF6 atmosphere. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 234–240. [Google Scholar] [CrossRef]
- Chu, F.Y. SF6 decomposition in gas-insulated equipment. IEEE Trans. Dielectr. Electr. Insul. 1986, 21, 693–725. [Google Scholar] [CrossRef]
- Van Brunt, R.J.; Herron, J.T. Fundamental processes of SF6 decomposition and oxidation in glow and corona discharges. IEEE Trans. Dielectr. Electr. Insul. 1990, 25, 75–94. [Google Scholar] [CrossRef]
- Chang, C.; Chang, C.S.; Jin, J.; Hoshino, T.; Hanai, M.; Kobayashi, N. Source classification of partial discharge for gas insulated substation using wave shape pattern recognition. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 374–386. [Google Scholar] [CrossRef]
- Dreisbusch, K.; Kranz, H.G.; Schnettler, A. Determination of a failure probability prognosis based on PD-diagnostics in GIS. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1707–1714. [Google Scholar] [CrossRef]
- Istad, M.; Runde, M. Thirty-six years of service experience with a national population of gas-insulated substations. IEEE Trans. Power Del. 2010, 25, 2448–2454. [Google Scholar] [CrossRef]
- Van Brunt, R.J. Production rates for oxy-fluorides SOF2, SO2F2 and SOF4 in SF6 corona discharges. J. Res. Natl. Bur. Stand. 1985, 90, 229–253. [Google Scholar] [CrossRef]
- Piemontesi, M.; Niemeyer, L. Sorption of SF6 and SF6 decomposition products by activated alumina and molecular sieve 13X. In Proceedings of the 1996 IEEE International Symposium on Electrical Insulation, Montreal, QC, Canada, 16–19 June 1996; pp. 828–838. [Google Scholar]
- Van Brunt, R.J.; Herron, J.T. Plasma chemical model for decomposition of SF6 in a negative glow corona discharge. Phys. Scr. 1994, 53, 9–29. [Google Scholar] [CrossRef]
- Prakash, K.S.; Srivastava, K.D.; Morcos, M.M. Movement of particles in compressed SF6 GIS with dielectric coated enclosure. IEEE Trans. Dielectr. Electr. Insul. 1997, 4, 344–347. [Google Scholar] [CrossRef]
- Tang, J.; Liu, F.; Zhang, X.X.; Meng, Q.H.; Zhou, J.B. Partial discharge recognition through an analysis of SF6 decomposition products part 1: Decomposition characteristics of SF6 under four different partial discharges. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 29–36. [Google Scholar] [CrossRef]
- Tang, J.; Liu, F.; Meng, Q.H.; Zhang, X.X.; Tao, J.G. Partial discharge recognition through an analysis of SF6 decomposition products part 2: Feature extraction and decision tree-based pattern recognition. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 37–44. [Google Scholar] [CrossRef]
- Tang, J.; Yang, X.; Ye, G.X.; Yao, Q.; Miao, Y.L.; Zeng, F.P. Decomposition characteristics of SF6 and partial discharge recognition under negative DC conditions. Energies 2017, 10, 556. [Google Scholar] [CrossRef]
- Majidi, M.; Oskuoee, M. Improving pattern recognition accuracy of partial discharges by new data preprocessing methods. Electr. Power Syst. Res. 2015, 119, 100–110. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, F.P.; Pan, J.Y.; Zhang, X.X.; Yao, Q. Correlation analysis between formation process of SF6 decomposed components and partial discharge qualities. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 864–875. [Google Scholar] [CrossRef]
- Tang, J.; Pan, J.Y.; Zhang, X.X.; Zeng, F.P.; Yao, Q.; Hou, X.Z. Correlation analysis between SF6 decomposed components and charge magnitude of partial discharges initiated by free metal particles. IET Sci. Meas. Technol. 2014, 8, 170–177. [Google Scholar] [CrossRef]
- Ashkezari, A.D.; Ma, H.; Saha, T.K.; Cui, Y. Investigation of feature selection techniques for improving efficiency of power transformer condition assessment. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 836–844. [Google Scholar] [CrossRef]
- Peng, H.C.; Long, F.H.; Ding, C. Feature selection based on mutual information: Criteria of max-dependency, max-relevance, and min-redundancy. IEEE Trans. Pattern Anal. Mach. Intell. 2005, 27, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Yang, Y.T.; Li, G.Y. A fault diagnosis scheme for planetary gearboxes using modified multi-scale symbolic dynamic entropy and mRMR feature selection. Mech. Syst. Signal. Pr. 2017, 91, 295–312. [Google Scholar] [CrossRef]
- Escalona-Vargas, D.; Siegel, E.R.; Murphy, P.; Lowery, C.L.; Eswaran, H. Selection of reference channels based on mutual information for frequency-dependent subtraction method applied to fetal biomagnetic signals. IEEE Trans. Biomed. Eng. 2017, 64, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ding, Y.X.; Ding, J.; Wu, L.Y.; Xue, Y. Mal-Lys: Prediction of lysine malonylation sites in proteins integrated sequence-based features with mRMR feature selection. Sci. Rep. 2016, 6, 38318. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Gallego, S.; Lastra, I.; Martinez-Rego, D.; Bolon-Canedo, V.; Benitez, J.M.; Herrera, F.; Alonso-Betanzos, A. Fast-mRMR: Fast minimum redundancy maximum relevance algorithm for high-dimensional big data. Int. J. Intell. Syst. 2017, 32, 134–152. [Google Scholar] [CrossRef]
- Aminian, M.; Aminian, F. Neural-network based analog-circuit fault diagnosis using wavelet transform as preprocessor. IEEE Trans. Circuits Syst. II Analog Digit. Signal Process. 2000, 47, 151–156. [Google Scholar] [CrossRef]
- Karnin, E.D. A simple procedure for punning back-propagation trained neural networks. IEEE Trans. Neural Netw. 1990, 1, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Mas’ud, A.A.; Albarracín, R.; Ardila-Rey, J.A.; Muhammad-Sukki, F.; Illias, H.A.; Bani, N.A.; Munir, A.B. Artificial neural network application for partial discharge recognition: Survey and future directions. Energies 2016, 9, 574–591. [Google Scholar] [CrossRef]
- Majidi, M.; Fadali, M.S.; Etezadi-Amoli, M.; Oskuoee, M. Partial discharge pattern recognition via sparse representation and ANN. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1061–1070. [Google Scholar] [CrossRef]
- Gulski, E.; Krivda, A. Neural networks as a tool for recognition of partial discharges. IEEE Trans. Dielectr. Electr. Insul. 1993, 28, 984–1001. [Google Scholar] [CrossRef]
- Satish, L.; Zaengl, W.S. Artificial neural networks for recognition of 3-D partial discharge patterns. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 265–275. [Google Scholar] [CrossRef]
- Hozumi, N.; Okamoto, T.; Imajo, T. Discrimination of partial discharge patterns using a neural network. IEEE Trans. Dielectr. Electr. Insul. 1992, 27, 550–556. [Google Scholar] [CrossRef]
- Leng, X.Z.; Wang, J.H.; Ji, H.B.; Wang, Q.G.; Li, H.M.; Qian, X.; Li, F.Y.; Yang, M. Prediction of size-fractionated airborne particle-bound metals using MLR, BP-ANN and SVM analyses. Chemosphere 2017, 180, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhuo, R.; Wang, D.B.; Wu, J.R.; Zhang, X.X. Application of SA-SVM incremental algorithm in GIS PD pattern recognition. J. Electr. Eng. Technol. 2016, 11, 192–199. [Google Scholar] [CrossRef]
- Tang, J.; Tao, J.G.; Zhang, X.X.; Liu, F. Multiple SVM-RFE for feature subset selection in partial discharge pattern recognition. Int. Rev. Electr. Eng. I. 2012, 7, 5240–5246. [Google Scholar]
- Tang, J.; Wang, D.B.; Fan, L.; Zhuo, R.; Zhang, X.X. Feature parameters extraction of GIS partial discharge signal with multifractal detrended fluctuation analysis. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3037–3045. [Google Scholar] [CrossRef]
- Naseri, F.; Jafari, F.; Mohseni, E.; Tang, W.C.; Feizbakhsh, A.; Khatibinia, M. Experimental observations and SVM-based prediction of properties of polypropylene fibres reinforced self-compacting composites incorporating nano-CuO. Coustr. Build. Mater. 2017, 143, 589–598. [Google Scholar] [CrossRef]
- Wei, Q.; Wu, B.; Xu, D.W.; Zargari, N.R. Natural sampling SVM-based common-mode voltage reduction in medium-voltage current source rectifier. IEEE Trans. Power Electr. 2017, 32, 7553–7560. [Google Scholar] [CrossRef]
- Gonzalez-Costa, J.J.; Reigosa, M.J.; Matias, J.M.; Covelo, E.F. Soil Cd, Cr, Cu, Ni, Pb and Zn sorption and retention models using SVM: Variable selection and competitive model. Sci. Total Environ. 2017, 593, 508–522. [Google Scholar] [CrossRef] [PubMed]
- IEC 60270:2000 High-voltage Test Techniques—Partial Discharge Measurements. Available online: http://www.doc88.com/p-868119919771.html (accessed on 20 June 2017).
- DL/T 596–1996 Preventive Test Code for Electric Power Equipment. Available online: http://www.doc88.com/p-9763610841190.html (accessed on 20 June 2017).
- Zhou, W.J.; Zheng, Y.; Yang, S.; Li, H.; Wang, B.S.; Qiao, S.Y. Detection of intense partial discharge of epoxy insulation in SF6 insulated equipment using carbonyl sulfide. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2942–2948. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, F.P.; Zhang, X.X.; Pan, J.Y.; Yao, Q.; Hou, X.Z.; Tang, Y. Relationship between decomposition gas ratios and partial discharge energy in GIS, and the influence of residual water and oxygen. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1226–1234. [Google Scholar] [CrossRef]
- Kraskov, A.; Stögbauer, H.; Grassberger, P. Estimating mutual information. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2004, 69, 066138. [Google Scholar] [CrossRef] [PubMed]
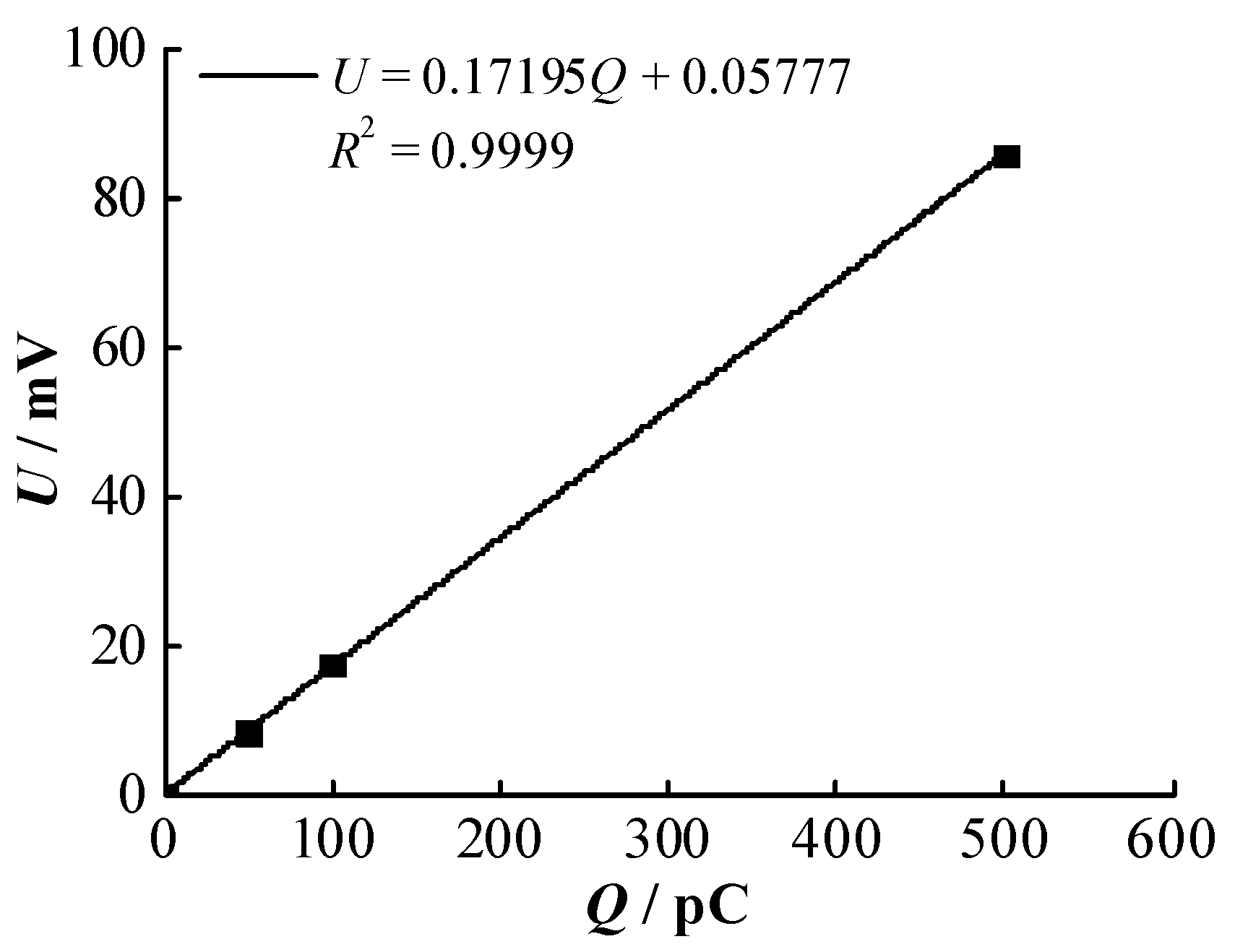
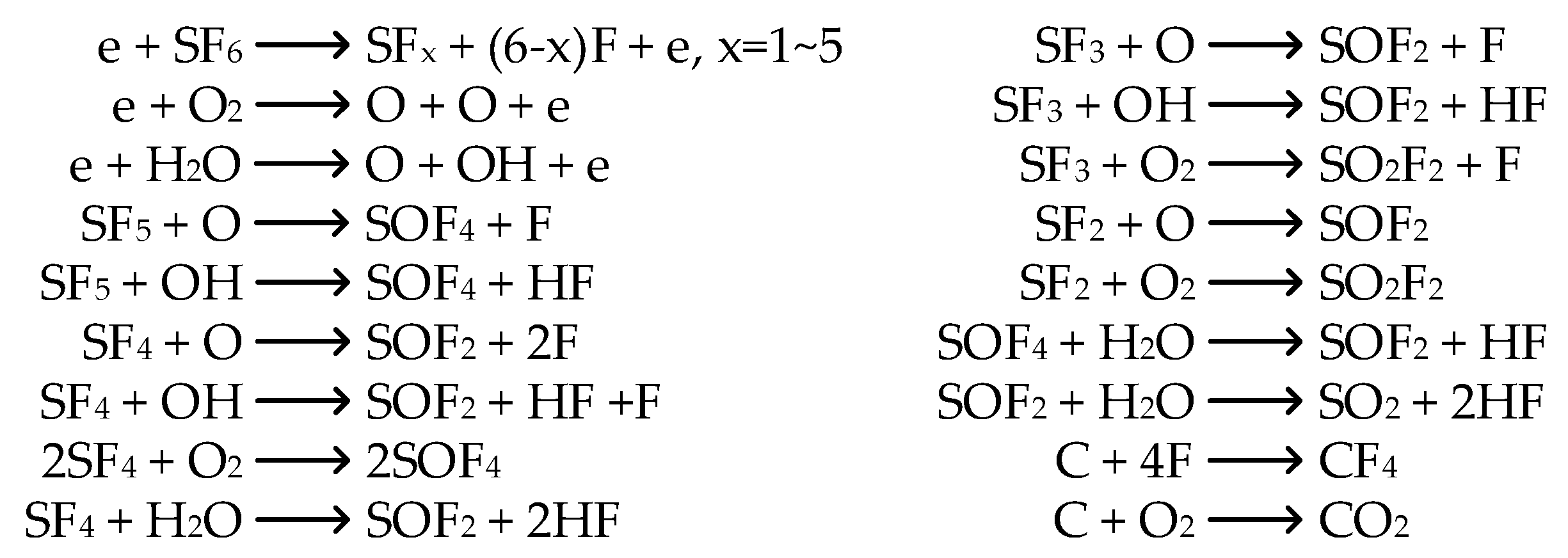
| Decomposed Components | Retention Time/s | Ki/10−5 | R2 |
|---|---|---|---|
| CF4 | 4.320 | 2.838 | 0.999 |
| CO2 | 4.815 | 2.525 | 0.999 |
| SO2F2 | 5.055 | 2.670 | 0.999 |
| SOF2 | 5.425 | 3.575 | 0.999 |
| SO2 | 7.095 | 2.472 | 0.999 |
| Qsec (pC/s) | U/kV | Region | PD Severity |
|---|---|---|---|
| 0–6 × 104 | 28, 30, 32, 34, 36, 38 | I | Mild PD |
| 6 × 104–12 × 104 | 40, 42, 44 | II | Medium PD |
| 12 × 104–18 × 104 | 46, 48, 50, 52 | III | Dangerous PD |
| Concentration Ratio | Label | Concentration Ratio | Label | Concentration Ratio | Label |
|---|---|---|---|---|---|
| C(CF4)/C(CO2) | 1 | C(CO2)/C(SOF2) | 8 | C(SO2F2)/CT2 | 15 |
| C(CF4)/C(SO2F2) | 2 | C(CO2)/C(SO2) | 9 | C(SOF2)/C(SO2) | 16 |
| C(CF4)/C(SOF2) | 3 | C(CO2)/CT1 | 10 | C(SOF2)/CT1 | 17 |
| C(CF4)/C(SO2) | 4 | C(CO2)/CT2 | 11 | C(SOF2)/CT2 | 18 |
| C(CF4)/CT1 | 5 | C(SO2F2)/C(SOF2) | 12 | C(SO2)/CT1 | 19 |
| C(CF4)/CT2 | 6 | C(SO2F2)/C(SO2) | 13 | C(SO2)/CT2 | 20 |
| C(CO2)/C(SO2F2) | 7 | C(SO2F2)/CT1 | 14 | CT1/CT2 | 21 |
| Feature Order | mRMR Principle | |
|---|---|---|
| MID | MIQ | |
| Concentration order | CF4, SOF2, CO2, SO2, CT2, SO2F2, CT1 | CF4, SOF2, SO2, CT2, CT1, SO2F2, CO2 |
| Concentration ratio order | 10, 4, 8, 1, 12, 5, 14, 2, 9, 3, 18, 7, 6, 11, 15, 16, 19, 21, 17, 20, 13 | 10, 4, 8, 1, 5, 12, 2, 3, 18, 14, 9, 6, 7, 11, 17, 15, 19, 21, 20, 16, 13 |
| mRMR Principle | Algorithm | Accuracy Order | Accuracy | Number of Features | Concentration Ratio Labels |
|---|---|---|---|---|---|
| MID | BPNN | 1 | 92.5% | 6 | 10, 4, 8, 1, 12, 5 |
| 2 | 91.75% | 4/14 | 10, 4, 8, 1/10, 4, 8, 1, 12, 5, 14, 2, 9, 3, 18, 7, 6, 11 | ||
| 3 | - | - | - | ||
| SVM | 1 | 97% | 12/18 | 10, 4, 8, 1, 12, 5, 14, 2, 9, 3, 18, 7/10, 4, 8, 1, 12, 5, 14, 2, 9, 3, 18, 7, 6, 11, 15, 16, 19, 21 | |
| 2 | - | - | - | ||
| 3 | 97.5% | 4 | 10, 4, 8, 1 | ||
| MIQ | BPNN | 1 | 92.75% | 14 | 10, 4, 8, 1, 5, 12, 2, 3, 18, 14, 9, 6, 7, 11 |
| 2 | 92.5% | 8 | 10, 4, 8, 1, 5, 12, 2, 3 | ||
| 3 | 92.25% | 4 | 10, 4, 8, 1 | ||
| SVM | 1 | 97.75% | 8 | 10, 4, 8, 1, 5, 12, 2, 3 | |
| 2 | 97.25% | 17 | 10, 4, 8, 1, 5, 12, 2, 3, 18, 14, 9, 6, 7, 11, 17, 15, 19 | ||
| 3 | 96.75% | 4/10 | 10, 4, 8, 1/10, 4, 8, 1, 5, 12, 2, 3, 18, 14 |
| Real PD Severity | Number of Samples in Each PD Level in the Diagnosis Result | ||
|---|---|---|---|
| Mild PD | Medium PD | Dangerous PD | |
| Mild PD | 7 | 1 | 0 |
| Medium PD | 0 | 7 | 1 |
| Dangerous PD | 0 | 0 | 8 |
| Real PD Severity | Number of Samples in Each PD Level in the Diagnosis Result | ||
|---|---|---|---|
| Mild PD | Medium PD | Dangerous PD | |
| Mild PD | 8 | 0 | 0 |
| Medium PD | 0 | 7 | 1 |
| Dangerous PD | 0 | 0 | 8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Yang, X.; Yang, D.; Yao, Q.; Miao, Y.; Zhang, C.; Zeng, F. Using SF6 Decomposed Component Analysis for the Diagnosis of Partial Discharge Severity Initiated by Free Metal Particle Defect. Energies 2017, 10, 1119. https://doi.org/10.3390/en10081119
Tang J, Yang X, Yang D, Yao Q, Miao Y, Zhang C, Zeng F. Using SF6 Decomposed Component Analysis for the Diagnosis of Partial Discharge Severity Initiated by Free Metal Particle Defect. Energies. 2017; 10(8):1119. https://doi.org/10.3390/en10081119
Chicago/Turabian StyleTang, Ju, Xu Yang, Dong Yang, Qiang Yao, Yulong Miao, Chaohai Zhang, and Fuping Zeng. 2017. "Using SF6 Decomposed Component Analysis for the Diagnosis of Partial Discharge Severity Initiated by Free Metal Particle Defect" Energies 10, no. 8: 1119. https://doi.org/10.3390/en10081119




