Biotechnological Utilization with a Focus on Anaerobic Treatment of Cheese Whey: Current Status and Prospects
Abstract
:1. Introduction
2. Composition of Cheese Whey
| Components | Sweet whey (g/L) | Acid whey (g/L) |
|---|---|---|
| Total solids | 63–70 | 63–70 |
| Lactose | 46–52 | 44–46 |
| Proteins | 6−10 | 6–8 |
| Calcium | 0.4–0.6 | 1.2–1.6 |
| Phosphate | 1–3 | 2–4.5 |
| Lactate | 2 | 6.4 |
| Chloride | 1.1 | 1.1 |
| Components | Value | References |
|---|---|---|
| BOD5 | 40,000–60,000 ppm 30,000–50,000 ppm >30,000 ppm | [34] [15,39] [17] |
| COD | 50,000–80,000 ppm 60,000–80,000 pp m60,000–100,000 ppm >60,000 ppm | [34,40] [15] [39] [17] |
3. Cheese Whey Management
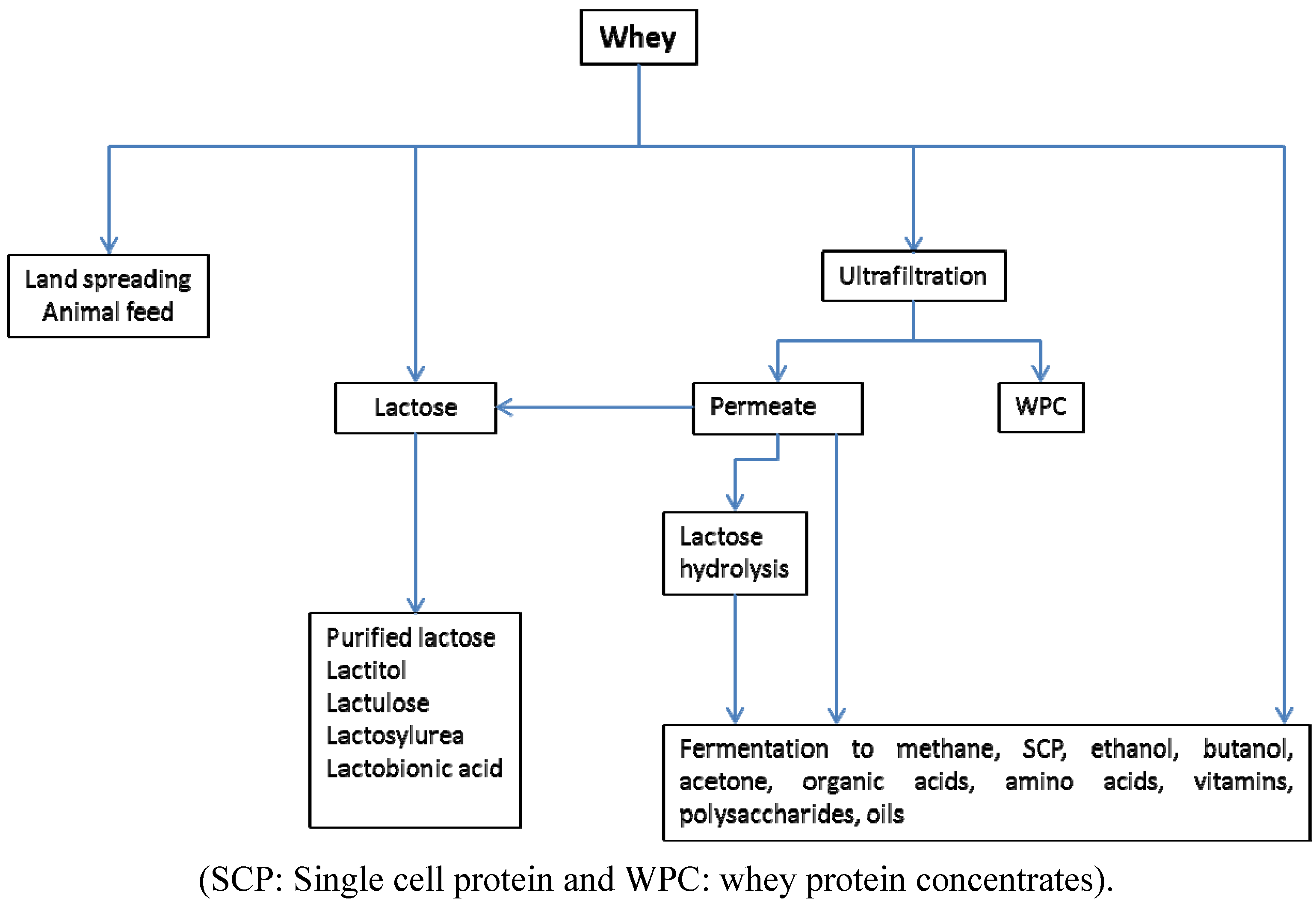
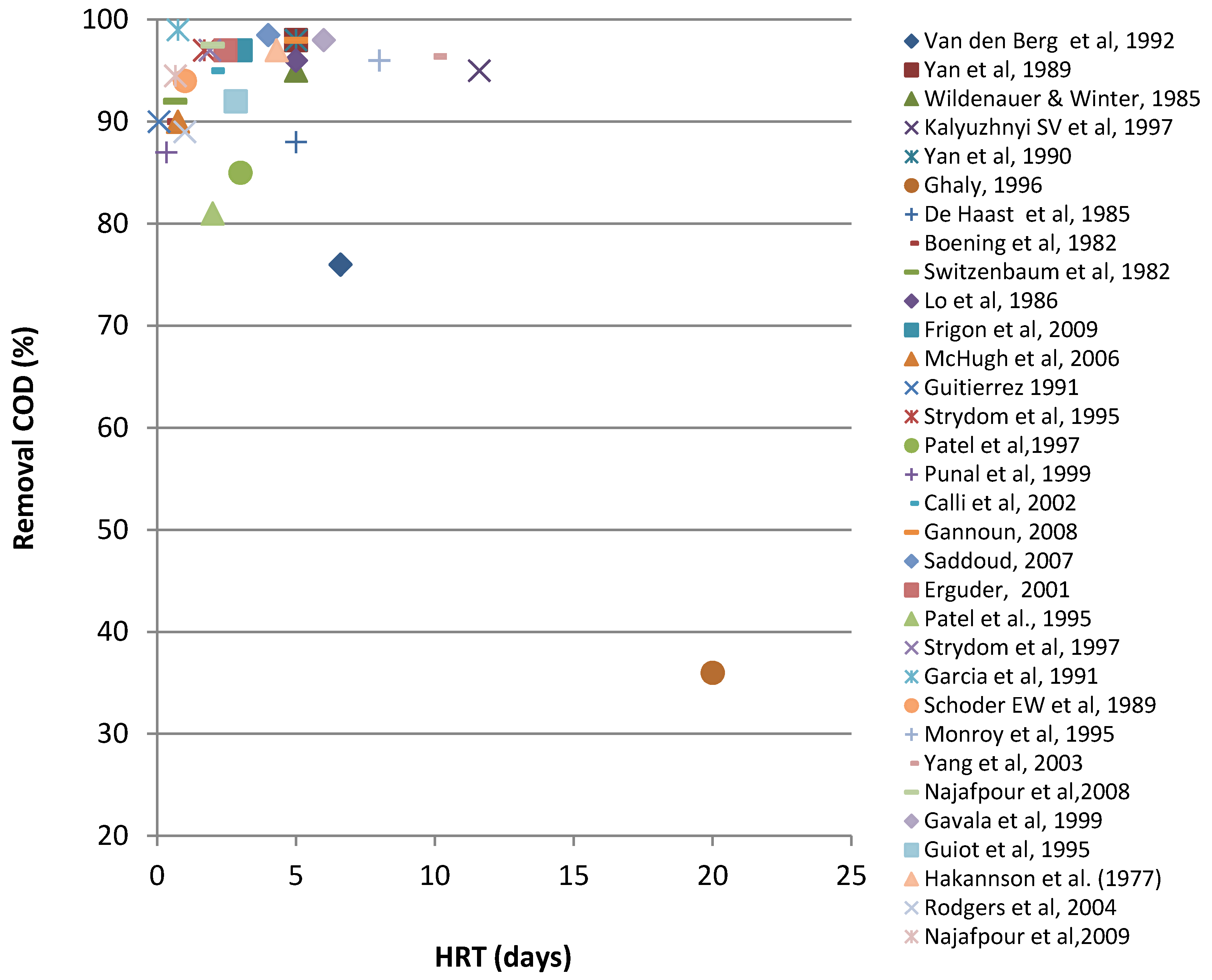
4. Use of Anaerobic Digestion Systems—Production of Biogas and Removal COD
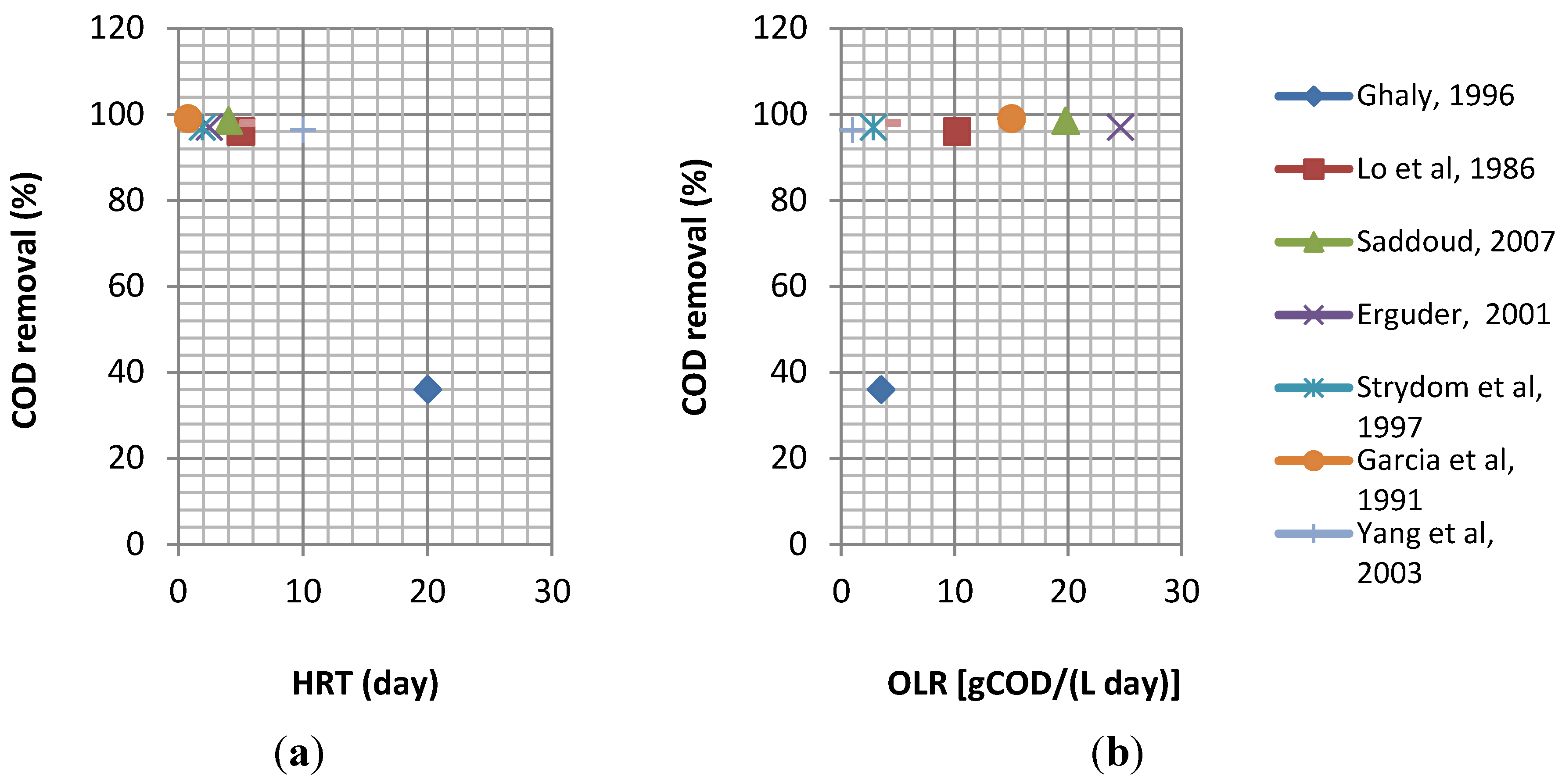
| Waste | Reactor | IC | pH | Biogas/CH4 yield | HRT | OLR | T | CR | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| CW | DFF | 66 | - | 0,28 m3CH4/kg CODrem | 4.9 | 13 | - | 75 | [71] |
| CW | DFF | 66 | - | 0,34 m3CH4/kg CODrem | 6.6 | 8.3 | - | 76 | [71] |
| CW | AF | - | - | - | 4 | - | 30–21–12.5 | 92–85–78 | [72,73] |
| CW | UASB | 28.7 | 7.18 | 9.57 L CH4/L feed/day | 5 | 5.96 | 33 | 98 | [74] |
| Sour(acidic)whey | UFFLR | 79 | 6.7 | 5.6 m3/m3day | 5 | 14 | 35 | 95 | [75] |
| CW high strength | UASB | 77 | - | - | 11.6 | 28.5 | 35 | 95 | [76] |
| CW | UASB | 28.8 | 7.15 | - | 5 | 5.96 | 33 | 98 | [77] |
| CW | UAR* | 72.2 | 4.5 | - | 20 | 3.5 | 35 | 36 | [78] |
| Deproteinated CW | DSFFR | 13 | - | - | 5 | 2.6 | 35 | 88 | [79] |
| Strength lactic casein whey permeate | FBR | 7 | 4.3 | 0.396 m3CH4/kg CODrem. | 0.4 | 7.7 | 35 | 90 | [80] |
| Sweet whey powder | AAFEB | 5 | 7 | - | 0.65 | 10 | 35 | 92 | [11] |
| Cheddar CW | AnRBC* | 64 | - | - | 5 | 10.2 | 35 | 96 | [81] |
| CW | SDFA | 69.8 | - | - | 4.3 | 16.1 | - | 99 | [82] |
| CW | (SBR)*** | 3.9 | 7 | - | 3 | 1.04 | 20 | 97 | [83] |
| Whey in a dried form | AHR (R1) | 1 | 7–8 | 0.69 (CH4yield) | 0.75 | 1.3 | 20 | 80 | [84] |
| Whey in a dried form | AHR (R2) | 10 | 7–8 | 0.55 (CH4yield) | 0.75 | 13.3 | 20 | 90 | [84] |
| Cheese was/ter | UASB | 2.05 | 6.7 | 0.32lCH4/gCODelim. | 0.07 | 31 | 35 | 90 | [85] |
| CW | AHR | 10 | - | 0.354 (CH4yield) | 1.7 | 6.11 | 35 | 97 | [86] |
| (Salty) CW | RBCR | 30 | 7 | 4.1 L/Ldigester/day | 3 | 10 | 37 | 85 | [87] |
| (Salty) CW | (MB) | - | - | 3.2 L/Ldigester/day | 2 | - | 37 | 83 | [88] |
| CW | UF (SFR & MFR) | 9 | 4-7 | - | 0.33 | 35 | - | 87 | [89] |
| CW | HBR | 22 | - | - | 2 | 11 | - | 95 | [90] |
| CW | EPFAUF)* | 20 | 7 | - | 5 | 4 | 34–36 | 98 | [4] |
| CW | ASBR | - | - | - | 0.33 | - | 28–32 | 90 | [91] |
| Raw CW | TSMAMD* | 68.6 | 7.9–8.5 | >0.70(CH4yield) | 4 | 19.78 | 37 | 98.5 | [34] |
| CW | UASB* | 58.4 | 7–8 | 0.77 (CH4yield ) | 2.46 | 24.6 | - | 97 | [10] |
| CW | DUHR | 68 | - | - | 7 | 10 | - | 97 | [70] |
| CW | UAFFR | 70 | - | 0.72 (CH4yield) | 2 | 35 | 37 | 81 | [92] |
| CW, butter, fresh milk | AHR* | 5.34 | 5.22 | 0.28–0.35 (CH4yield) | 1.9 | 2.82 | 35 | 97 | [93] |
| CW &diluted poultry manure | CSTR | 91 | - | 2.2 L/Lreactor/day | 18 | 4.9 | 35 | 77 | [39] |
| Whey mix & cow manure | AD1 | - | - | - | 14 | - | 35 | 74 | [94] |
| CW | UASB* | 10.82 | - | - | 0.75 | 15 | 34–36 | 99 | [95] |
| CW&dairy manure | CSTR | 29 | - | - | 10 | - | 34 | 54 | [28] |
| CW | FBR | 0.8–10 | - | - | 0.1–0.4 | 6–40 | 35 | 63–87 | [96] |
| Deprotainated CW | UASB | 11 | - | - | 1.5 | 7.1 | 35 | 94 | [97] |
| CW | AP ** | 4.4 | - | - | 8 | 0.55 | - | 96 | [98] |
| CW | CSTR & UAF* | - | - | 0.55m3/kg CODrem. | 4 | - | - | 95 | [99] |
| CW | 2 CSTRs* | 10 | - | - | 10 | 0.97 | 55 | 96.4 | [100] |
| CW | UASFF | 57 | - | 3.75 L/day | 2 | 25 | 36 | 97.5 | [40] |
| Diluted CW | UASB | 37 | 7.2 | - | 6 | 6.2 | 35 | 98 | [9] |
| CW | NMR | 42.5 | - | - | 2.83 | 15 | 34 | 92 | [101] |
| CW | AF | 8.1 | - | - | 4.3 | 1.9 | 22–25 | 97 | [102] |
| CW | NMABR | 10.2 | - | - | 1 | 11.3 | 35 | 89 | [103] |
| CW | NMABR | 10.2 | 7–8 | - | 0.6 | 15.3 | 35 | 81 | [103] |
| CW | UAPBR | 59.4 | 6.5 | - | 0.66 | 59.28 | 25 | 94.5 | [104] |
| Waste | Reactor | Inoculum | Effluent | Ref. |
|---|---|---|---|---|
| CW | UASB | Seed sludge from AnRBC used for treatment a mixture of CW and manure | COD: 457 mg/L, pH: 7.18, VFA: 18 mg/L | [74] |
| Sour (acidic) whey | UFFLR | Sewage sludge | COD: 3.9 g/L | [75] |
| CW (high strength) | UASB | Dispersed sludge from anaerobic lagoon treating meet industry wastewater | VFA < 0.5 gCOD/L | [76] |
| CW | UASB | Seed sludge from AnRBC used for treatment a mixture of CW and manure | COD: 457 mg/L, pH = 7.18, VFA: 18 mg/L | [77] |
| CW | UAR* | Seed material from unmixed An. Digester operating on dairy manure | COD: 33.02 g/L, Volatile solids concentration: 17.9 g/L | [78] |
| CW | (SBR)*** | Biomass for the inoculum came from a full scale anaer. digester treating fruit processing wastewaters | COD: 51 ± 56 mg/L after aerobic step | [83] |
| Whey in a dried form | AHR (R1) | Anaerobic granular sludge originating from an internal circulation reactor treating wastewater from a commercial lactose alcohol | sCOD: 150–300 mg/L | [84] |
| Whey in a dried form | AHR (R2) | Anaerobic granular sludge originating from an internal circulation reactor treating wastewater from a commercial lactose alcohol | propionate: 500 mg/L, acetate: 100 mg/L | [84] |
| Cheese production wastewater | UASB | Cleaning water from cheese factory (cheese production waastewaters) | acetic & propionic acid concentration: 0.01–0.02 mg/L, COD: 65 mg/L | [85] |
| CW | UF (SFR & MFR) | Seed sludge from UAF mesophilic reactor treating wastewaters from tuna processing factory | SFR COD: 5 g/L & MFR COD:2 g/L | [89] |
| Pretreated CW | (EPFAUF)* | Inoculum from an active biogas digester of fruit and vegetable waste treatment | - | [4] |
| CW | ASBR | Inoculum from UASB treating poultry slaugherhouse wastewater | - | [91] |
| Raw CW | TSMAMD* | Inoculum From full scale AD treatment plant | - | [34] |
| CW | UASB* | - | COD: 1.428–1.975 mgCOD/L | [10] |
| CW | UAFFR | Inoculum from operating whey reactor | VFA: 0.94 g/L, COD: 16.4 g/L | [92] |
| CW, butter, freshmilk | AHR* | Inoculum from sewage sludge, rumen fluid and effluent from two other mesophilic lab-scale reactor | - | [93] |
| CW | UASB* | Sludge from anaerobic reactor treating whey in a single step | - | [95] |
| CW | 2 CSTRs* | Inoculum from municipal wastewater treatment | - | [100] |
| CW | UASFF | Seed culture from wastewater treatment plant | - | [40] |
| Diluted CW | UASB | Inoculated with anaerobic mixed liquor from dairy wastewaters and glucose fed digesters | COD: 5g/L | [9] |
4.1. Conventional (Single Phase) Anaerobic Treatment of Cheese Whey

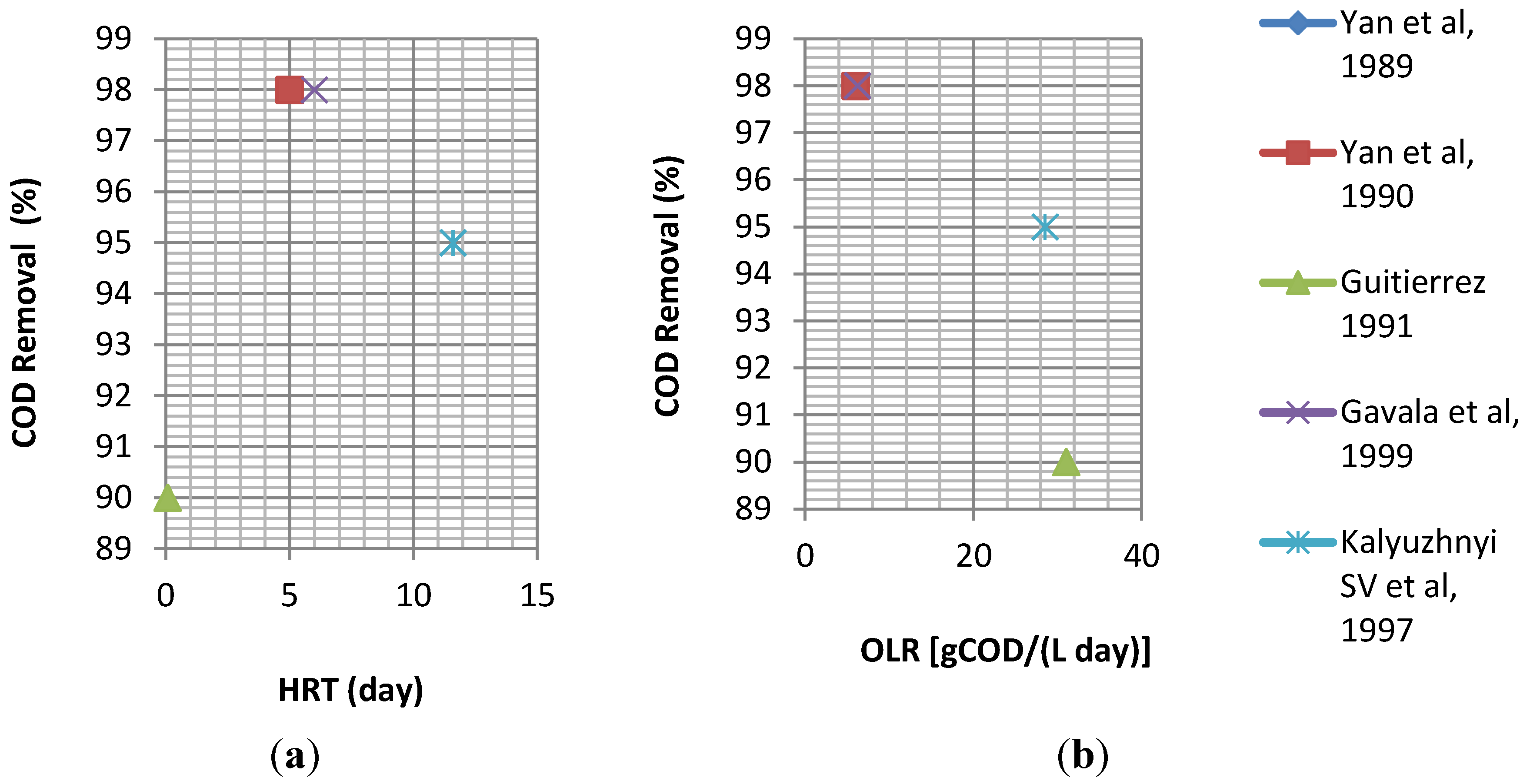

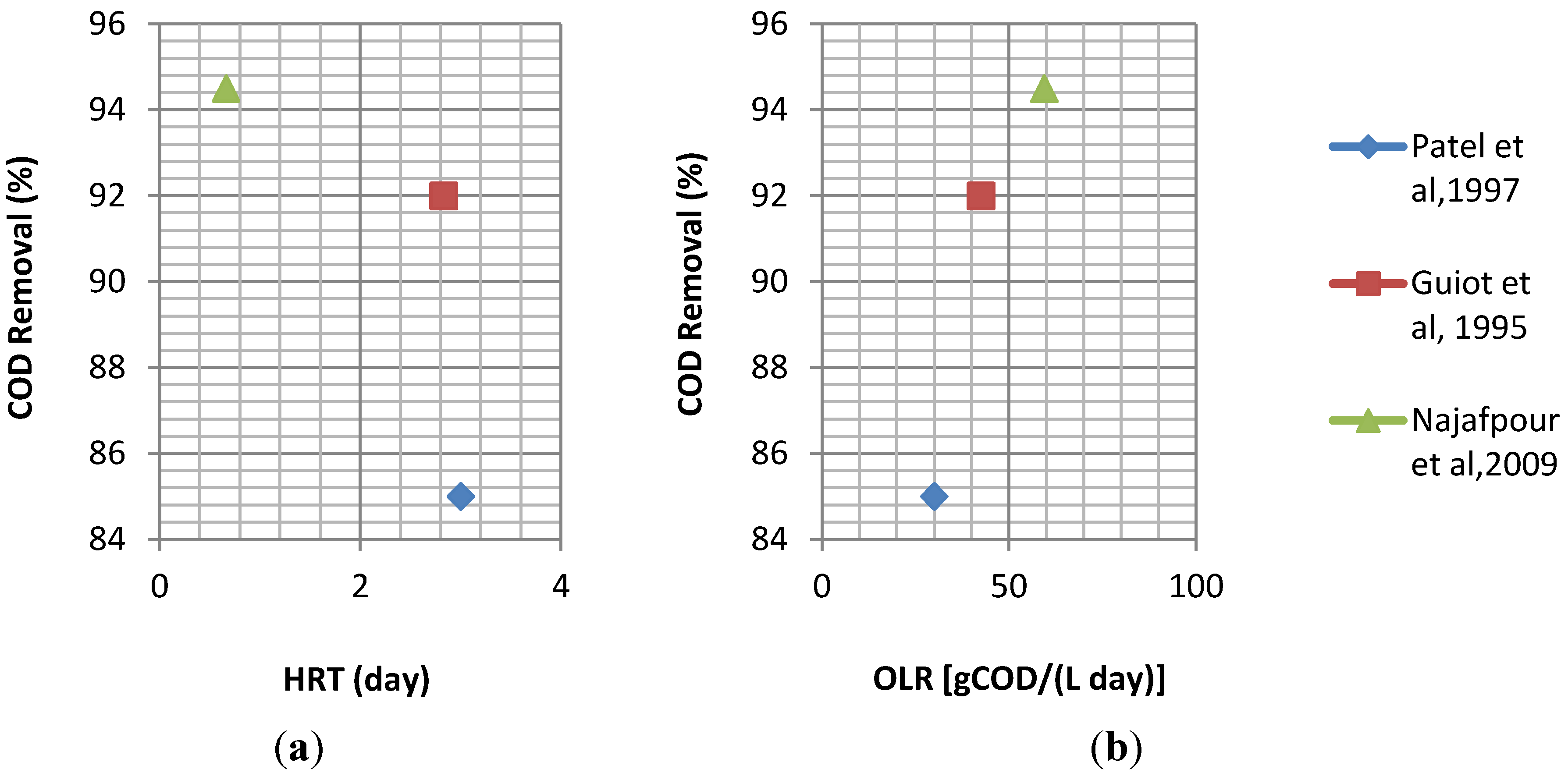
4.2. Two Phase (Two Stage) Anaerobic Treatment of Cheese Whey
4.3. Anaerobic/Aerobic Reactors
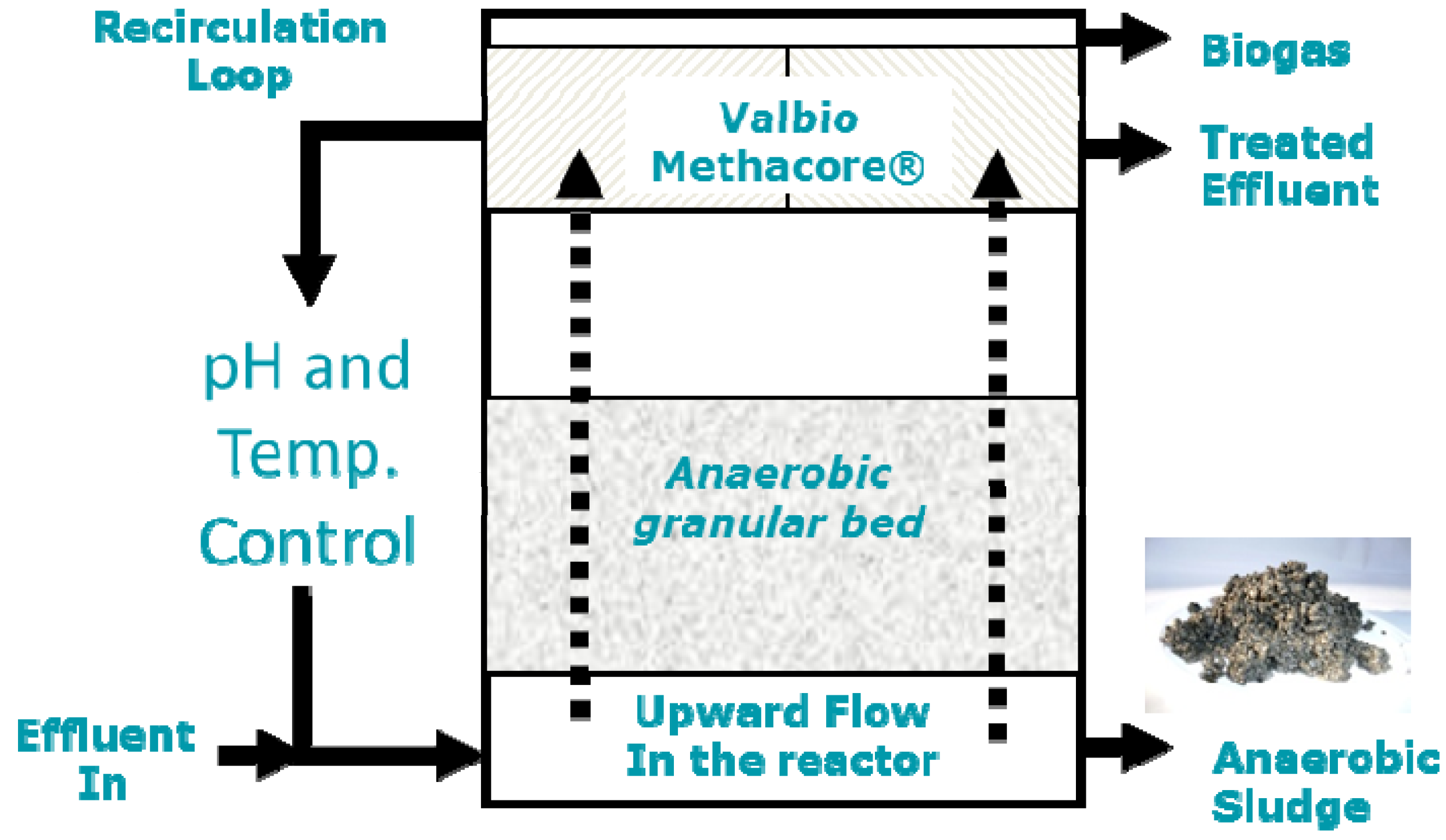
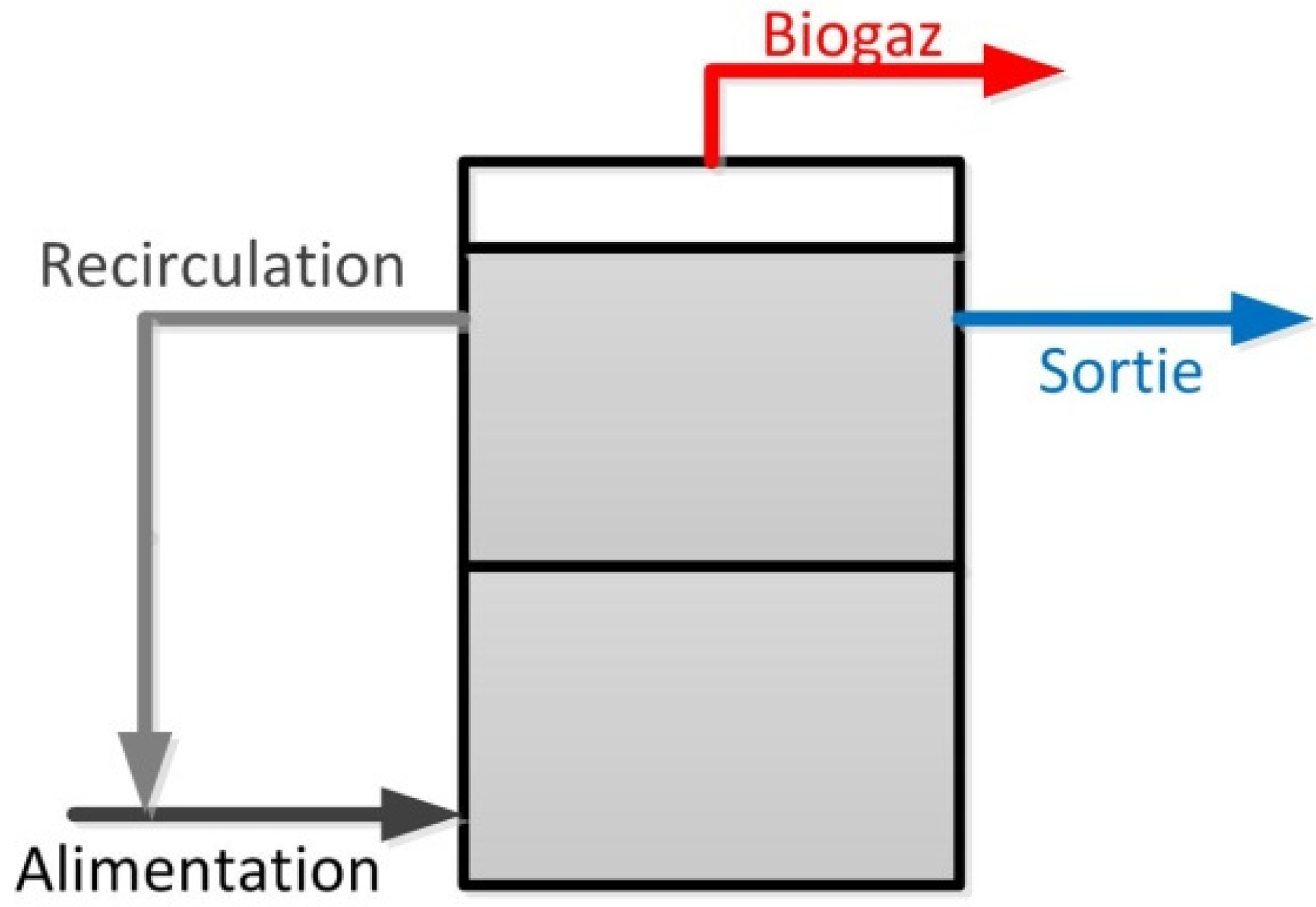
4.4. Dairy Industries Using Anaerobic Digestion Systems
5. Conclusions
Nomenclature
| AD | Anaerobic Digestion |
| BOD | Biological (Biochemical) Oxygen Demand |
| BOD5 | Five day Biological (Biochemical) Oxygen Demand |
| COD | Chemical Oxygen Demand |
| CR | COD Removal (%) |
| CW | Cheese Whey |
| HRT | Hydraulic Retention Time (day) |
| IC | Influent COD (g COD/L) |
| OLR | Organic Load Rate (g COD/(L day)) |
| T | Temperature (°C) |
Abbreviations
| AAFEB | Anaerobic Attached Film Expanded Bed |
| ABR | Anaerobic Bio film Reactor |
| AF | Anaerobic Filter |
| AHR | Anaerobic Hybrid Reactor |
| AMMBR | Anaerobic Moving Biofilm Reactor |
| AnRBC | Anaerobic Rotating Biological Contact Reactor |
| AP | Anaerobic Pond |
| ARBCR | Anaerobic Rotating Biological Contact Reactor |
| ASBBR | Anaerobic Sequencing Batch Biofilm Reactor |
| ASBR | Anaerobic Sequencing Batch Reactor |
| CSTR | Continuously Stirred Tank Reactor |
| DFFR | Downflow Fixed Film Reactor |
| DSFFR | Downflow Stationary Fixed Film Reactor |
| DUHR | Downflow-Upflow Hybrid Reactor |
| EPFAUF | Ecological Pretreatment Followed by Anaerobic Upflow Filter |
| FBR | Fluidized Bed Reactor |
| FFR | Fixed Film Reactor |
| HAR | Hybrid Anaerobic Reactor |
| HBR | Hybrid Bed Reactor |
| MAB | Multichamber Anaerobic Bioreactor |
| NMABR | Novel Moving Anaerobic Biofilm Reactor |
| NMR | Novel Multiplate Reactor |
| RBCR | Rotating Biological Contact Reactor |
| SDFA | Semi-continuous Digester and chemical Flocculant Addition |
| SMFBR | Sub-Merged Fixed Bio film Reactor |
| TSMAMD | Two Stage Mixed Anaerobic Membrane Digester |
| UAF | Upflow Anaerobic Filter |
| UAFFR | Upflow Anaerobic Fixed Film Reactor |
| UAPBR | Upflow Anaerobic Packed Bed Reactor |
| UAR | Unmixed Anaerobic Reactor |
| UASB | Upflow Anaerobic Sludge Blanket |
| UASFF | Upflow Anaerobic Sludge Fixed Film Reactor |
| UFFR | Upflow Fixed Film Reactor |
| UFFLR | Upflow Fixed Film Loop Reactor |
| UHR | Upflow Hybrid Reactor |
Acknowledgments
References
- Papachristou, E.; Lafazanis, C. Application of membrane technology in the pretreatment of cheese dairies wastes and co-treatment in a municipal conventional biological unit. Water Sci. Technol. 1997, 32, 361–367. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic treatment of dairy wastewaters: A review. Process Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Farizoglu, B.; Keskinler, B.; Yildiz, E.; Nuhoglu, A. Cheese whey treatment performance of an aerobic jet loop membrane bioreactor. Process Biochem. 2004, 39, 2283–2291. [Google Scholar] [CrossRef]
- Gannoun, H.; Khelifi, E.; Bouallagui, H.; Touhami, Y.; Hamdi, M. Ecological clarification of cheese whey prior to anaerobic digestion in upflow anaerobic filter. Bioresour. Technol. 2008, 99, 6105–6111. [Google Scholar] [CrossRef] [PubMed]
- Omil, F.; Garrido, J.M.; Arrojo, B.; Méndez, R. Anaerobic filter reactor performance for the treatment of complex dairy wastewater at industrial scale. Water Res. 2003, 37, 4099–4108. [Google Scholar] [CrossRef] [PubMed]
- Wust, E.L. Single-Phase and Two-Phase Cheese Wastewater Treatment by Anaerobic SBRs. Ph.D. Disseration, Marquette University, Milwaukee, WI, USA, 2003. [Google Scholar]
- Orhon, D.; Görgün, E.; Germirli, F.; Artan, N. Biological treatability of dairy wastewaters. Water Res. 1993, 27, 625–633. [Google Scholar] [CrossRef]
- Vidal, G.; Carvalho, A.; Méndez, R.; Lema, J.M. Influence of the content in fats and proteins on the anaerobic biodegradability of dairy wastewaters. Bioresour. Technol. 2000, 74, 231–239. [Google Scholar] [CrossRef]
- Gavala, H.N.; Kopsinis, H.; Skiadas, I.V.; Stamatelatou, K.; Lyberatos, G. Treatment of dairy wastewater using an upflow anaerobic sludge blanket reactor. J. Agr. Eng. Res. 1999, 73, 59–63. [Google Scholar] [CrossRef]
- Ergüder, T.H.; Tezel, U.; Güven, E.; Demirer, G.N. Anaerobic biotransformation and methane generation potential of cheese whey in batch and UASB reactors. Waste Manag. 2001, 21, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Switzenbaum, M.S.; Danskin, S.C. Anaerobic expanded bed treatment of whey. Agric. Wastes 1982, 4, 411–426. [Google Scholar] [CrossRef]
- Marshall, K.R.; Harper, W.J. The treatment of wastes from the dairy industry. In Surveys in Industrial Wastewater Treatment—Food and Allied Industries; Barnes, D., Forster, S.T., Hurdley, S.E., Eds.; Pitman Publishing: London, UK, 1984; Volume 1, pp. 296–376. [Google Scholar]
- Mawson, A.J. Bioconversions for whey utilization and waste abatement. Bioresour. Technol. 1994, 47, 195–203. [Google Scholar] [CrossRef]
- Sienkiewicz, T.; Riedel, C.L. Whey and Whey Utilization: Possibilities for Utilization in Agriculture and Foodstuffs Production, 2nd ed.; Th. Mann Gelsenkirchen-Buer: Berlin, Germany, 1990. [Google Scholar]
- Siso, M.I.G. The biotechnological utilization of cheese whey: A review. Bioresour. Technol. 1996, 57, 1–11. [Google Scholar] [CrossRef]
- Durham, R.J.; Hourigan, J.A. Waste management and co-product recovery in dairy processing. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Waldron, K., Ed.; Woodhead Publishing: Cambridge, UK, 2007. [Google Scholar]
- Smithers, G.W. Whey and whey proteins—From “gutter-to-gold”. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Audic, J.L.; Chaufer, B.; Daufin, G. Non-food applications of milk components and dairy co-products: A review. Lait 2003, 83, 417–438. [Google Scholar] [CrossRef]
- Rajeshwari, K.V.; Balakrishnan, M.; Kansal, A.; Lata, K.; Kishore, V.V.N. State-of-the-art of anaerobic digestion technology for industrial wastewater treatment. Renew. Sustain. Energy Rev. 2000, 4, 135–156. [Google Scholar] [CrossRef]
- Saleh, M.M.A.; Mahmood, U.F. Anaerobic digestion technology for industrial wastewater treatment. In Proceedings of Eighth International Water Technology Conference (IWTC8), Alexandria, Egypt, 27 March 2004.
- Demirel, B.; Yenigün, O. Two-phase anaerobic digestion processes: A review. J. Chem. Technol. Biotechnol. 2002, 77, 743–755. [Google Scholar] [CrossRef]
- Ke, S.; Shi, Z.; Fang, H.H.P. Applications of two-phase anaerobic degradation in industrial wastewater treatment. Int. J. Environ. Pollut. 2005, 23, 65–80. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.E.; Kamal, M.A. Submerged yeast fermentation of acid cheese whey for protein production and pollution potential reduction. Water Res. 2004, 38, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Ferchichi, M.; Crabbe, E.; Gil, G.-H.; Hintz, W.; Almadidy, A. Influence of initial pH on hydrogen production from cheese whey. J. Biotechnol. 2005, 120, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Kisaalita, W.S.; Pinder, K.L.; Lo, K.V. Acidogenic fermentation of lactose. Biotechnol. Bioeng. 1987, 30, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zadow, J.G. Utilization of milk components: Whey. In Modern Dairy Technology, Advances in Milk Processing, 2nd ed.; Robinson, R.K., Ed.; Chapman & Hall: London, UK, 1994; Volume 1. [Google Scholar]
- Kavacik, B.; Topaloglu, B. Biogas production from co-digestion of a mixture of cheese whey and dairy manure. Biomass Bioenergy 2010, 34, 1321–1329. [Google Scholar] [CrossRef]
- Venetsaneas, N.; Antonopoulou, G.; Stamatelatou, K.; Kornaros, M.; Lyberatos, G. Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Bioresour. Technol. 2009, 100, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Panesar, P.S.; Kennedy, J.F.; Gandhi, D.N.; Bunko, K. Bioutilisation of whey for lactic acid production. Food Chem. 2007, 105, 1–14. [Google Scholar] [CrossRef]
- Bylund, G. Dairy Processing Handbook; Tetra Pak Processing Systems: Lund, Sweden, 1995. [Google Scholar]
- Jelen, P. Whey processing. In Encyclopedia of Dairy Sciences; Roginski, H., Fuquay, J.W., Fox, P.F., Eds.; London Academic Press: London, UK, 2003; Volume 4, pp. 2739–2751. [Google Scholar]
- Ben-Hassan, R.; Ghaly, A. Continuous propagation of Kluyveromyces fragilis in cheese whey for pollution potential reduction. Appl. Biochem. Biotechnol. 1994, 47, 89–105. [Google Scholar] [CrossRef]
- Saddoud, A.; Hassaïri, I.; Sayadi, S. Anaerobic membrane reactor with phase separation for the treatment of cheese whey. Bioresour. Technol. 2007, 98, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Kisaalita, W.S.; Lo, K.V.; Pinder, K.L. Influence of whey protein on continuous acidogenic degradation of lactose. Biotechnol. Bioeng. 1990, 36, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Hansen, C.L. Characterization of and bioproduction of short-chain organic acids from mixed dairy-processing wastewater. Trans. ASAE 1998, 41, 795–802. [Google Scholar] [CrossRef]
- Vasala, A.; Panula, J.; Neubauer, P. Efficient lactic acid production from high salt containing dairy by-products by Lactobacillus salivarius ssp. salicinius with pre-treatment by proteolytic microorganisms. J. Biotechnol. 2005, 117, 421–431. [Google Scholar]
- Guimarães, P.M.R.; Teixeira, J.A.; Domingues, L. Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol. Adv. 2010, 28, 375–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Mavris, V. Optimization of biogas production by co-digesting whey with diluted poultry manure. Renew. Energy 2007, 32, 2147–2160. [Google Scholar] [CrossRef]
- Najafpour, G.D.; Hashemiyeh, B.A.; Asadi, M.; Ghasemi, M.B. Biological treatment of dairy wastewater in an upflow anaerobic sludge-fixed film bioreactor. Am. Eurasian J. Agric. Environ. Sci. 2008, 4, 251–257. [Google Scholar]
- Cimino, G.; Caristi, C. Acute toxicity of heavy metals to aerobic digestion of waste cheese whey. Biol. Wastes 1990, 33, 201–210. [Google Scholar] [CrossRef]
- Ayar, A.; Sert, D.; Akin, N. The trace metal levels in milk and dairy products consumed in middle Anatolia—Turkey. Environ. Monit. Assess. 2009, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- OECD-FAO Agricultural Outlook 2008–2017. Highlights. Paris. Available online: http://www.agri-outlook.org/dataoecd/54/15/40715381.pdf (accessed on 5 September 2012).
- Karadima, K. Toxicity Estimation of Various Stages of Cheese Making Effluent Treatment Units Using Bioindicators. PhD Dissertation, University of Patra, Patra, Greece, 2009. [Google Scholar]
- FAO Food Outlook No. 2. Available online: http://www.fao.org/docrep/009/j8126e/j8126e11.htm (accessed on 5 September 2012).
- Kotoupas, A.; Rigas, F.; Chalaris, M. Computer-aided process design, economic evaluation and environmental impact assessment for treatment of cheese whey wastewater. Desalination 2007, 213, 238–252. [Google Scholar] [CrossRef]
- Gekas, V.; Lopez-Leiva, M. Hydrolysis of lactose: A literature review. Process Biochem. 1985, 20, 2–12. [Google Scholar]
- Kosikowski, F. Whey utilization and whey products. J. Dairy Sci. 1979, 62, 1149–1160. [Google Scholar] [CrossRef]
- Yang, S.T.; Silva, E.M. Novel Products and new technologies for use of a familiar carbohydrate, milk lactose. J. Dairy Sci. 1995, 78, 2541–2562. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.; Dupont, C.; Lemieux, P. Whey proteins and peptides: Beneficial effects on immune health. Therapy 2006, 3, 69–78. [Google Scholar] [CrossRef]
- Yalçin, A.S. Emerging therapeutic potential of whey proteins and peptides. Curr. Pharm. Des. 2006, 12, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Riechel, P.; Weiss, T.; Weiss, M.; Ulber, R.; Heinrich, B.; Scheper, T. Determination of the minor whey protein bovine lactoferrin in cheese whey concentrates with capillary electrophoresis. J. Chromatogr. A 1998, 817, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Zall, R.R. Trends in whey fractionation and utilization, a global perspective. J. Dairy Sci. 1984, 67, 2621–2629. [Google Scholar] [CrossRef]
- Kosaric, N.; Asher, Y. The utilization of cheese whey and its components. In Agricultural Feedstock and Waste Treatment and Engineering; Springer: Berlin, Germany, 1985; Volume 32, pp. 25–60. [Google Scholar]
- Gänzle, M.G.; Haase, G.; Jelen, P. Lactose: Crystallization, hydrolysis and value-added derivatives. Int. Dairy J. 2008, 18, 685–694. [Google Scholar] [CrossRef]
- Zadow, J.G. Lactose: Properties and uses. J. Dairy Sci. 1984, 67, 2654–2679. [Google Scholar] [CrossRef]
- Ling, K.C.; Liebrand, C.B. Whey to Ethanol: A Biofuel Role for Dairy Cooperatives; United States Department of Agriculture: Beltsville, MD, USA, 2008. [Google Scholar]
- Pesta, G.; Meyer-Pittroff, R.; Russ, W. Utilization of whey. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer: New York, NY, USA, 2007; pp. 193–207. [Google Scholar]
- Antonopoulou, G.; Stamatelatou, K.; Bebelis, S.; Lyberatos, G. Electricity generation from synthetic substrates and cheese whey using a two chamber microbial fuel cell. Biochem. Eng. J. 2010, 50, 10–15. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Antonopoulou, G.; Tremouli, A.; Lyberatos, G. Production of Gaseous biofuels and electricity from cheese whey. Ind. Eng. Chem. Res. 2010, 50, 639–644. [Google Scholar] [CrossRef]
- Ferchichi, M.; Crabbe, E.; Hintz, W.; Gil, G.-H.; Almadidy, A. Influence of culture parameters on biological hydrogen production by Clostridium saccharoperbutylacetonicum ATCC 27021. World J. Microbiol. Biotechnol. 2005, 21, 855–862. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, R.; McGarvey, J.A.; Benemann, J.R. Biohydrogen production from cheese processing wastewater by anaerobic fermentation using mixed microbial communities. Int. J. Hydrog. Energy 2007, 32, 4761–4771. [Google Scholar] [CrossRef]
- Davila-Vazquez, G.; Alatriste-Mondragón, F.; de León-Rodríguez, A.; Razo-Flores, E. Fermentative hydrogen production in batch experiments using lactose, cheese whey and glucose: Influence of initial substrate concentration and pH. Int. J. Hydrog. Energy 2008, 33, 4989–4997. [Google Scholar] [CrossRef]
- Azbar, N.; Çetinkaya-Dokgöz, F.T.; Keskin, T.; Korkmaz, K.S.; Syed, H.M. Continuous fermentative hydrogen production from cheese whey wastewater under thermophilic anaerobic conditions. Int. J. Hydrog. Energy 2009, 34, 7441–7447. [Google Scholar] [CrossRef]
- Rosales-Colunga, L.M.; Razo-Flores, E.; Ordonez, L.G.; Alatriste-Mondragón, F.; de León-Rodríguez, A. Hydrogen production by Escherichia coli DhycA DlacI using cheese whey as substrate. Int. J. Hydrog. Energy 2010, 35, 491–499. [Google Scholar] [CrossRef]
- Kargi, F.; Eren, N.S.; Ozmihci, S. Hydrogen gas production from cheese whey powder (CWP) solution by thermophilic dark fermentation. Int. J. Hydrog. Energy 2012, 37, 2260–2266. [Google Scholar] [CrossRef]
- Spachos, T.; Stamatis, A. Thermal analysis and optimization of an anaerobic treatment system of whey. Renew. Energy 2011, 36, 2097–2105. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’Dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in poultry litter disposal technology—A review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Stronach, S.M.; Rudd, T.M.; Lester, J.N. Anaerobic Digestion Processes in Industrial Wastewater Treatment; Springer-Verlag: Berlin, Germany, 1986. [Google Scholar]
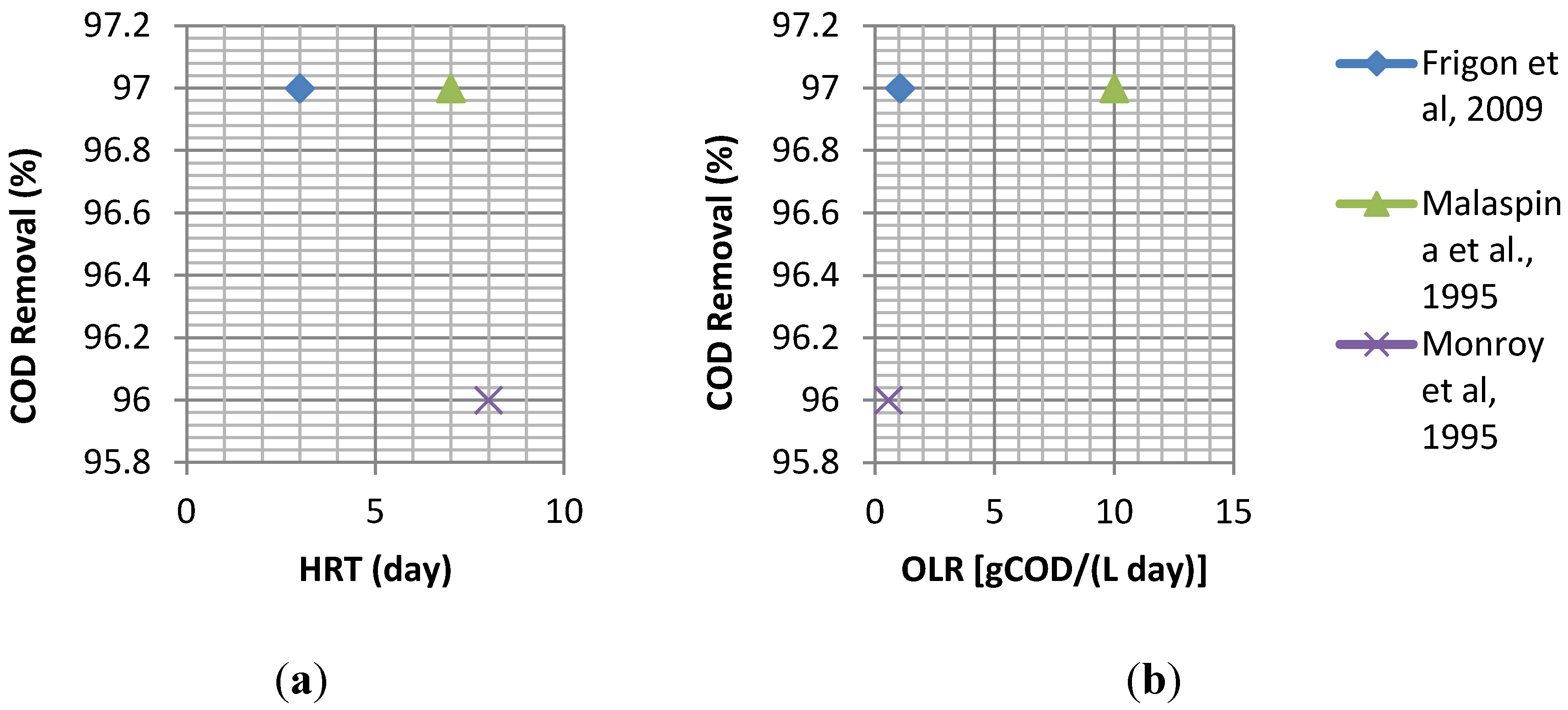
- Malaspina, F.; Stante, L.; Cellamare, C.M.; Tilche, A. Cheese whey and cheese factory wastewater treatment with a biological anaerobic—Aerobic process. Water Sci. Technol. 1995, 32, 59–72. [Google Scholar] [CrossRef]
- Van den Berg, L.; Kennedy, K.J. Dairy waste treatment with anaerobic stationary fixed film reactors. In Design of Anaerobic Processes for the Treatment of Industrial and Municipal Wastes; Malina, J.F., Pohland, F.G., Eds.; Technomic Publishing Company: Lancaster, PA, USA, 1992; pp. 89–96. [Google Scholar]
- Viraraghavan, T.; Kikkeri, S.R. Effect of temperature on anaerobic filter treatment of dairy wastewater. Water Sci. Technol. 1990, 22, 191–198. [Google Scholar]
- Viraraghavan, T.; Kikkeri, S.R. Dairy wastewater treatment using anaerobic filters. Can. Agr. Eng. 1991, 33, 143–149. [Google Scholar]
- Yan, J.Q.; Lo, K.V.; Liao, P.H. Anaerobic digestion of cheese whey using up-flow anaerobic sludge blanket reactor. Biol. Wastes 1989, 27, 289–305. [Google Scholar] [CrossRef]
- Wildenauer, F.X.; Winter, J. Anaerobic digestion of high-strength acidic whey in a pH-controlled up-flow fixed film loop reactor. Appl. Microbiol. Biotechnol. 1985, 22, 367–372. [Google Scholar] [CrossRef]
- Kalyuzhnyi, S.V.; Martinez, E.P.; Martinez, J.R. Anaerobic treatment of high-strength cheese-whey wastewaters in laboratory and pilot UASB-reactors. Bioresour. Technol. 1997, 60, 59–65. [Google Scholar] [CrossRef]
- Yan, J.Q.; Lo, K.V.; Liao, P.H. Anaerobic digestion of cheese whey using an upflow anaerobic sludge blanket reactor: III. Sludge and substrate profiles. Biomass 1990, 21, 257–271. [Google Scholar] [CrossRef]
- Ghaly, A.E. A comparative study of anaerobic digestion of acid cheese whey and dairy manure in a two-stage reactor. Bioresour. Technol. 1996, 58, 61–72. [Google Scholar] [CrossRef]
- De Haast, J.; Britz, T.J.; Novello, J.C.; Verwey, E.W. Anaerobic digestion of deproteinated cheese whey. J. Dairy Res. 1985, 22, 457–467. [Google Scholar]
- Boening, P.H.; Larsen, V.F. Anaerobic fluidized bed whey treatment. Biotechnol. Bioeng. 1982, 24, 2539–2556. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.V.; Liao, P.H. Digestion of cheese whey with anaerobic rotating biological contact reactors. Biomass 1986, 10, 243–252. [Google Scholar] [CrossRef]
- Barford, J.P.; Cail, R.G.; Callander, I.J.; Floyd, E.J. Anaerobic digestion of high-strength cheese whey utilizing semicontinuous digesters and chemical flocculant addition. Biotechnol. Bioeng. 1986, 28, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Frigon, J.C.; Breton, J.; Bruneau, T.; Moletta, R.; Guiot, S.R. The treatment of cheese whey wastewater by sequential anaerobic and aerobic steps in a single digester at pilot scale. Bioresour. Technol. 2009, 100, 4156–4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHugh, S.; Collins, G.; O’Flaherty, V. Long-term, high-rate anaerobic biological treatment of whey wastewaters at psychrophilic temperatures. Bioresour. Technol. 2006, 97, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.L.R.; Encina, P.A.G.; Fdz-Polanco, F. Anaerobic treatment of cheese-production wastewater using a UASB reactor. Bioresour. Technol. 1991, 37, 271–276. [Google Scholar] [CrossRef]
- Strydom, J.P.; Mostert, J.F.; Britz, T.J. Anaerobic treatment of a synthetic dairy effluent using a hybrid digester. Water S.A. 1995, 21, 125–130. [Google Scholar]
- Patel, C.; Madamwar, D. Biomethanation of salty cheese whey using an anaerobic rotating biological contact reactor. J. Ferment. Bioeng. 1997, 83, 502–504. [Google Scholar] [CrossRef]
- Patel, C.; Madamwar, D. Biomethanation of salty cheese whey using multichamber anaerobic bioreactor. Energy Environ. 1998, 9, 225–231. [Google Scholar]
- Puñal, A.; Méndez-Pampín, R.J.; Lema, J.M. Characterization and comparison of biomasses from single- and multi-fed upflow anaerobic filters. Bioresour. Technol. 1999, 68, 293–300. [Google Scholar] [CrossRef]
- Calli, B.; Yukselen, M.A. Anaerobic Treatment by a hybrid reactor. Environ. Eng. Sci. 2004, 19, 143–150. [Google Scholar] [CrossRef]
- Mockaitis, G.; Ratusznei, S.M.; Rodrigues, J.A.D.; Zaiat, M.; Foresti, E. Anaerobic whey treatment by a stirred sequencing batch reactor (ASBR): Effects of organic loading and supplemented alkalinity. J. Environ. Manag. 2006, 79, 198–206. [Google Scholar] [CrossRef]
- Patel, P.; Desai, M.; Madamwar, D. Biomethanation of cheese whey using anaerobic upflow fixed film reactor. J. Ferment. Bioeng. 1995, 79, 398–399. [Google Scholar] [CrossRef]
- Strydom, J.P.; Britz, T.J.; Mostert, J.F. Two-phase anaerobic digestion of three different effluents using a hybrid bioreactor. Water S.A. 1997, 23, 151–156. [Google Scholar]
- Comino, E.; Rosso, M.; Riggio, V. Development of a pilot scale anaerobic digester for biogas production from cow manure and whey mix. Bioresour. Technol. 2009, 100, 5072–5078. [Google Scholar] [CrossRef] [PubMed]
- García, P.A.; Rico, J.L.; Fdz-Polanco, F. Anaerobic treatment of cheese whey in a two-phase uasb reactor. Environ. Technol. 1991, 12, 355–362. [Google Scholar] [CrossRef]
- Denac, M.; Dunn, I.J. Packed- and fluidized-bed biofilm reactor performance for anaerobic wastewater treatment. Biotechnol. Bioeng. 1988, 32, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Schroder, E.W.; de Haast, J. Anaerobic digestion of deproteinated cheese whey in an up-flow sludge blanket reactor. J. Dairy Res. 1989, 56, 129–139. [Google Scholar] [CrossRef]
- Monroy, H.O.; Vázquez, M.F.; Derramadero, J.C.; Guyot, J.P. Anaerobic-aerobic treatment of cheese wastewater with national technology in Mexico: The case of “El Sauz”. Water Sci. Technol. 1995, 32, 149–156. [Google Scholar]
- Yilmazer, G.; Yenigün, O. Two-phase anaerobic treatment of cheese whey. Water Sci. Technol. 1999, 40, 289–295. [Google Scholar] [CrossRef]
- Yang, K.; Yu, Y.; Hwang, S. Selective optimization in thermophilic acidogenesis of cheese-whey wastewater to acetic and butyric acids: Partial acidification and methanation. Water Res. 2003, 37, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Guiot, S.R.; Safi, B.; Frigon, J.C.; Mercier, P.; Mulligan, C.; Tremblay, R.; Samson, R. Performances of a full-scale novel multiplate anaerobic reactor treating cheese whey effluent. Biotechnol. Bioeng. 1995, 45, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Hakannson, H.; Frostell, B.; Norrman, J. Anaerobic Treatment of Whey and Whey Permeate in Submerged Filters; Inst. Vatten-Luftvardsforsl: Stockholm, Sweden, 1977. [Google Scholar]
- Rodgers, M.; Zhan, X.-M.; Dolan, B. Mixing Characteristics and whey wastewater treatment of a novel moving anaerobic biofilm reactor. J. Environ. Sci. Health Part A 2004, 39, 2183–2193. [Google Scholar] [CrossRef]
- Najafpour, G.D.; Tajallipour, M.; Komeili, M.; Mohammadi, M. Kinetic model for an up-flow anaerobic packed bed bioreactor: Dairy wastewater treatment. Afr. J. Biotechnol. 2009, 8, 3590–3596. [Google Scholar]
- Mateescu, C.; Constantinescu, I. Comparative analysisof inoculum biomass for biogas potential in the anaerobic digestion. UPB Sci. Bull. 2011, 73, 99–104. [Google Scholar]
- Bouallagui, H.; Ben, C.R.; Marouani, L.; Hamdi, M. Mesophilic biogas production from fruit and vegetable waste in a tubular digester. Bioresour. Technol. 2003, 86, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Tay, J.H.; Tay, S.T.L.; Hung, Y.T. Environmental Bioengineering, 1st ed.; Humana Press: New York, NY, USA, 2010. [Google Scholar]
- Ahring, B.K. Biomethanation II; Springer: Berlin, Germany, 2003. [Google Scholar]
- Nayono, S.E. Anaerobic Digestion of Organic Solid Waste for Energy Production. Ph.D. Dissertation, Karlsruhe Institute of Technology, Karlsruhe, Germany, 2009. [Google Scholar]
- Chernicharo, C.A.D.L. Anaerobic Reactors; IWA: London, UK, 2007; Volume 4. [Google Scholar]
- Qi, Y. Effect of centrifugal dewatering on the regrowth of fecal coliforms and salmonella in anaerobically digested biosolids. Ph.D. Dissertation, University of Delaware: Delaware, NJ, USA, 2008. [Google Scholar]
- Kikkeri, S.R.; Viraraghavan, T. Startup of anaerobic filters treating dairy wastewater: Effect of temperature and shock load. J. Environ. Sci. Health Part A 1991, 26, 287–300. [Google Scholar] [CrossRef]
- Hickey, R.F.; Owens, R.W. Methane generation from high-strength industrial wastes with the anaerobic biological fluidized bed. Biotechnol. Bioeng. Symp. 1981, 11, 399–413. [Google Scholar]
- Sutton, P.M.; Li, A. Anitron and oxitron systems: High-rate anaerobic biological treatment systems for industry. In Proceedings of 36th Industrial Waste Conference, Purdue University, West Lafayette, IN, USA, 12–14 May 1981.
- Shirazi, S.A.M. The new methods for purifying the industrial effluents by submerged biofilm reactors. J. Environ. Prot. 2011, 2, 996–1001. [Google Scholar] [CrossRef]
- Ratusznei, S.M.; Rodrigues, J.A.D.; Zaiat, M. Operating feasibility of anaerobic whey treatment in a stirred sequencing batch reactor containing immobilized biomass. Water Sci. Technol. 2003, 48, 179–186. [Google Scholar] [PubMed]
- Cocci, A.A.; Burke, B.F.; Landine, R.C.; Blickenstaff, D.L. Anaerobic-aerobic pretreatment of a dairy waste; a case history. Dairy Food Environ. Sanit. 1991, 11, 505–509. [Google Scholar]
- Lettinga, G.; van Velsen, A.F.M.; Hobma, S.W.; de Zeeuw, W.; Klapwijk, A. Use of the upflow sludge blanket (USB) reactor concept for biological wastewater treatment, especially for anaerobic treatment. Biotechnol. Bioeng. 1980, 22, 699–734. [Google Scholar] [CrossRef]
- Yan, J.Q.; Lo, K.V.; Pinder, K.L. Instability caused by high strength of cheese whey in a UASB reactor. Biotechnol. Bioeng. 1993, 41, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, M.C.; Teixeira, G.A.; Freire, D.M.G. Enzymatic pre-hydrolysis and anaerobic degradation of wastewaters with high fat contents. Biotechnol. Lett. 2001, 23, 1591–1595. [Google Scholar] [CrossRef]
- Elmitwalli, T.A.; Otterpohl, R. Anaerobic biodegradability and treatment of grey water in upflow anaerobic sludge blanket (UASB) reactor. Water Res. 2007, 41, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhnyi, S.; Fedorovich, V.; Lens, P. Dispersed plug flow model for upflow anaerobic sludge bed reactors with focus on granular sludge dynamics. J. Ind. Microbiol. Biotechnol. 2006, 33, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Zaiat, M.; Rodrigues, J.A.D.; Ratusznei, S.M.; de Camargo, E.F.M.; Borzani, W. Anaerobic sequencing batch reactors for wastewater treatment: A developing technology. Appl. Microbiol. Biotechnol. 2001, 55, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, L.H.S.; Rodrigues, J.A.D.; Ratusznei, S.M.; Zaiat, M.; Foresti, E. Effects of feeding time and organic loading in an anaerobic sequencing batch biofilm reactor (ASBBR) treating diluted whey. J. Environ. Manag. 2007, 85, 927–935. [Google Scholar] [CrossRef]
- Pohland, F.G.; Ghosh, S. Developments in Anaerobic stabilization of organic wastes—The two-phase concept. Environ. Lett. 1971, 1, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, I.E.; Anderson, G.K.; Evison, L.M. Design of pre-acidification reactors for the anaerobic treatment of industrial wastewaters. Water Sci. Technol. 1994, 29, 199–204. [Google Scholar]
- Hall, E.R. Anaerobic treatment of wastewaters in suspended growth and fixed film processes. In Water Quality Management Library; Malina, J.F., Pohland, F.G., Eds.; Technomic Publishing Company Inc.: Lancaster, PA, USA, 1992; Volume 7, pp. 41–119. [Google Scholar]
- Cohen, A.; Thiele, J.H.; Zeikus, J.G. Pilot-scale anaerobic treatment of cheese whey by the substrate shuttle process. Water Sci. Technol. 1994, 30, 433–442. [Google Scholar]
- Yu, Y.; Hansen, C.L.; Hwang, S. Biokinetics in acidogenesis of highly suspended organic wastewater by adenosine 5′ triphosphate analysis. Biotechnol. Bioeng. 2002, 78, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Frigon, J.C.; Bruneau, T.; Moletta, R.; Guiot, S.R. Coupled anaerobic-aerobic treatment of whey wastewater in a sequencing batch reactor: Proof of concept. Water Sci. Technol. 2007, 55, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D.G. A review on anaerobic-aerobic treatment of industrial and municipal wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Valbio Canada Inc. Available online: http://www.valbio.ca/en/solutions/for-industries/cheese-producers.html (accessed on 5 September 2012).
- Naskeo Environnement. Available online: http://www.naskeo.com/wastewater_references.html (accessed on 5 September 2012).
- ESi.info. Available online: http://www.esi.info/detail.cfm/SHE-UK-Ltd/Wheywastewater-treatment-for-cottage-cheese-production/_/R-201.2898 (accessed on 5 September 2012).
- Procorp Enterprises LLC. Available online: http://procorp.com/wastewater-processes/anaerobic-digestion.html (accessed on 5 September 2012).
- Navaratnasamy, M.; Edeogu, I.; Papworth, L. Economic Feasibility of Anaerobic Digesters; Practical Inforamtion for Alberta’s Agriculture Industry; Alberta Agriculture and Rural Development: Edmonton, AB, Canada, 2008. Available online: http://www1.agric.gov.ab.ca/$Department/deptdocs.nsf/all/agdex12280 (accessed on 5 September 2012).
- Al Seadi, T.; Rutz, D.; Prassl, H.; Köttner, M.; Finsterwalder, T.; Volk, S.; Janssen, R. Biogas Handbook; University of Southern Denmark: Esbjerg, Denmark, 2008. [Google Scholar]
- Greer, D. Anaerobic Digestion for smaller dairies. BioCycle 2010, 51, 24–26. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chatzipaschali, A.A.; Stamatis, A.G. Biotechnological Utilization with a Focus on Anaerobic Treatment of Cheese Whey: Current Status and Prospects. Energies 2012, 5, 3492-3525. https://doi.org/10.3390/en5093492
Chatzipaschali AA, Stamatis AG. Biotechnological Utilization with a Focus on Anaerobic Treatment of Cheese Whey: Current Status and Prospects. Energies. 2012; 5(9):3492-3525. https://doi.org/10.3390/en5093492
Chicago/Turabian StyleChatzipaschali, Aspasia A., and Anastassios G. Stamatis. 2012. "Biotechnological Utilization with a Focus on Anaerobic Treatment of Cheese Whey: Current Status and Prospects" Energies 5, no. 9: 3492-3525. https://doi.org/10.3390/en5093492




