Effects of Organic Loading Rate on the Performance of a Pressurized Anaerobic Filter in Two-Phase Anaerobic Digestion
Abstract
: The effect of organic loading rate (OLR) on a pressurized anaerobic filter was studied in a laboratory two-phase anaerobic digestion system. The anaerobic filter was operated successively at two working pressures (9 bar and 1.5 bar). The OLR(COD) for each pressure was increased from 5 to 17.5 kg·m−3·day−1. The best performance of the reactor at 9 bar was observed at OLR(COD) of 12.5 kg·m−3·day−1 and hydraulic retention time (HRT) of 1.8 day, with specific biogas productivity (SBP) of 5.3 L·L−1·day−1 and COD degradation grade of 90.6%. At higher OLRs and shorter HRTs, the process became unstable. In contrast, there was no indication of digester failure during the experiments at 1.5 bar. The SBP peaked at OLR(COD) of 17.5 kg·m−3·day−1 with 8.2 L·L−1·day−1, where COD degradation grade was 90.4%. The biogas collected from the reactor at 9 bar and 1.5 bar contained approximately 74.5% CH4 and 66.2% CH4, respectively, regardless of OLR variation. At OLR(COD) of 5–12.5 kg·m−3·day−1, the reactor at 9 bar had the same specific methane yield as at 1.5 bar, which was in the range of 0.31–0.32 LN·g−1COD. Raising the working pressure in the reactor resulted in an increase of methane content of the produced biogas. However, the low pH value (approximately 6.5) inside the reactor, induced by high CO2 partial pressure seemed to limit the reactor performance at high OLRs and short HRT.1. Introduction
Biogas, an important renewable energy source, is produced through the process of anaerobic digestion of biodegradable substances. It is primarily comprised of methane (CH4) and carbon dioxide (CO2). Biogas can be used for electricity and/or heat generation. However, with a large portion of CO2, the utilization of biogas is limited due to its low calorific value. Therefore, the removal of CO2 from raw biogas is essential. After the proper purification, drying and pressurization, the biogas can be even directly injected into existing natural gas pipelines or used as fuel for vehicles [1]. This further widens the range of biogas application and increases the overall energy utilization efficiency. Generally, the applied external biogas purification and upgrading technologies, such as water scrubbing, pressure swing adsorption, cryogenic separation and membrane separation, combined with gas compression to inject biomethane into the gas grid, all consume high amounts of energy [2]; therefore, economical feasibility is always a big concern.
Since most microbes in anaerobic digesters are inoculated from sewage slurry, excrement or wastewater treatment sludge under atmospheric pressure, they are normally piezosensitive or piezotolerant species [3]. According to Abe and Horikoshi's [4] research, methanogens are metabolically active at pressures of up to 100 bar. This provides a theoretical base for the innovative concept of pressurized anaerobic digestion (pAD), developed at the University of Hohenheim in cooperation with the DVGW-research center [5]. In pressurized anaerobic digestion, the pressure of the biogas is gradually built up during fermentation. Under the auto-generative pressure, part of the CO2 can be directly removed from the reactor with the liquid effluent due to its high gas solubility. The methane-rich biogas can be treated and supplied to the grid without an additional post-compression process. In this way, biogas production, purification and pressurization are integrated in one system, and the expenses involved in the subsequent treatment can be reduced considerably.
Although pressurized anaerobic digestion has several advantages over the conventional process, lab-scale experiments in this field are scarce. The reported few trials showed that pressurized anaerobic digestion had the potential to produce biogas of natural gas quality [6,7]. However, all the experiments were carried out under low organic loading rate (OLR) and a fixed pH value. Apparently, those experiments lacked enough data to get a better understanding of the overall digester performance. As an important operational parameter, OLR represents the biological conversion capacity of an anaerobic digestion system. With low OLR, the anaerobic digester is running inefficiently, while with high OLR, a risk of system failure due to overloading exists [8]. For example, Rincón et al. mentioned that the OLR(COD) from 1.5 to 9.2 kg·m−3·day−1 during the digestion of olive mill solid residue in an anaerobic stirred tank reactor caused an increase in the methane productivity of more than 400%, while attempts to further increase the OLR led to process instability [9]. In a research project on the anaerobic digestion of distillery wastewater in the fixed-film reactor, it was reported that the biogas productivity was rising from 4.5 to 8 L·L−1·day−1, but the specific methane yield was decreasing from 0.288 to 0.216 LN·g−1 COD, as OLR(COD) was increased from 9.2 to 20.4 kg·m−3·day−1 [10]. Therefore, it is necessary to investigate the OLR effect on pressurized anaerobic digesters, which can further reveal the technical and economical potential feasibility of the novel technology.
The experiments in this study were conducted successively at an absolute working pressure of 9 bar and 1.5 bar in a lab-scale pressurized anaerobic filter reactor in a two-phase process. Six different OLR(COD)s (5, 7.5, 10, 12.5, 15, and 17.5 kg·m−3·day−1) were applied to the anaerobic filter at both pressures. The OLR was increased by increasing flow rate. That correspondingly reduced hydraulic retention time (HRT) from 3.9 day to 1.2 day at 9 bar and from 3.9 day to 1.3 day at 1.5 bar. By this means, the influence of OLRs on the system stability and performance of the pressurized anaerobic filter was explored. Gas production, gas quality, pH-value, volatile fatty acids (VFAs), chemical oxygen demand (COD), degradation grade and buffer capacity were analyzed and compared.
2. Materials and Methods
2.1. Reactors and Substrate
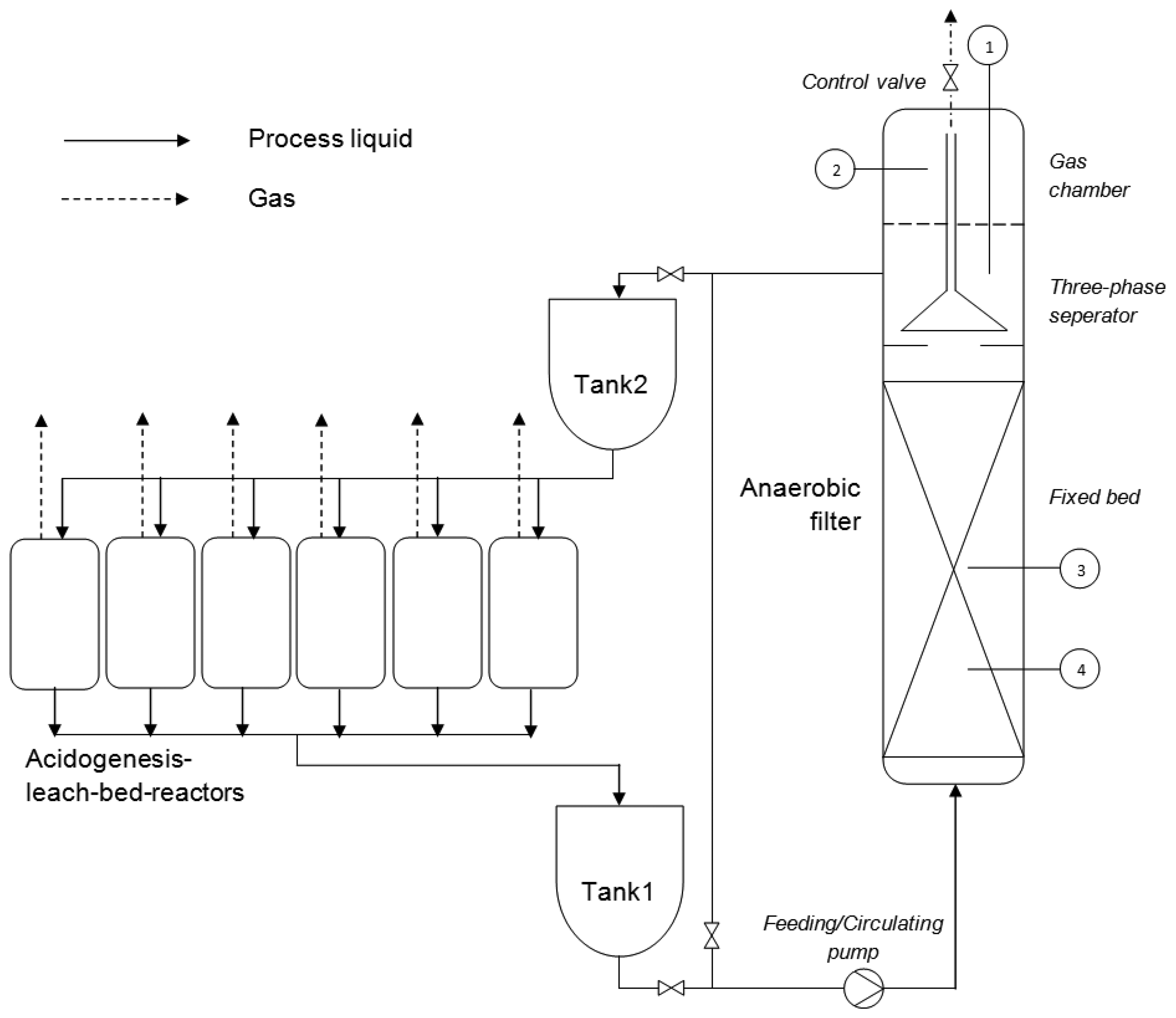
The laboratory scale experimental setup in this study was mainly composed of six parallel-operated leach-bed batch reactors as the first phase for hydrolysis and acidogenesis, and one pressurized upflow anaerobic filter reactor as the second phase for acidification and methanogenesis (Figure 1). Each leach-bed reactor had a fifty-liter volume. The reactors were operated under thermophillic conditions (55 °C); the temperature was controlled by a water bath system (Ecoline Staredition E200, Lauda, Lauda-Königshofen, Germany) equipped with a thermostat and a pump.
The anaerobic filter reactor was filled with approximately 20 L of sintered glass cylinders (Sera Siporax, Heinsberg, Germany) as a carrier material, which had a 270 m2·L−1 biologically effective surface. Above the fixed bed, a three-phase separator was installed, which prevented the suspended biomass from leaving the reactor with the effluent. At the headspace, there was a gas chamber, accounting for about 15% of the reactor volume. The temperature of the anaerobic filter reactor was maintained at 37 °C with heating foil (480 W, Thermo, Rohrbach, Germany) and a temperature sensor (GTF 103 Pt100, Greisinger, Regenstauf, Germany). In this study, the experiment was performed at the absolute pressure of 9 bar and subsequently 1.5 bar. The working pressures were generated by biogas production. Unlike in conventional anaerobic digesters, the produced biogas did not immediately leave the anaerobic filter until the desired pressure (9 bar or 1.5 bar) in the reactor was reached. Once the gas started to be released, the pressure in the reactor dropped, and the gas outlet was closed again. The process occurred instantaneously, and therefore, the pressure drop due to the valve opening was small (approximately 0.1 bar). The entire process was controlled by a pressure sensor (Ceraphant T PTC31, Endress+Hauser, Reinach, Switzerland) and a control valve (Bürkert 2712, Ingelfingen, Germany).
Both the anaerobic filter and the leach-bed reactors had gas outlets that were connected to gasbags for gas quality and gas quantity measurement. In order to provide good contact between the microbes and the substrate, the process liquid in each reactor was internally recycled five minutes every ten minutes at a flow rate of 0.6 L·min−1.
Alternating weekly two of the six leach-bed reactors were emptied and fed with 10 kg (fresh mass) maize silage from the agricultural experimental station of the University of Hohenheim (Unterer Lindenhof, Eningen, Germany). The composition and the characteristics of the maize silage are given in Table 1. Together with the fresh maize silage, a certain amount of micronutrients were also added to the reactors, providing necessary nutrients for microbial growth and avoiding biological process disturbances. The dosage and the composition of the micronutrients was based on Vintiloiu et al.'s [11] recommendation. The maize silage in the leach-bed reactors was slowly decomposed and turned into a leachate rich in organic acids and alcohols. Twice a week, approximately 12 liters of the leachate from each leach-bed reactor flowed through a 100 μm filter and were collected in a tank (Tank1 in Figure 1). There, the leachate was stored and homogenized by a circulating pump, and used as an intermediate substrate for further acetogenesis and methanogenesis in the pressurized anaerobic filter. At lower OLR(COD) (5, 7.5 and 10 kg·m−3·day−1), the anaerobic filter was fed every six hours, while at higher OLR(COD) (12.5, 15 and 17.5 kg·m−3·day−1), the feeding interval was reduced to three hours. For the volumetric balance, every time in feeding, the same amount of process liquid was eluted from the anaerobic filter and collected in the other tank (Tank2 in Figure 1) and then distributed to the leach-bed reactors evenly. The working volume of the anaerobic filter was constantly controlled by a liquid level sensor (Liquicap T FMI21, Endress + Hauser, Reinach, Switzerland).
2.2. Experimental Procedure
At the beginning of the experiment, the anaerobic filter was inoculated with 26 liters of effluent from another fixed-bed anaerobic reactor that had been running for more than two years. The general composition of the inoculum can be referred to Zielonka et al. [12]. The start-up period lasted approximately four months, allowing biofilm development in the anaerobic filter. After that, the experiment on the OLR effects on the pressurized anaerobic filter in a two-phase anaerobic digestion process began.
The experiment on OLR effect on the performance of the pressurized anaerobic filter was divided into 12 runs. From run 1 to run 6, the absolute working pressure of the anaerobic filter was maintained at 9 bar. The OLR(COD) applied to the anaerobic filter reactor was stepwise increased from 5 kg·m−3·day−1 to 17.5 kg·m−3·day−1 by increasing the influent flow rate to the reactor. The influent COD concentration varied between 19.1 and 23.6 kg·m−3. Due to the increase of the OLR at a relatively constant COD concentration in the leachate, the HRT was correspondingly reduced from 3.9 to 1.2 day. After run 6, the anaerobic filter entered recovery phase, in which it was operated at 1.5 bar with OLR(COD) of 5 kg·m−3·day−1. In this way, the accumulated acids from previous experiment runs were removed and the anaerobic filter returned to normal condition and was well prepared for the subsequent experiments. At runs 7–12, the working pressure of the anaerobic filter reactor was kept at 1.5 bar. The same OLR(COD) (5, 7.5, 10, 12.5, 15 and 17.5 kg·m−3·day−1) were tested. Each experimental run lasted for at least 18 days, in order to ensure that a minimum 12-day steady state was reached at a particular experimental condition. The whole experimental design is summarized in Table 2. Throughout the experiments, no additional caustic chemicals were added for pH adjustment or to maintain process stability.
2.3. Analytical Methods and Data Acquisition
In this study, a pH-sensor (Endress+Hauser CPS11D), a pressure sensor (Endress+Hauser Ceraphant T PTC31) and a temperature sensor (Greisinger GTF 103 Pt100) continuously kept track of the system operation in the anaerobic filter, and the data were recorded using Labview 11.0.1 (National Instruments, Austin, TX, USA). The quantity and quality of the collected gas was analyzed every six hours. The gas volume was measured by a gas meter (Ritter TG20/5, Bochum, Germany), and corrected to dry gas at a standard temperature and pressure (STP). The gas composition was determined with an infrared gas analyzer (Sick Maihak S710, Waldkirch, Germany).
Every other day, the effluent from the anaerobic filter was sampled and underwent chemical analyses on COD, VFAs and the content of sugar, alcohol and total inorganic carbon (TIC). The collected leachate from the leach-bed-reactors was analyzed for the same parameters on a weekly basis. The content of organic dry matter (ODM), VFAs, sugar and alcohol in the maize silage was measured once a week. ODM was evaluated according to the VDI-Guideline 3475. COD was assessed by a specific COD analysis system (Hach Lange Company, Düsseldorf, Germany), equipped with pre-dosed reagents (LCK014, 1000–10,000 mg·L−1), a high temperature thermostat (HT 200 S) and a sensor array photometer (LASA 20). Volatile fatty acids were detected with capillary column gas chromatography (Varian CP-3800, Palo Alto, CA, USA). By means of high-performance liquid chromatography (Bischoff Company, Leonberg, Germany), dl-lactic acid, formic acid, sucrose, glucose, fructose, ethanol and propylene glycol content were assessed. Prior to gas chromatography and high-performance liquid chromatography analyses, the soluble analytes from maize silage were extracted. TIC was determined with a titrator (785 DMP Titrino, Metrohm, Herisau, Switzerland). All the obtained data were statistically analyzed with the ANOVA program in R-Studio.
3. Results
3.1. Influent to the Pressurized Anaerobic Filter
In the leach-bed reactors, maize silage was broken into organic acids and alcohols through microbial activity. The amount of the acids formed and the proportion of the individual acids were dependent on substrate properties. The entire experiment in this study lasted approximately one year. As summarized in Table 1, the composition of the maize silage adopted in this experiment varied in acids and sugar content to some extent. Therefore, the leachate from the leach-bed reactors that was used as substrate for the pressurized anaerobic filter underwent corresponding slight changes in acid composition as well as COD concentration (Table 3). Among them, the variation of acetic acid-, n-butyric acid- and dl-lactic acid concentration was significant. The influent COD concentration during the experiment at working pressure of 9 bar and 1.5 bar ranged from 19.1 to 23.6 kg·m−3, and 19.5 to 23.4 kg·m−3, respectively.
3.2. Effect of OLR on Gas Production
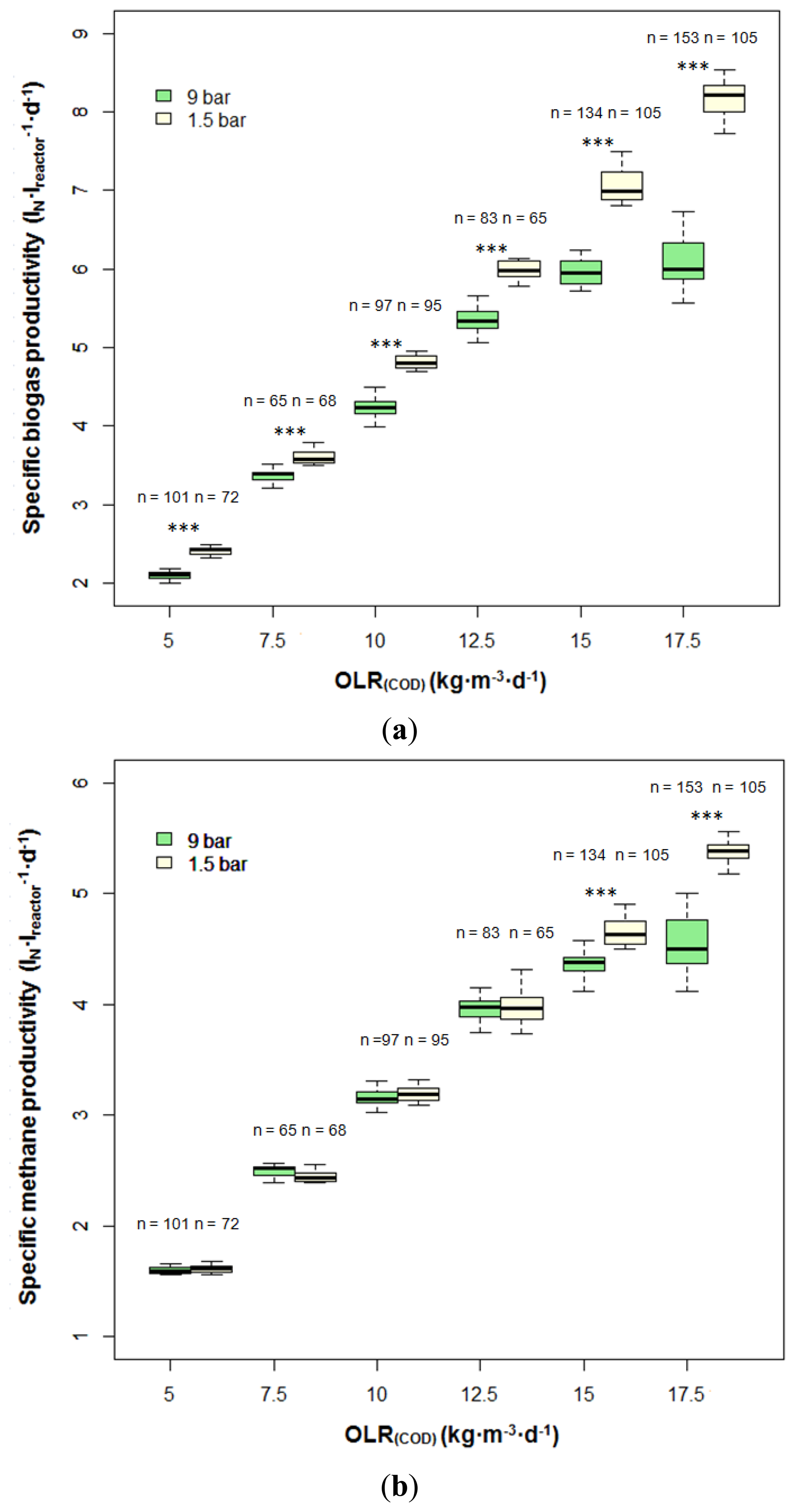
Figure 2 illustrates specific biogas productivity (SBP) and specific methane productivity (SMP) under different OLRs and different pressures. In this study, SBP and SMP stand for the daily collected biogas and methane amount per active working volume of the anaerobic filter. From OLR(COD) of 5 to 12.5 kg·m−3·day−1, the SBP at 1.5 bar and 9 bar showed a clear upward tendency, gradually increasing from 2.4 ± 0.05 to 6.0 ± 0.2 L·L−1·day−1 and from 2.1 ± 0.05 to 5.3 ± 0.2 L·L−1·day−1, respectively. However, the increase in SBP at 9 bar retarded significantly with OLR further rising. At OLR(COD) of 15 and 17.5 kg·m−3·day−1, the SBPs at 9 bar were only 6.0 ± 0.2 L·L−1·day−1 and 6.1 ± 0.3 L·L−1·day−1 respectively. In contrast, the SBP at 1.5 bar continued strong growth, rising further to 8.2 ± 0.2 L·L−1·day−1. Compared with 9 bar, the pressurized anaerobic filter running at 1.5 bar featured a higher SBP at each OLR. Nevertheless, the SMP at both pressures did not differentiate until the OLR(COD) of 15 kg·m−3·day−1 was reached. It was observed that the reactor at 9 bar had less SMP at OLR(COD) higher than 15 kg·m−3·day−1.
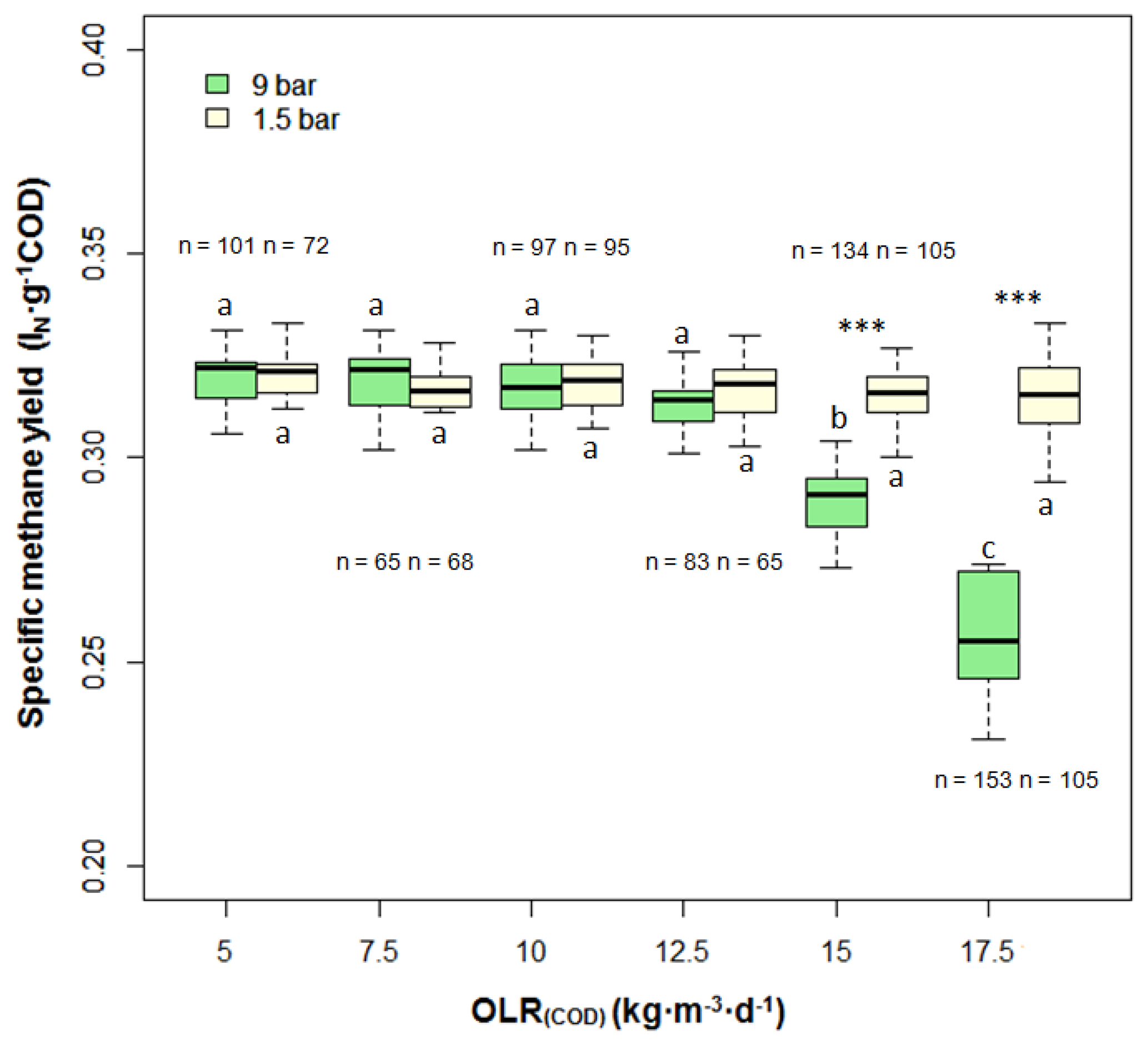
The standard methane yield in terms of one gram fed COD is expressed as specific methane yield (SMY), which is demonstrated in Figure 3. At OLR(COD) of 5–12.5 kg·m−3·day−1, the SMY gained from 9 bar and 1.5 bar remained constant at 0.32 ± 0.01 LN·g−1COD. As OLR was further increased, the SMY at 9 bar slumped and ended at 0.25 ± 0.01 LN·g−1COD at OLR(COD) of 17.5 kg·m−3·day−1. In contrast, the SMY at 1.5 bar was more stable at 0.31 ± 0.01 LN·g−1COD. Statistically, the SMY between the pressures did not show any significant difference until OLR was raised to 15 kgCOD·m−3·day−1. After that, the reactor running at pressure of 9 bar was characterized by much lower SMY.
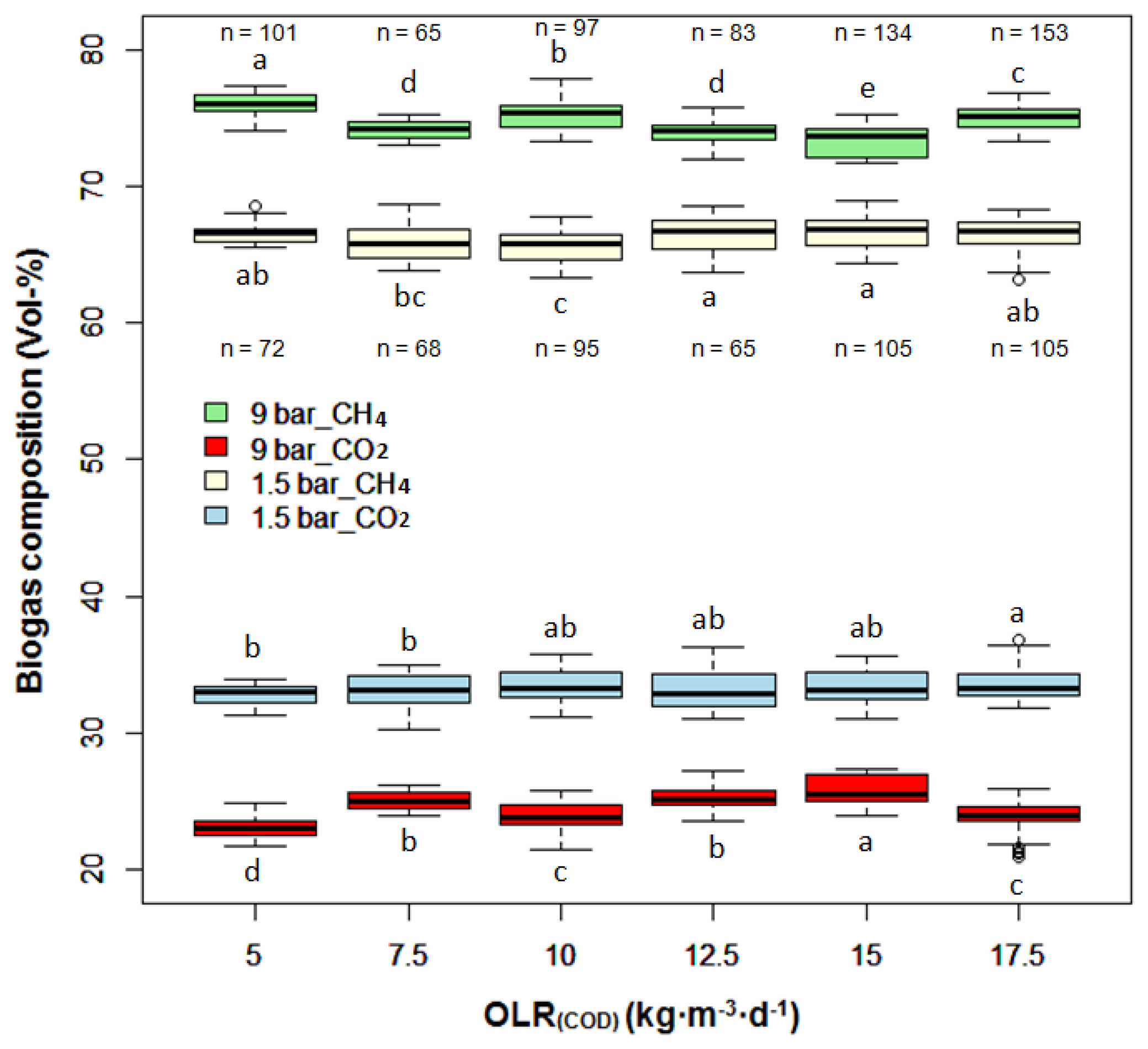
As shown in Figure 4, the biogas composition was quite stable at a given working pressure despite OLR increasing. In comparison with OLR, pressure had a larger impact on biogas composition. During the entire experiment, the biogas produced at 9 bar comprised of 74.5% ± 1.3% CH4 and 24.6% ± 1.3% CO2, while CH4, and CO2 accounted respectively for 66.2% ± 1.1% and 33.3% ± 1.1% of the biogas produced at 1.5 bar.
3.3. Effect of OLR on pH Value and Acid Accumulation
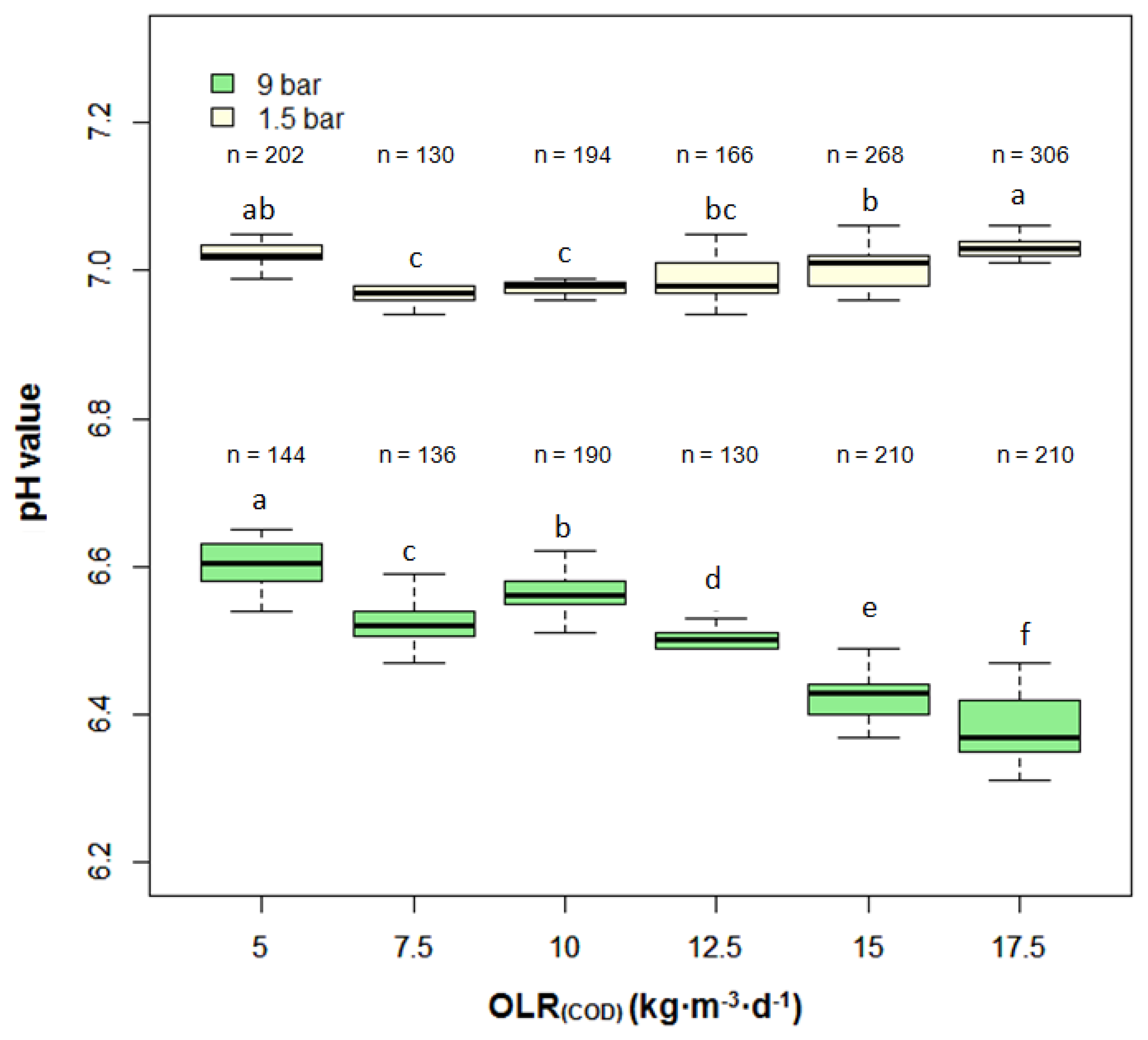
Figure 5 shows the average pH value for every three hours in the anaerobic filter. It was observed that the pH value at 9 bar was much lower than that at 1.5 bar. Despite a small fluctuation, the pH value of the anaerobic filter at 9 bar gradually decreased from 6.6 to 6.4, with OLR(COD) increasing from 5 kg·m−3·day−1 to 17.5 kg·m−3·day−1. In contrast, the pH-value at 1.5 bar was relatively stable, remaining at approximately 7.0 during the entire experiment.
At the pressure of 9 bar, there was no distinct VFA accumulation until OLR(COD) of 15 kg·m−3·day−1. Afterwards, the concentration of VFAs in the effluent, especially acetic acid, propionic acid and n-butyric acid rose sharply (Table 4). Meanwhile, the COD degradation grade fell to 76% and the VFA/TIC ratio rose to 0.52. That contrasted with the effluent of the reactor running at 1.5 bar, where small amounts of VFAs were detected and overall COD degradation grade was more than 90% at each OLR level. The VFA/TIC ratio was always below 0.2 throughout the experiments.
4. Discussion
4.1. Critical OLR and HRT
In this study, the OLR was gradually increased by increasing the influent flow rate, and thus HRT decreased correspondingly. As shown in the results, daily biogas and methane production at 9 bar peaked at OLR(COD) of 17.5 kg·m−3·day−1 with HRT of 1.2 day. However, at OLR(COD) of higher than 15 kg·m−3·day−1, SMY and COD degradation grade declined noticeably and VFAs built up in the anaerobic filter, indicating that the substrate could not be decomposed in time, and the reactor performance was limited. Under this condition, 12.5 kgCOD·m−3·day−1 and 1.8 d could be taken as the critical OLR and HRT for the anaerobic filter running at 9 bar, where the reactor was characterized by high SMP and SMY, high reduction in COD and high alkaline buffer capacity. In the experiment of 1.5 bar, maximal daily biogas and methane production were observed at OLR(COD) of 17.5 kg·m−3·day−1 with HRT of 1.3 day. There, SMY, COD degradation grade and alkaline buffer capacity stayed at as high a level as in previous runs and no apparent acid accumulation was detected. It can be inferred that the anaerobic filter reactor at 1.5 bar had not reached its critical OLR and HRT, and it had potential to perform at even higher OLR with shorter HRT.
However, even in the case of the anaerobic filter operated at 9 bar, the critical OLR(COD) and HRT was outstanding, in comparison to most fixed bed reactors in practice, which are reported to perform generally at 6–10 kg·m−3·day−1 with HRT between 0.75 and 7 day [13]. That benefited primarily from the two-phase anaerobic digestion system. The substrate fed into the pressurized anaerobic filter was already pre-digested in the first phase. Therefore, the substrate became more easily degradable and could be completely converted into biogas in a shorter time [14]. In addition, due to the large and porous specific surface, the use of sintered glass as a carrier material in bioreactors could be another reason for achieving a higher COD degradation rate, compared with conventional carrier materials [15]. Both factors helped the reactor to proceed at a relatively high OLR with short HRT.
4.2. Reactor Performance and Stability
At lower OLR(COD) (up to 12.5 kg·m−3·day−1) and longer HRTs (more than 1.8 day), the anaerobic filter was running smoothly and efficiently at both pressures. SMYs of 9 bar and 1.5 bar were almost the same, approximately 0.32 LN·g−1COD. The pressure seemed to have little impact on SMY, which is in agreement with the observation from a previous study [5]. High CO2 partial pressure brought the pH value of the reactor at 9 bar down to 6.5. Generally, such a low pH value favored unionized VFAs to penetrate the microbial cell wall and inhibited the growth rate of the methanogens [16]. Apparently, in this study, the inhibition was not significant enough to hinder the methanogens converting VFAs to gas products. At OLR(COD) up to 12.5 kg·m−3·day−1, COD degradation grade remained more than 90%, and almost no VFA built up in the reactor. This might be attributed to the thick biofilm formed in the reactor with the help of the sintered glass as a carrier material. Several papers reported that there were pH gradients in the biofilm system and the pH value inside methanogenic aggregates was higher than that in the process liquid [17,18]. Thus, the adverse impact might have been smoothed.
4.3. Biogas Composition
The biogas collected at 9 bar contained significantly more methane than that collected at 1.5 bar in each studied OLR. It can be attributed to the remarkable difference in solubility between CH4 and CO2 under pressure. At a higher pressure, more CO2 is dissolved in the liquid and then CH4 becomes dominant in the gas phase. Methane enrichment in anaerobic digestion under pressure was also documented elsewhere [5–7,19]. However, in terms of the absolute value, the methane content was not as high as reported in other studies. It is mainly due to the low pH-value of the substrate in the reactor. In this study, the anaerobic filter was running at pH lower than 6.5 at 9 bar. The decrease of the pH value tends to result in a reduction of CO2-solubility [20]. Calculations based on Henry's Law show at the same pressure, the solubility of CO2 at a pH value of 7.5 is about seven times as much as that at a pH value of 6.5. The drop in pH value counteracts the pressure effect on CO2-solubility, and thus the methane enrichment in this study was not that apparent.
Despite a slight fluctuation, the biogas composition at both pressures, as a whole, remained on the same level throughout the entire experiment. This could be explained from the perspective of the interaction of OLR, influent COD concentration and pH value. Based on Henry's Law, the amount of dissolved CO2 and CH4 is directly proportional to their respective partial pressure in the anaerobic filter. That means, the solution is saturated with CO2 and CH4 in a dynamic equilibrium with the gas phase. Only the leachate newly introduced from the pressure-free tank into the pressurized anaerobic filter, as feeding, has a capacity to absorb CO2 and CH4. With the constant operational parameters (temperature, pressure and pH value), the amount of dissolved CO2 is dependent on the volume of the leachate fed to the anaerobic filter, in other words, the influent COD concentration.
After reaching the critical OLR, both COD degradation grade and SMY started to diminish noticeably at 9 bar. On the one hand, a unit of feeding volume produced less gas. Then, the part of CO2 that stayed in the gas phase in the previous gas-liquid equilibrium could enter the liquid phase, while the movement of CH4 could be neglected due to its extremely low solubility. This tended to decrease the CO2 percentage in the gas phase. On the other hand, the undigested acids accumulated in the reactor, bringing down the pH value, which in turn decreased CO2 solubility [20]. The two factors counteracted each other, and thus the biogas composition still stayed in the same range despite overloading.
In comparison, the biogas quality and pH value at 1.5 bar were much more stable. There are two possible reasons for this. Firstly, the reactor did not reach its critical OLR. Few acids accumulated in the anaerobic filter, which ensured a stable pH value and thereby the CO2 stored in the liquid was fairly constant. Secondly, the low total working pressure resulted in a low CO2 partial pressure. Thus, less CO2 is dissolved as carbonic acid at 1.5 bar. Considering the absolute amount, it was less of a burden on the alkaline buffer capacity. In other words, the reactor at 1.5 bar could better resist the changes of pH value. Therefore, the Henry coefficient of CO2 and CO2 solubility did not fluctuate much at 1.5 bar. As a result, the biogas quality at 1.5 bar did not respond so strongly to the influent COD concentration as it did at 9 bar.
5. Conclusions and Outlook
The study compared the performance of an anaerobic filter operated at two different pressures (9 bar and 1.5 bar) with OLR(COD) stepwise increasing up to 17.5 kg·m−3·day−1. The pressure itself did not exert a direct effect on the process stability of anaerobic digestion. However, the low pH value inside the reactor induced by high CO2 partial pressure, seemed to limit the reactor's performance at high OLRs with short HRTs. The reactor at 9 bar proceeded well until the critical OLR(COD) (12.5 kg·m−3·day−1) and HRT (1.8 day) was reached, regardless of pH value as low as 6.5. The SMY lay between 0.31 and 0.32 LN·g−1COD and COD degradation grade was over 90%, and both values were in accordance with those of the reactor at 1.5 bar. At OLR(COD) higher than 12.5 kg·m−3·day−1, process inhibition was observed at the reactor at 9 bar, while the SMY and COD degradation grade of the reactor at 1.5 bar remained at the same level as previously, even at OLR(COD) of 17.5 kg·m−3·day−1, with HRT of 1.3 day. In terms of biogas quality, the biogas collected at 9 bar featured a higher methane content at each studied OLR. In addition, the biogas composition at 9 bar seemed more sensitive to influent COD concentration. Therefore, it is essential to enhance the performance stability of the reactor operated under high pressure. It could be done with relevant research on how to economically and effectively avoid pH value drop inside the pressurized reactor. Furthermore, a research approach to explore OLR effect on pressurized anaerobic digestion with fixed HRT and same influent chemical concentration is recommended. With a proper HRT, the pressurized anaerobic filter might perform well at an even higher OLR(COD) (more than 12.5 kg·m−3·day−1). Thus, an economical and efficient performance of pressurized anaerobic digestion in a large-scale plant might be also expected.
Acknowledgments
The authors gratefully acknowledge the financial support of the work by the German Ministry of Education and Research within the joint research project B2G.
Conflicts of Interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work; there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
References
- Graf, F.; Bajohr, S. Biogas—Erzeugung, Aufbereitung, Einspeisung; Oldenbourg Industrieverlag: München, Germany, 2011. [Google Scholar]
- Köppel, W.; Götz, M.; Graf, F. Biogas upgrading for injection into the gas grid. gwf-Gas|Erdgas 2009, 150, 26–35. [Google Scholar]
- Aertsen, A.; Meersman, F.; Hendrickx, M.E.G.; Vogel, R.F.; Michiels, C.W. Biotechnology under high pressure: Applications and implications. Trends Biotechnol. 2009, 27, 434–441. [Google Scholar]
- Abe, F.; Horikoshi, K. The biotechnological potential of piezophiles. Trends Biotechnol. 2001, 19, 102–108. [Google Scholar]
- Chen, Y.; Röβler, B.; Zielonka, S.; Lemmer, A.; Wonneberger, A.-M.; Jungbluth, T. The pressure effects on two-phase anaerobic digestion. Appl. Energy 2014, 116, 409–415. [Google Scholar]
- Hayes, T.D.; Isaacson, H.R.; Pfeffer, J.T.; Liu, Y.M. In situ methane enrichment in anaerobic digestion. Biotechnol. Bioeng. 1990, 35, 73–86. [Google Scholar]
- Lindeboom, R.E.F.; Fermoso, F.G.; Weijma, J.; Zagt, K.; van Lier, J.B. Autogenerative high pressure digestion: Anaerobic digestion and biogas upgrading in a single step reactor system. Water Sci. Technol. 2011, 64, 647–653. [Google Scholar]
- Braun, R. Biogas—Methangärung organischer Abfallstoffe: Grundlagen und Anwendungsbeispiele; Springer-Verlag: Vienna, Austria, 1982; in German. [Google Scholar]
- Rincón, B.; Borja, R.; González, J.M.; Portillo, M.C.; Sáiz-Jiménez, C. Influence of organic loading rate and hydraulic retention time on the performance, stability and microbial communities of one-stage anaerobic digestion of two-phase olive mill solid residue. Biochem. Eng. J. 2008, 40, 253–261. [Google Scholar]
- Bories, A.; Raynal, J.; Bazile, F. Anaerobic digestion of high-strength distillery wastewater (cane molasses stillage) in a fixed-film reactor. Biol. Wastes 1988, 23, 251–267. [Google Scholar]
- Vintiloiu, A.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Mineral substances and macronutrients in the anaerobic conversion of biomass: An impact evaluation. Eng. Life Sci. 2012, 12, 287–294. [Google Scholar]
- Zielonka, S.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Energy balance of a two-phase anaerobic digestion process for energy crops. Eng. Life Sci. 2010, 10, 515–519. [Google Scholar]
- Young, J.C. Factors affecting the design and performance of upflow anaerobic filters. Water Sci. Technol. 1991, 24, 133–155. [Google Scholar]
- Demirel, B.; Yenigün, O. Two-phase anaerobic digestion processes: A review. J. Chem. Technol. Biotechnol. 2002, 77, 743–755. [Google Scholar]
- Breitenbucher, K.; Siegl, M.; Knupfer, A.; Radke, M. Open-pore sintered glass as a high-efficiency support medium in bioreactors: New results and long-term experiences achieved in high-rate anaerobic digestion. Water Sci. Technol. 1990, 22, 25–32. [Google Scholar]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar]
- De Beer, D.; Huisman, J.W.; van den Heuvel, J.C.; Ottengraf, S.P.P. The effect of pH profiles in methanogenic aggregates on the kinetics of acetate conversion. Water Res. 1992, 26, 1329–1336. [Google Scholar]
- Vroom, J.M.; de Grauw, K.J.; Gerritsen, H.C.; Bradshaw, D.J.; Marsh, P.D.; Watson, G.K.; Birmingham, J.J.; Allison, C. Depth penetration and detection of pH gradients in biofilms by two- photon excitation microscopy. Appl. Environ. Microbiol. 1999, 65, 3502–3511. [Google Scholar]
- Friedmann, H.; Märkl, H. Der Einfluβ von erhöhtem hydrostatischen Druck auf die Biogasproduktion. Wasser Abwasser 1993, 134, 689–698, in German. [Google Scholar]
- Wonneberger, A.-M.; Graf, F.; Bajohr, S.; Lemmer, A.; Reimert, R. Process Development of Two-Phase Pressure Fermentation—Influence of Gas Solubilities, Proceedings of the International Congress on Progress in Biogas, Stuttgart, Germany, 30 March–1 April 2011; Part 1. pp. 176–182.
| Parameter | Content (g·kg−1) |
|---|---|
| ODM | 265.6 ± 19.5 |
| Acetic acid | 7.7 ± 1.2 |
| Propionic acid | 0.3 ± 0.2 |
| iso-Butyric acid | 0 |
| n-Butyric acid | 0.04 ± 0.02 |
| iso-Valeric acid | 0 |
| n-Valeric acid | 0 |
| Capronic acid | 0 |
| dl-Lactic acid | 18.8 ± 2.1 |
| Fructose | 2.5 ± 0.4 |
| Ethanol | 1.2 ± 0.3 |
| Propylene glycol | 2.2 ± 0.7 |
| Run | Working pressure (bar) | OLR(COD) (kg·m−3·day−1) | Duration (day) | HRT (day) |
|---|---|---|---|---|
| 1 | 9 | 5.0 ± 0.1 | 28 | 3.9 ± 0.2 |
| 2 | 9 | 7.5 ± 0.1 | 18 | 3.2 ± 0.2 |
| 3 | 9 | 10.0 ± 0.1 | 27 | 2.3 ± 0.1 |
| 4 | 9 | 12.5 ± 0.1 | 23 | 1.8 ± 0.03 |
| 5 | 9 | 15.0 ± 0.2 | 42 | 1.4 ± 0.1 |
| 6 | 9 | 17.5 ± 0.3 | 51 | 1.2 ± 0.1 |
| Recovery | 1.5 | 5–10 | 23 | - |
| 7 | 1.5 | 5.0 ± 0.1 | 19 | 3.9 ± 0.2 |
| 8 | 1.5 | 7.5 ± 0.1 | 19 | 2.6 ± 0.1 |
| 9 | 1.5 | 10.0 ± 0.1 | 25 | 2.3 ± 0.1 |
| 10 | 1.5 | 12.5 ± 0.2 | 18 | 1.6 ± 0.04 |
| 11 | 1.5 | 15.0 ± 0.3 | 29 | 1.5 ± 0.1 |
| 12 | 1.5 | 17.5 ± 0.2 | 31 | 1.3 ± 0.1 |
| Run | COD (kg·m−3) | Acetic acid (g·kg−1) | Propionic acid (g·kg−1) | n-Butyric acid (g·kg−1) | Capronic acid (g·kg−1) | dl-lactic acid (g·kg−1) | Ethanol (g·kg−1) |
|---|---|---|---|---|---|---|---|
| 1 | 19.1 ± 0.2 | 2.4 ± 0.1 | 0.6 ± 0.05 | 3.4 ± 0.3 | 0.6 ± 0.1 | 0.4 ± 0.3 | 0.5 ± 0.07 |
| 2 | 23.6 ± 0.2 | 3.6 ± 0.1 | 0.6 ± 0.1 | 4.4 ± 0.2 | 0.6 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 |
| 3 | 22.6 ± 0.2 | 3.5 ± 0.1 | 0.4 ± 0.02 | 4.9 ± 0.2 | 0.4 ± 0.05 | 0.5 ± 0.1 | 0.9 ± 0.1 |
| 4 | 22.2 ± 0.1 | 4.4 ± 0.1 | 0.6 ± 0.04 | 4.4 ± 0.4 | 0.6 ± 0.04 | 0.3 ± 0.1 | 0.6 ± 0.02 |
| 5 | 21.0 ± 0.2 | 3.6 ± 0.2 | 0.6 ± 0.05 | 5.0 ± 0.3 | 0.5 ± 0.03 | 0.8 ± 0.2 | 0.7 ± 0.1 |
| 6 | 19.3 ± 0.3 | 2.4 ± 0.1 | 0.4 ± 0.01 | 4.3 ± 0.2 | 0.3 ± 0.02 | 0.6 ± 0.1 | 0.5 ± 0.04 |
| 7 | 20.1 ± 0.2 | 2.3 ± 0.3 | 0.5 ± 0.1 | 4.0 ± 0.5 | 0.5 ± 0.3 | 1.8 ± 0.3 | 0.6 ± 0.1 |
| 8 | 19.5 ± 0.4 | 2.5 ± 0.1 | 0.2 ± 0.02 | 2.8 ± 0.1 | 0.1 ± 0.02 | 3.2 ± 0.3 | 0.8 ± 0.06 |
| 9 | 22.9 ± 0.6 | 2.7 ± 0.1 | 0.3 ± 0.06 | 3.7 ± 0.2 | 0.2 ± 0.03 | 2.9 ± 0.4 | 1.0 ± 0.1 |
| 10 | 20.8 ± 0.1 | 1.9 ± 0.1 | 0.4 ± 0.02 | 3.2 ± 0.3 | 0.1 ± 0.01 | 4.3 ± 0.2 | 0.3 ± 0.05 |
| 11 | 23.2 ± 0.1 | 2.2 ± 0.1 | 0.4 ± 0.04 | 5.3 ± 0.1 | 0.3 ± 0.02 | 2.0 ± 0.3 | 0.8 ± 0.06 |
| 12 | 23.4 ± 0.2 | 2.5 ± 0.1 | 0.4 ± 0.06 | 4.7 ± 0.3 | 0.2 ± 0.04 | 2.5 ± 0.3 | 1.0 ± 0.04 |
| Parameter | Pressure (bar) | OLR(COD) (kg·m−3·day−1) | |||||
|---|---|---|---|---|---|---|---|
| 5 | 7.5 | 10 | 12.5 | 15 | 17.5 | ||
| Acetic acid (g·kg−1) | 1.5 | 0 | 0 | 0 | 0.12 ± 0.01 | 0.14 ± 0.03 | 0.11 ± 0.03 |
| 9 | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.05 ± 0.04 | 0.11 ± 0.02 | 0.56 ± 0.03 | 0.69 ± 0.03 | |
| Propionic acid (g·kg−1) | 1.5 | 0 | 0 | 0 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.01 |
| 9 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.24 ± 0.02 | 0.47 ± 0.03 | |
| n-Butryric acid (g·kg−1) | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0.08 ± 0.01 | 0.31 ± 0.04 | |
| n-Valeric acid (g·kg−1) | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0.03 ± 0.01 | 0.10 ± 0.01 | |
| Carpronic acid (g·kg−1) | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0.02 ± 0.01 | 0.06 ± 0.01 | |
| VFA/TIC | 1.5 | 0.14 ± 0.02 | 0.13 ± 0.01 | 0.13 ± 0.02 | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.14 ± 0.01 |
| 9 | 0.22 ± 0.01 | 0.23 ± 0.01 | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.33 ± 0.01 | 0.52 ± 0.05 | |
| COD degradation grade (%) | 1.5 | 90.9 ± 0.2 | 90.4 ± 0.2 | 90.7 ± 0.2 | 90.9 ± 1.0 | 90.2 ± 1.1 | 90.4 ± 2.4 |
| 9 | 91.9 ± 0.6 | 91.0 ± 1.3 | 90.5 ± 1.7 | 90.6 ± 0.8 | 83.5 ± 1.8 | 75.9 ± 3.4 | |
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.; Rößler, B.; Zielonka, S.; Wonneberger, A.-M.; Lemmer, A. Effects of Organic Loading Rate on the Performance of a Pressurized Anaerobic Filter in Two-Phase Anaerobic Digestion. Energies 2014, 7, 736-750. https://doi.org/10.3390/en7020736
Chen Y, Rößler B, Zielonka S, Wonneberger A-M, Lemmer A. Effects of Organic Loading Rate on the Performance of a Pressurized Anaerobic Filter in Two-Phase Anaerobic Digestion. Energies. 2014; 7(2):736-750. https://doi.org/10.3390/en7020736
Chicago/Turabian StyleChen, Yuling, Benjamin Rößler, Simon Zielonka, Anna-Maria Wonneberger, and Andreas Lemmer. 2014. "Effects of Organic Loading Rate on the Performance of a Pressurized Anaerobic Filter in Two-Phase Anaerobic Digestion" Energies 7, no. 2: 736-750. https://doi.org/10.3390/en7020736




