1. Introduction
Monoesters produced by the transesterification of vegetable oil with alcohol are known as biodiesel fuels [
1]. Biodiesel is a biodegradable and renewable fuel. It contributes no net carbon dioxide or sulfur to the atmosphere and emits less gaseous pollutants than normal diesel. Carbon dioxide, aromatics, polycyclic aromatic hydrocarbons (PAHS) and partially burned or unburned hydrocarbons emissions are all reduced in vehicles operating on biodiesel [
2]. Biodiesel is an environmentally friendly liquid fuel with similar combustion properties to petrol diesel. Increasing environmental concern, diminishing petroleum reserves, and the agriculture-based economy of our country are the driving forces to promote biodiesel as an alternative fuel [
3]. Other advantages of biodiesel are its good lubrication properties that help to extend engine life, high cetane number, high flash point, and acceptable cold filter plugging point (CFPP), which make it very attractive as an alternative fuel [
4,
5]. Biodiesel derived from vegetable oil and animal fats is used in the USA and Europe to reduce air pollution and to reduce the dependence on fossil fuels. In the USA and Europe, the surplus edible oils like soybean oil, sunflower oil, and rapeseed oil are being used as feedstock for the production of biodiesel [
6,
7]. However, vegetable oil is about ten times more viscous than diesel. As a result, vegetable oils can cause poor fuel atomization, incomplete combustion, and carbon deposition on the injector and valve seats; all resulting in serious engine fouling. Common methods employed to reduce the viscosity of vegetable oils include blending with diesel, emulsification, pyrolysis, cracking, and transesterification. Among these, the transesterification of vegetable oils to alkyl esters appears to be the best method [
8]. Use of a solid base catalyst offers several process advantages, including the elimination of a quenching step to isolate the products - and the associated contaminated water waste - as well as the opportunity to operate a continuous process [
9]. Such solid bases, including zeolites [
10], alkali earth oxides [
11], and hydrotalcites [
12] have been utilized in transesterification reactions, and recently tuneable solid bases, such as Li-doped CaO and Mg-hydrotalcites, for tributyrin transesterification [
13].
Biodiesel is usually prepared in the presence of a homogenous base or acid catalyst. The acid catalyzed process often uses sulfonic acid and hydrochloric acid as the catalyst; however, the reaction time is very long (48–96 hr) even at the temperature of methanol reflux, and a high molar ratio of methanol to oil is needed (30–150:1, by mol) [
14]. Potassium hydroxide, sodium hydroxide, and their carbonates, as well as potassium and sodium alkoxides such as NaOCH
3, are the base catalysts usually used for the reaction [
15,
16]. Most biodiesel fuels are currently synthesized using alkaline catalysts because the transesterification reaction is much faster than with acid catalysts [
17,
18]. Moreover, acid catalysts are more corrosive, and thus base catalysis is preferred commercially.
There have been several comprehensive studies on base catalyzed transesterification to produce fatty acid methyl esters (FAMEs) [
19]. A higher conversion of vegetable oil has been reported when strong base catalysts such as sodium hydroxide and potassium hydroxide are used. Furthermore, basic catalysts (for example KOH) can convert 97–99 wt% in 0.5–1 hr [
19]. However, the base catalyzed process still has two limitations: the base catalyzed transesterification of vegetable oil is sensitive to both moisture and to free fatty acids (FFA). This means that the raw materials must be refined; the moisture level must be no more than 0.06 wt% and FFA levels no more than 0.5 m wt% [
20]. A small amount of moisture can initiate oil hydrolyzation to form soap and glycerol. Vegetable oils, particularly cooking oil, also contain significant amounts of FFA, which incur significant processing problems in standard biodiesel manufacturing. FFA are saponified by homogenous alkaline catalysts. These soaps can bring about the emulsification between glycerol and FAME, which will make the separation even more difficult when the water washing method is used. Furthermore, the saponification leads to a loss of catalyst and an increased purification cost [
21]. If the FFA content in vegetable oil is more than 0.5 wt%, the simplest approach to improve the processing is to first esterify these FFA in the vegetable oil to their alkyl esters using an acid catalyst. Such a pretreatment process adds a neutralization step as well as a separation step to the overall process. Thus, development of solid acid catalysts for esterification pretreatments is crucial to improve the efficiency of biodiesel production [
22].
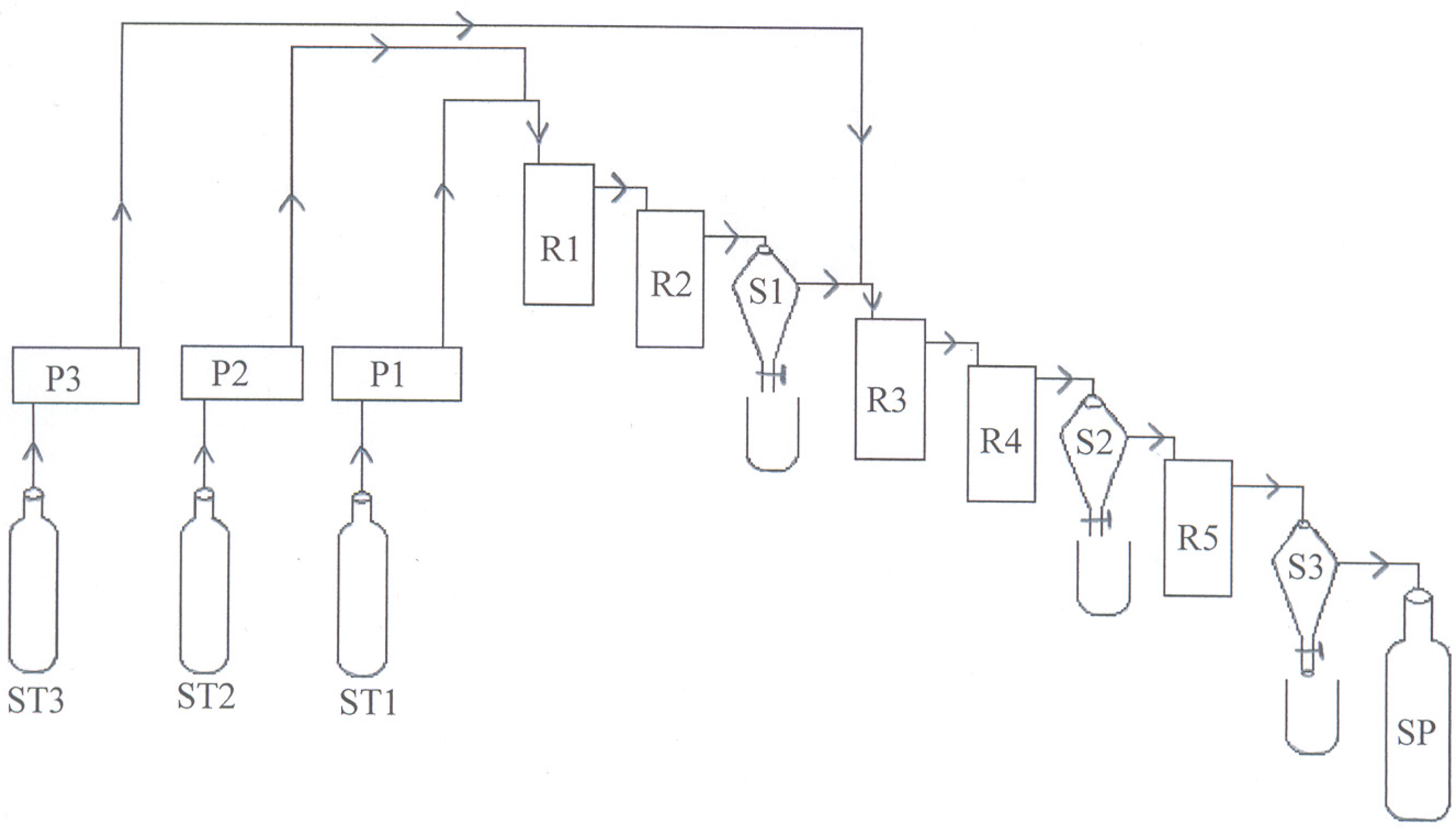
The present work studies the experimental conditions such as catalyst concentration, molar ratio (oil:methanol), reaction temperature, total flow rate, mixing intensity,and the residence time on biodiesel production from coconut oil, and the produced biodiesel, is analyzed by different tools.
2. Results and Discussion
2.1. Catalyst type and concentration
Catalysts used for the transesterification of vegetable oils are classified as alkali, acid, enzyme or heterogeneous catalysts; of which alkali catalysts like sodium hydroxide, potassium hydroxide, and potassium methoxide are more effective. If the oil has a high FFA content and a high concentration of water, acid catalyzed transesterification is preferred. For an alkaline catalysis, sodium hydroxide or potassium hydroxide is mostly used.
In the present work, the continuous transesterification of coconut oil is studied. For this, alkaline catalyst is more suitable due to the presence of very low FFA. For the methanolysis of coconut oil, potassium hydroxide gives the best results and viscosity of the esters. When optimizing catalyst concentration, a stirring speed of 800rpm with a mechanical stirrer, a flow rate of 142 mL/min, a temperature of 65 °C, and a molar ratio of 1:7 oil:methanol was held constant.
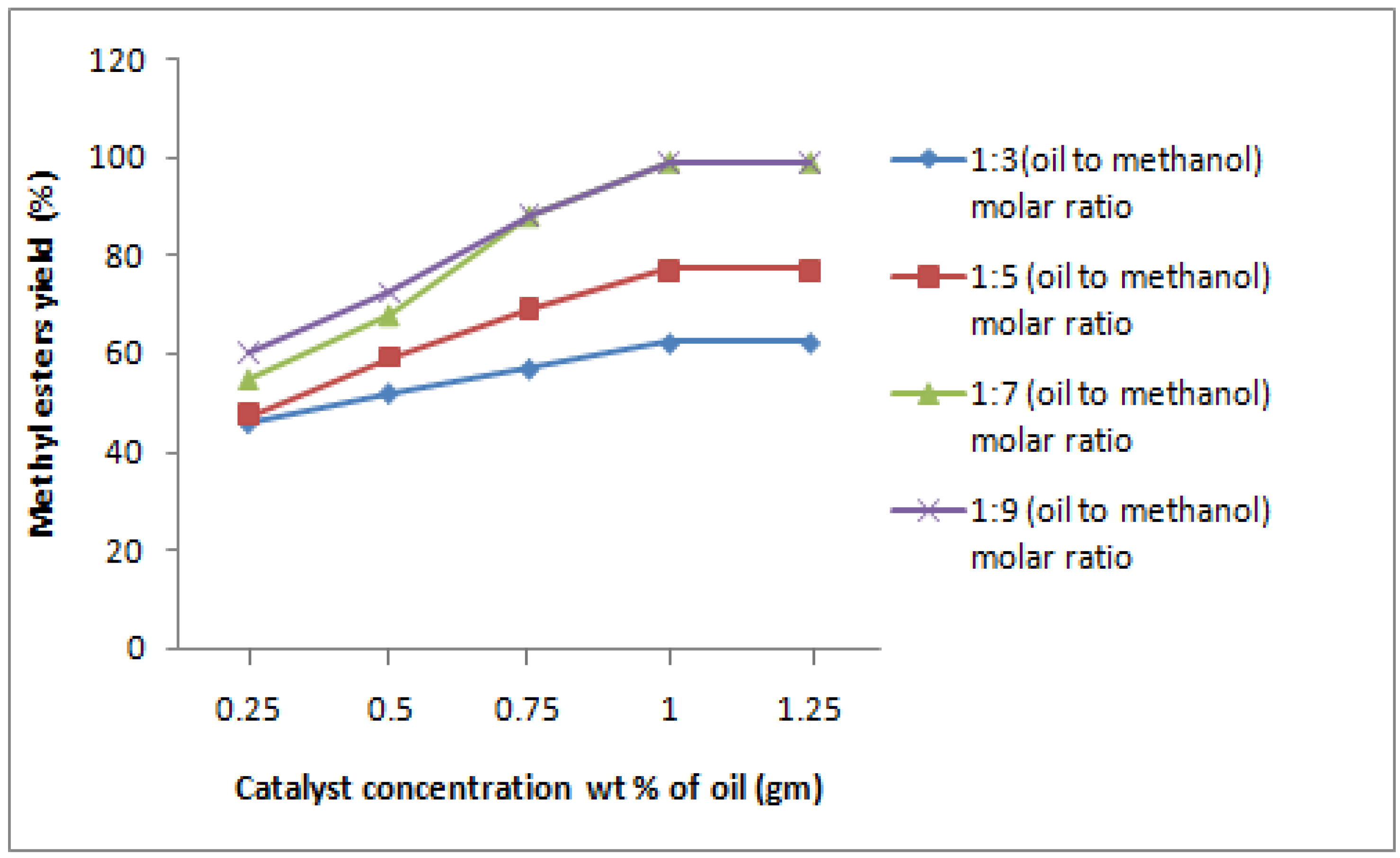
Figure 1 summarizes the experimental effect of varying the catalyst (KOH) concentration from 0.25 to 1.25 wt% of oil. When the catalyst was 0.25 wt%, the conversion was 75%. Increasing the catalyst concentration increased the conversion rate: a 1.00 wt% of catalyst gave the best conversion rate of 99.04 ± 0.05%. There are however drawbacks to the excessive use of alkaline catalysts in the transesterification reaction. A higher catalyst concentration increases the solubility of methyl esters in the glycerol phase of the final product. As a result, a significant amount of methyl esters remain in the glycerol phase after the phase separation.
2.2. Molar ratio
One of the most important variables affecting the yield of ester is the molar ratio of alcohol to oil. The stoichiometric ratio for transesterification requires three moles of alcohol and one mole of triglyceride to yield the three moles of fatty acid methyl esters and one mole of glycerol. However, transesterification is an equilibrium reaction in which a high molar ratio is used to drive the reaction to the right, enhancing the solubility and increasing the contact between the triglycerides and alcohol molecules. Experiments were conducted with molar ratios of 1:3, 1:5, 1:7 and 1:9 using a constant mixing speed of 800rpm and catalyst concentration of 1.00 wt% of oil. The results are shown in figure 1. At the molar ratio 1:7 (oil:methanol), the conversion of triglycerides was maximal ≥99%. In this experiment, methanol is fed in two parts: 5 moles in the first reactor and 2 moles in the third. Molar ratio has no effect on acid, peroxide, saponification, or iodine values of methyl esters [
23]. However, the high molar ratio of alcohol to vegetable oil interferes with the separation of glycerol because there is an increase in solubility. When glycerol remains in solution, it helps derive the equilibrium bask to the left, lowering the yield of esters. The production capacity of the unit was 6.4 L/hr.
Figure 1.
Effect of catalyst concentration (wt% of oil) and molar ratio (oil:methanol) on the yield of methyl esters from coconut oil (at constant temperature of 65 °C, flow rate of 142 mL/min, stirring speed of 800rpm, residence time 21.04 min).
Figure 1.
Effect of catalyst concentration (wt% of oil) and molar ratio (oil:methanol) on the yield of methyl esters from coconut oil (at constant temperature of 65 °C, flow rate of 142 mL/min, stirring speed of 800rpm, residence time 21.04 min).
2.3. Reaction temperature
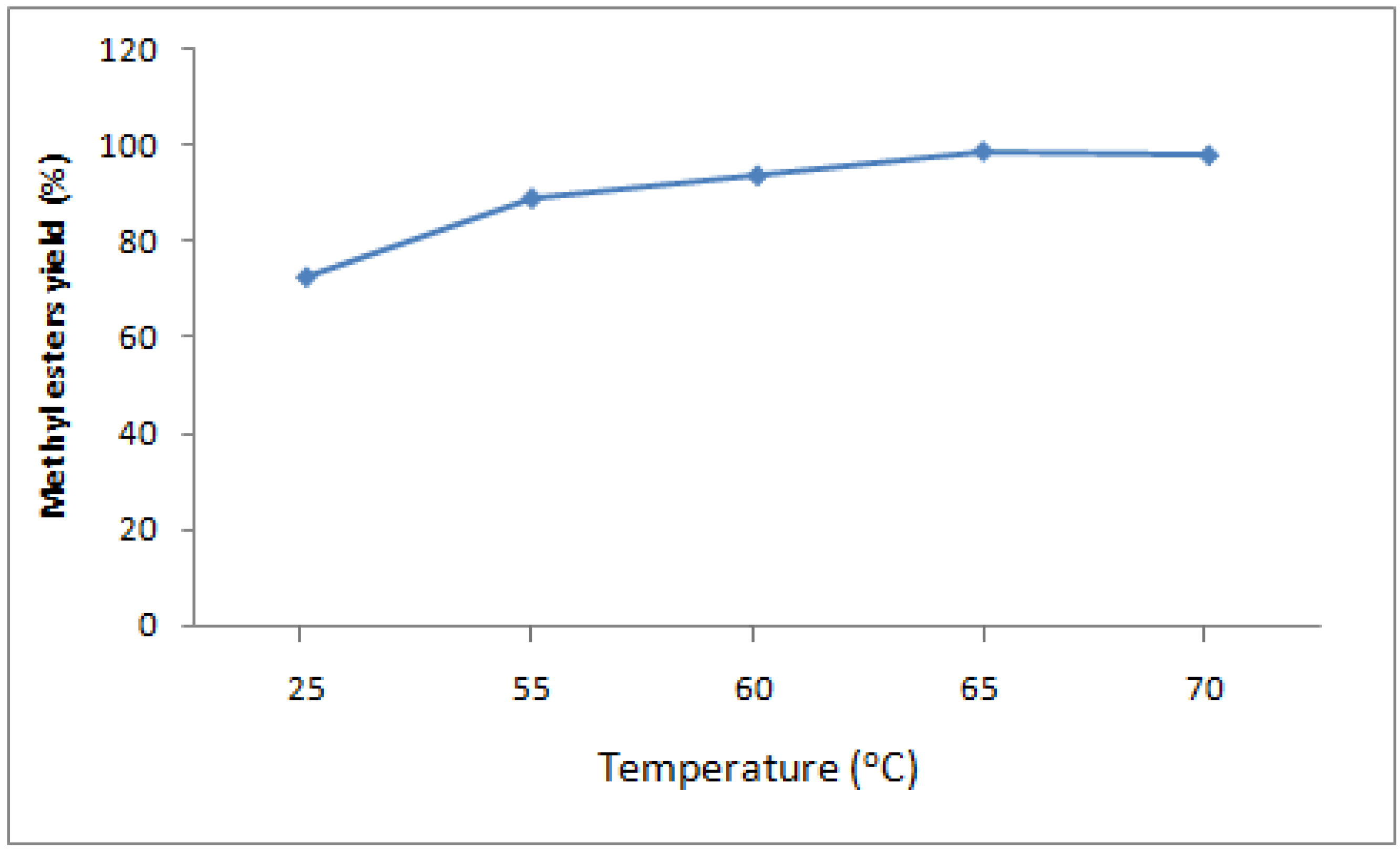
To test the effect of reaction temperature, experimental trials were carried out at room temperature (25 °C), and at 55 °C, 60 °C, 65 °C and 70 °C. In all experiments, a constant methanol to oil ratio of 7:1 and a 1 wt% KOH catalyst concentration was used, based on the optimal conditions in the previous section). As shown in figure 2, the conversion of coconut oil into fatty acid methyl esters increased with reaction temperature. The yield of methyl esters at 25 °C, 55 °C, and 60 °C was 60, 85, and 94%, respectively. The maximum yield of ≥99% was obtained at the temperature 65 °C. Methanolysis of vegetable oil is normally performed near the boiling point of the alcohol. A reaction temperature above the boiling point of alcohol, 64 °C, was ignored, because high temperatures lead to the acceleration of the saponification of glycerides in the presence of a base catalyst before completion of the methanolysis.
Figure 2.
Effect of reaction temperature on yield of methyl esters from coconut oil. (constant catalyst concentration of 1 wt% of oil, molar ratio methanol:oil of 7:1, flow rate 142 mL/min, stirring speed 800rpm, residence time 21.04 min).
Figure 2.
Effect of reaction temperature on yield of methyl esters from coconut oil. (constant catalyst concentration of 1 wt% of oil, molar ratio methanol:oil of 7:1, flow rate 142 mL/min, stirring speed 800rpm, residence time 21.04 min).
2.4. Flow rate
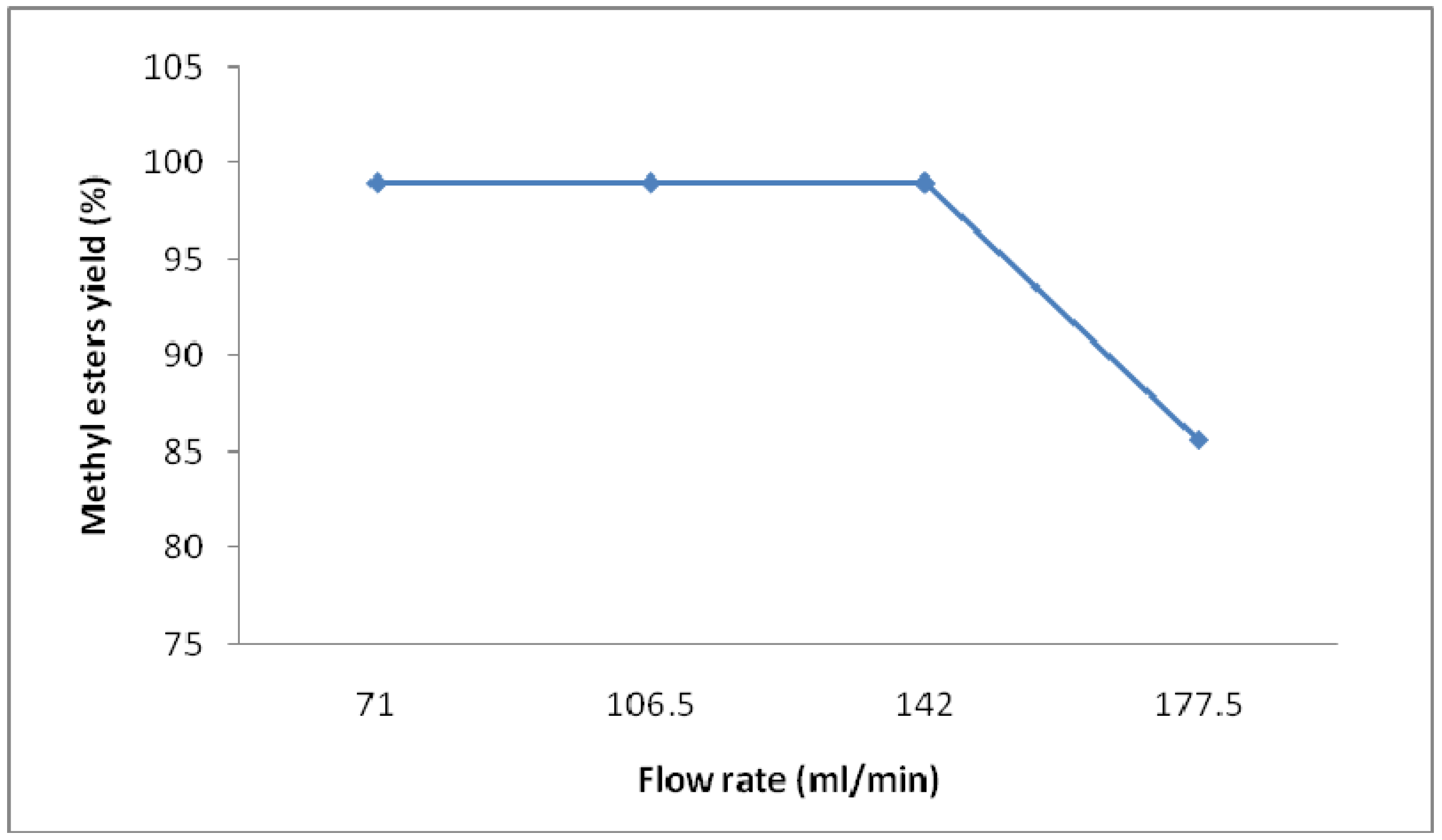
To optimize the flow rate for the process of continuous transesterification of coconut oil, total flow rates ranging from 70 mL/min to 180 mL/min were tested. All experiments were conducted with a molar ratio of methanol:oil 7:1, a 1 wt% KOH catalyst concentration, and a reaction temperature of 65 °C. The effect of flow rate on the biodiesel yield is presented in
Figure 3 and the total flow rate of oil and methanol is given in
Table 1. A combined total flow rate of oil and methanol was 142 mL/min (100 mL oil and 42 mL methanol) gave the best conversion of ≥99.
Table 1.
Effect of flow rate and residence time on yield of methyl esters from coconut oil. (Molar ratio oil:methanol 1:7, catalyst concentration 1 wt% of oil, mixing intensity 800 rpm, reaction temperature 65 °C).
Table 1.
Effect of flow rate and residence time on yield of methyl esters from coconut oil. (Molar ratio oil:methanol 1:7, catalyst concentration 1 wt% of oil, mixing intensity 800 rpm, reaction temperature 65 °C).
| Flow rate (ml/min) | Residence time (min) | Conversion % |
|---|
| Oil (ml) | MeOH (ml) | Total (ml) | Reactor R1 | Reactor R2 | Reactor R3 | Reactor R4 | Reactor R5 | Total | S1 | S2 | S3 |
| 50 | 21.0 | 71.0 | 8.07 | 8.07 | 8.4 | 8.4 | 9.85 | 42.79 | 82.5 | 97.0 | 99 |
| 75 | 31.57 | 106.5 | 5.1 | 5.1 | 5.6 | 5.6 | 6.57 | 27.97 | 82.5 | 96.8 | 99 |
| 100 | 42.0 | 142.0 | 3.84 | 3.84 | 4.22 | 4.22 | 4.92 | 21.04 | 82.6 | 96.8 | 99 |
| 125 | 52.5 | 177.5 | 3.07 | 3.07 | 3.38 | 3.38 | 3.94 | 16.84 | 75.2 | 80.0 | 85.6 |
Figure 3.
Effect of flow rate on yield of methyl esters from coconut oil (Catalyst concentration 1 wt% of oil, molar ratio methanol:oil 7:1, temperature 65 °C, stirring speed 800 rpm, residence time 21.04 min).
Figure 3.
Effect of flow rate on yield of methyl esters from coconut oil (Catalyst concentration 1 wt% of oil, molar ratio methanol:oil 7:1, temperature 65 °C, stirring speed 800 rpm, residence time 21.04 min).
2.5. Mixing intensity
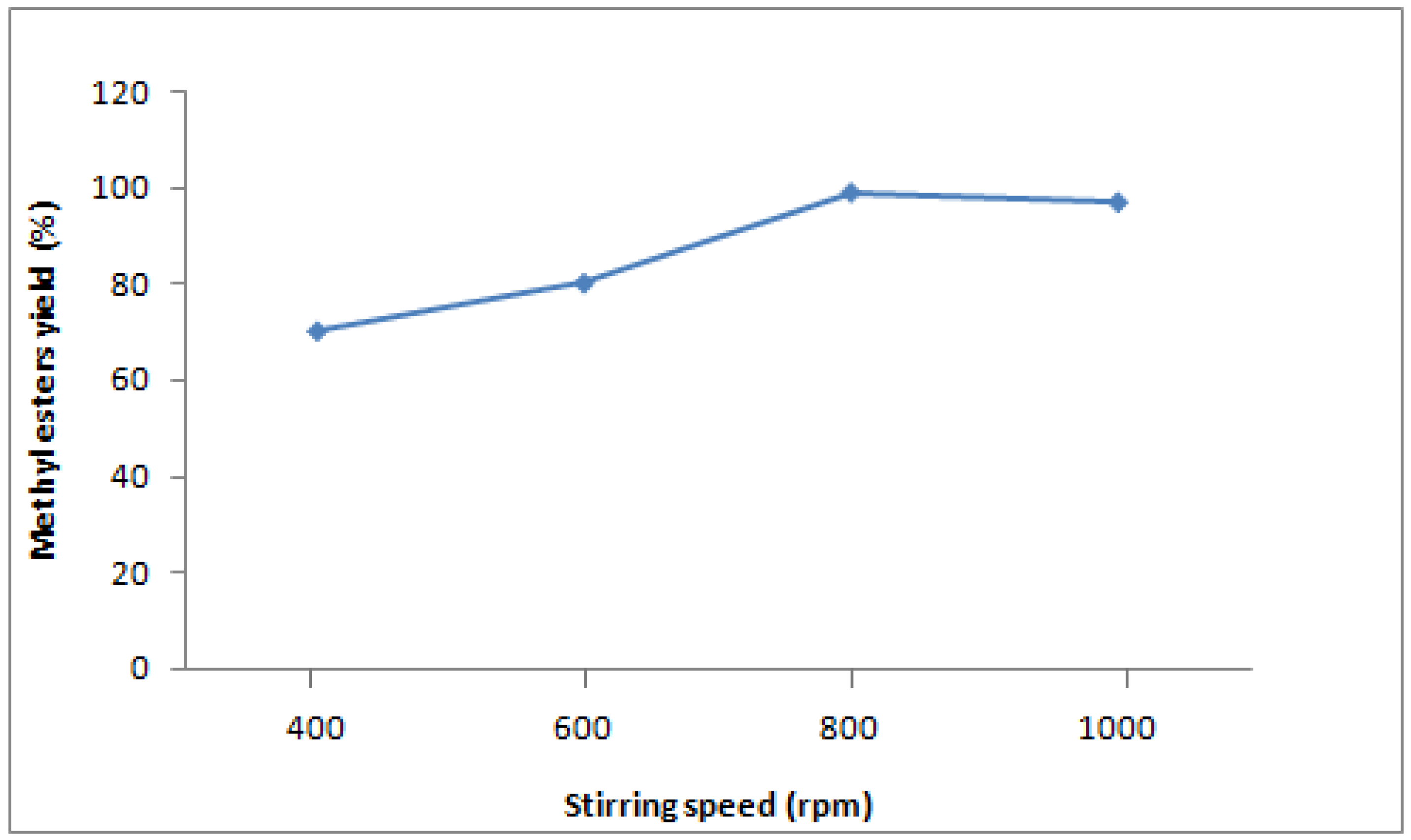
Mixing is a very important factor in the transesterification reaction. Without mixing, the reaction occurs only at the interface between the two layers and is considered too slow to be feasible. In the present work, the effect of mixing intensity at various catalyst concentrations was examined. Methanolysis was conducted with stirring rates of 200, 400, 600, 800 and 1,000 revolutions per minute (rpm) and catalyst concentrations of 0.25%, 0.50%, 0.75%, 1% and 1.25%. Based on the observations above that 1wt% of catalyst give the best conversion, the yield of methyl ester at different mixing intensities (rpm) using the catalyst concentration of 1wt% is shown in
Figure 4. The methanolysis reaction was partially incomplete at 200 rpm, only exhibiting a conversion of 85.5%. The conversion increased as mixing intensity was accelerated. The best yield of methyl esters was achieved at 800 rpm and 1% catalyst, up to 99%.
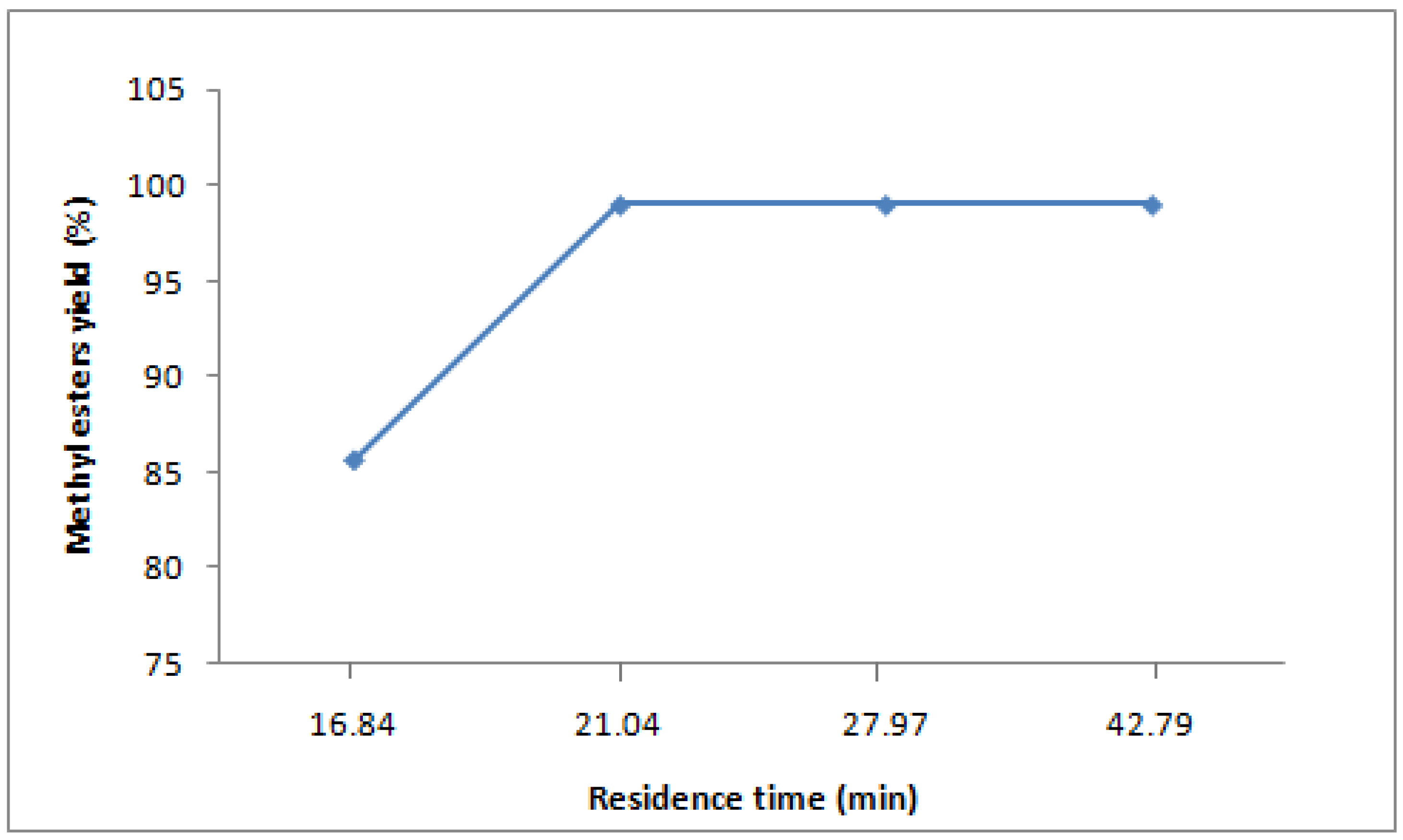
2.6. Residence time
The residence time of the reaction is the effective volume of the reactors divided by the total flow rate. The effective volume of the five CSTRs is 500 mL, 500 mL, 600 mL, 600 mL, and 700 mL, respectively. The desired total flow rate of oil and alkaline methanol were 71 mL/min, 106.5 mL/min, 142 mL/min and 177.5 mL/min for residence times of 42.79min, 27.97 min, 21.04 min and 16.84 min, respectively. The calculated molar ratio of methanol to oil in all experiments was 7:1, the flow rate was calculated based on the available density of coconut oil at 20 °C (0.92 gm/mL) and the density of methanol at 20 °C (0.792 gm/mL). The yield of biodiesel at different residence times is shown in
Figure 5. A flow rate of 142 ± 0.5 mL and a residence time of 21.04 gave the best conversion of ≥99%. When the total flow rate of oil and methanol was 142 mL/min, the residence time of each reactor was 3.84 min, 3.84 min, 4.22 min, 4.22 min and 4.92 min, respectively.
Figure 4.
Effect of stirring speed on yield of methyl esters from coconut oil (catalyst concentration 1wt% of oil, molar ratio methanol:oil 7:1, temperature 65°C, flow rate 142 mL/min, residence time 21.04 min).
Figure 4.
Effect of stirring speed on yield of methyl esters from coconut oil (catalyst concentration 1wt% of oil, molar ratio methanol:oil 7:1, temperature 65°C, flow rate 142 mL/min, residence time 21.04 min).
Figure 5.
Effect of residence time on yield of methyl esters to coconut oil. (catalyst concentration 1 wt% of oil, molar ratio methanol:oil 7:1, temperature 65 °C, flow rate 142 mL/min, stirring speed 800 rpm).
Figure 5.
Effect of residence time on yield of methyl esters to coconut oil. (catalyst concentration 1 wt% of oil, molar ratio methanol:oil 7:1, temperature 65 °C, flow rate 142 mL/min, stirring speed 800 rpm).
2.7. HPLC analysis
Reverse phase high performance liquid chromatography (RP-HPLC) was used to analyze the purity, conversion and FAME composition of the biodiesel esters produced. RP-HPLC separates different components according to their polarity. The chromatographic apparatus was from Waters, Milford MA, USA. It consisted of a model waters 600 pump with waters 600 controller, waters 2,996 photodiode array detector, a nova-pack®, 3.9 × 150 mm column with guard column of dimension 3.9 × 20 mm, both packed with C18 particle with diameter 4 µm. The RP-HPLC conditions used for the separation and determination of the compounds produced during the methanolysis of coconut oil in all the experiments were: a flow rate of 1 mL/min, an injection volume of 5µL, a column temperature of 45 °C, UV detection at 215 nm and a 40 min gradient mobile phase 15% H
2O + 85% CH
3OH in 10 min, 100% CH
3OH in 0 min, 60% CH
3OH + 15% hexane + 25% propane-2-ol in 30 min and for the last 10 min system back to initial state 15% H
2O + 85% CH
3OH.
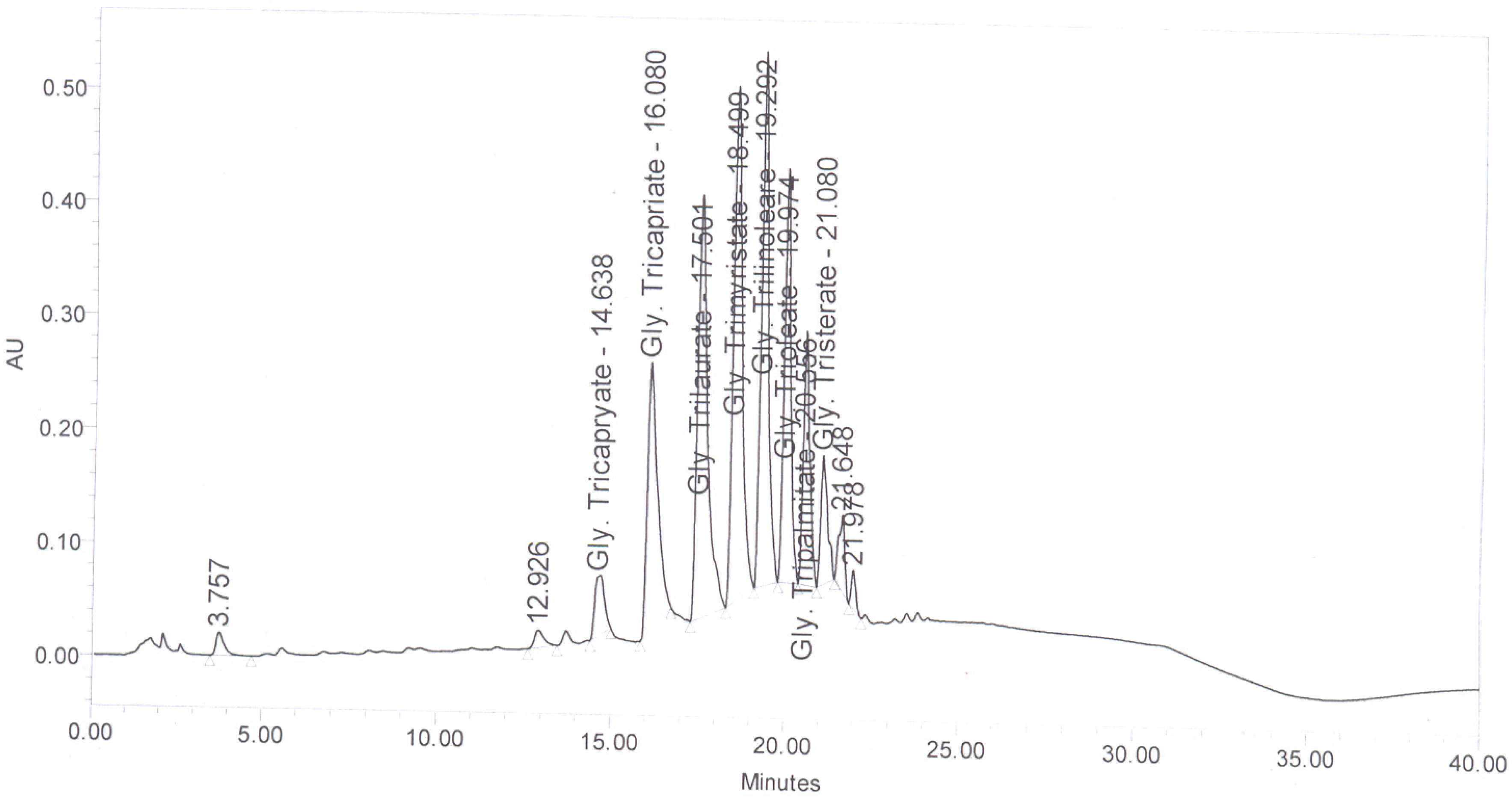
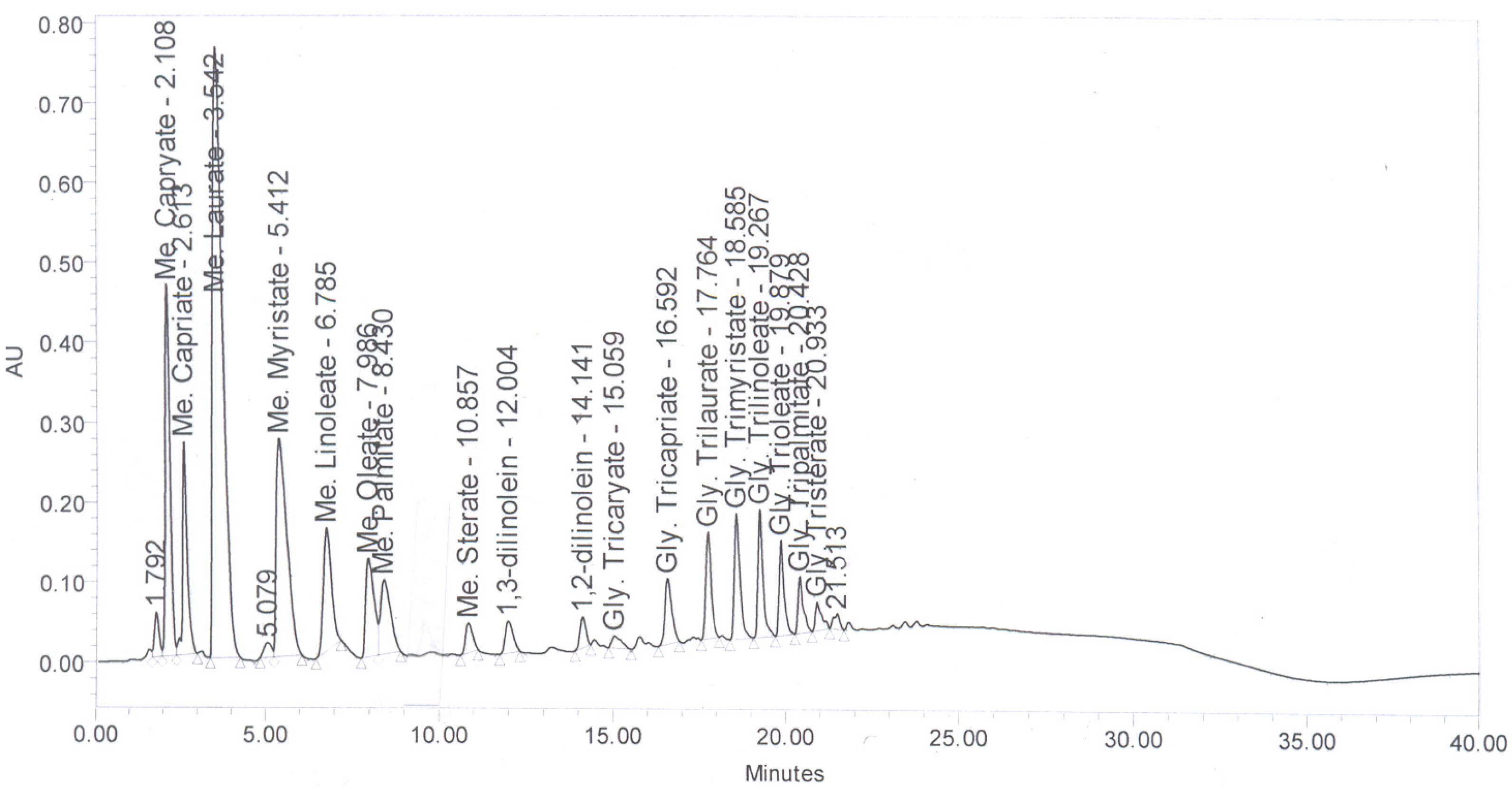
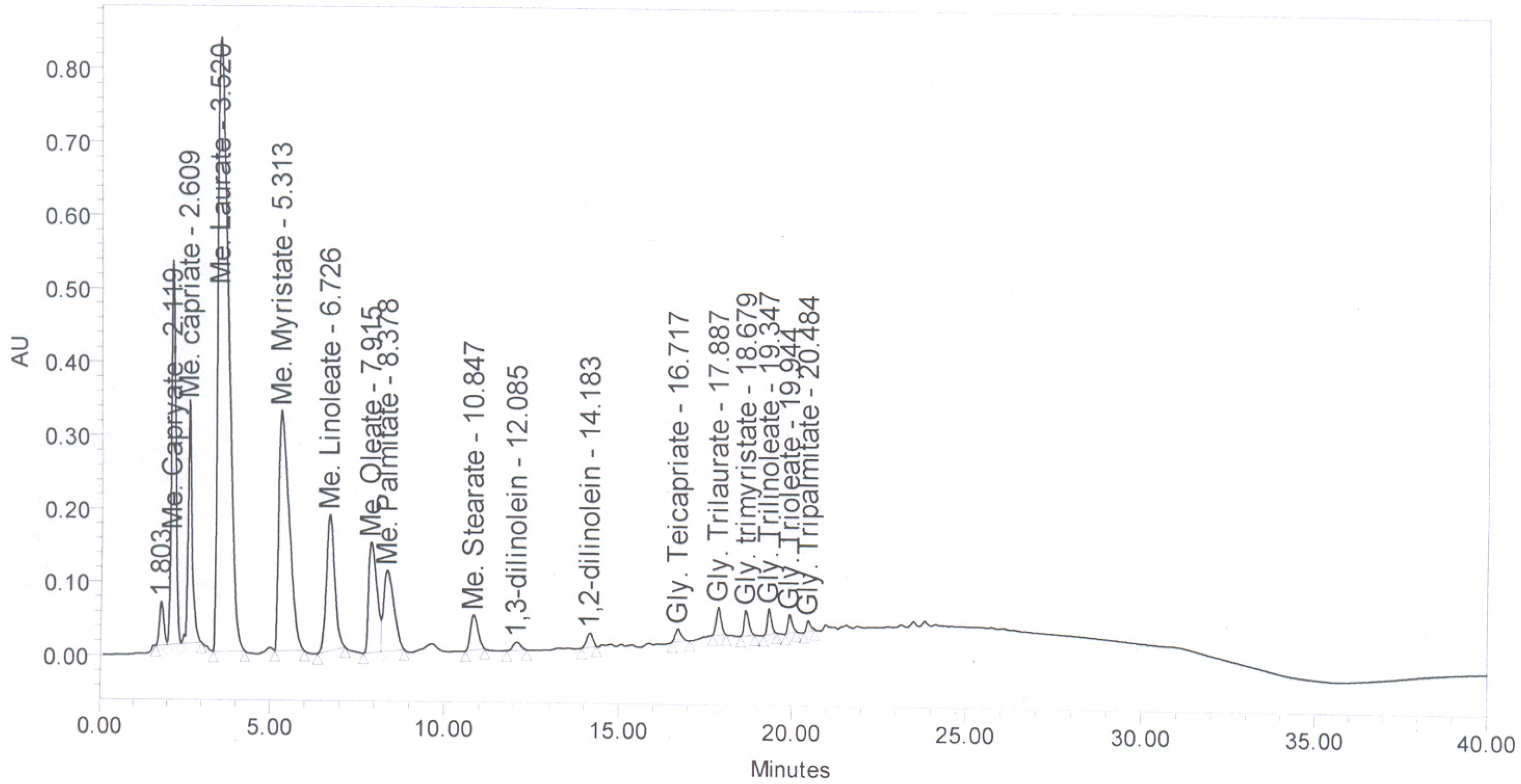
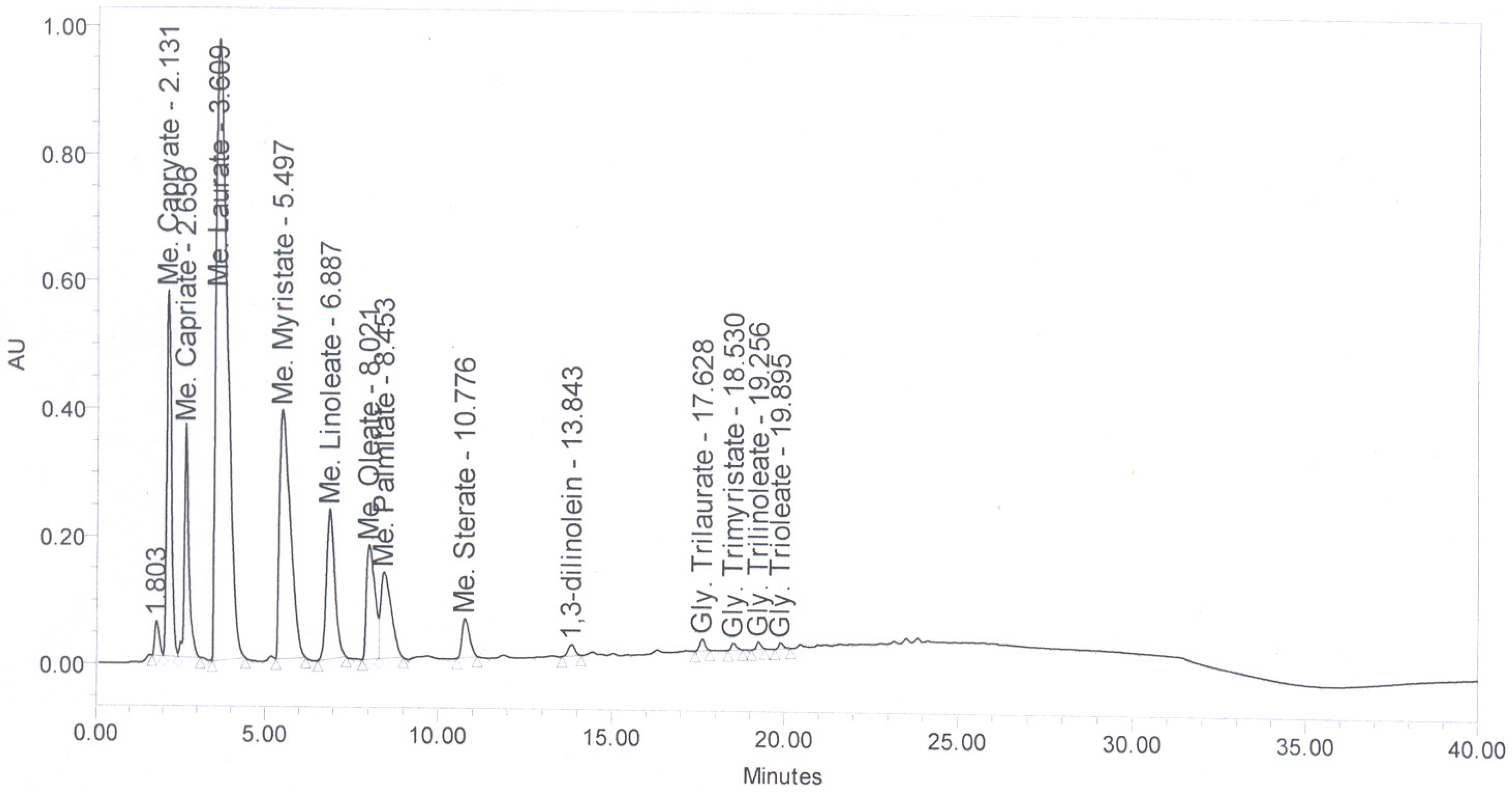
Figure 6 shows the HPLC chromatogram of coconut oil. The FAME present in coconut oi-based biodiesel are presented in
Table 2.
Figure 6.
HPLC Chromatogram of coconut oil.
Figure 6.
HPLC Chromatogram of coconut oil.
Table 2.
Fatty acid methyl esters (FAME) composition of coconut oil-based biodiesel.
Table 2.
Fatty acid methyl esters (FAME) composition of coconut oil-based biodiesel.
| Fatty acid | Percentage |
| Caprylic (C8:0) | 8.86 |
| Capric (C10:0) | 6.17 |
| Lauric (C12:0) | 48.83 |
| Myristic (C14:0) | 19.97 |
| Palmitic (C16:0) | 7.84 |
| Stearic (C18:0) | 3.06 |
| Oleic (C18:1) | 4.44 |
| Linoleic (C18:2) | 0.76 |
2.8. Characterization
Kinematic Viscosity, Acid number and oxidation stability
All the physicochemical properties of the coconut-based biodiesel are given in
Table 3. The kinematic viscosity of biodiesel at 40 °C was determined following ASTM D 445 [
24] using a Rheotek AKV 800 automated kinematic viscometer (Poulten Selfe and Lee Ltd., Essex, England). The acid number of biodiesel was determined according to ASTM D 664 [
25] using a Brinkman/Metrohn 809 Titrando (Westbury, NY). The oxidation stability of biodiesel was determined as the induction period (IP) according to EN 14112 [
26] using a Metrohn 743 Rancimat instrument (Herisau, Switzerland).
Table 3.
Physical properties of coconut biodiesel.
Table 3.
Physical properties of coconut biodiesel.
| Property | ASTM method | ASTMS pecification | Oil | Biodiesel |
|---|
| Viscosity 40 °C (mm2/s) | D 445 | 1.9–6.0 | 27.23 | 2.83 |
| Acid number (mgKOH/g) | D 664 | 0.5 | 2.1 | 0.18 |
| Free glycerin (mass %) | D 6584 | 0.020 | ---- | 0.008 |
| Total glycerin (mass %) | D 6584 | 0.24 | ---- | 0.24 |
| Oxidation stability (IP, h) | EN 14112 | 3 minimum | ---- | 9.2 |
| Cloud point | D 2500-05 | –3 to +12 | | _3 |
| Pour point | D 97-96a | –15 to +10 | _6 | _12 |
| Cetane number | D 613 | >47 | 60 | 51 |
| Cold filter plugging point | D 6371 | –4 to –9 | ---- | _5 |
| Density at 15 °C (Kg/m3) | D 976 | 0.575 to 0.900 | 0.9251 | 0.8733 |
| Flash point ( °C) | D 93 | >100 | 266 | 110 |
| Carbon residue (wt%) | D 4530 | <0.02 | 0.01 | 0.005 |
| Copper strip corrosion (3 hrs at 100 °C) | D6751 | <No. 1.0 | 1.0 | 1.0 |
| Water content (ppm) | D 95 | <500 | 1,293 | 230 |
Cold flow properties:
The cloud point (CP), pour point (PP) and cloud filter plugging point (CFPP) measurements were done as per ASTM standards, D 2500-05 [
27], D 97-96a [
28] and D 6371-05 [
29], respectively. A Lawler model DR-34H automated cold properties analyzed (Lawler Manufacturing Corporation, Edison, NJ) was used to measure the cold flow properties.