1. Introduction
Oil is currently indisputably the main source of energy, with a demand of about 12 million tons per day, and a projected increase to 16 million tons per day by 2030 [
1]. It is estimated that within 50 years, some oil reserves in some countries will be depleted, and many of the remaining sites (located at great depth and/or in oceans) will be inaccessible, which will inevitably cause rising oil prices as well as increasing anthropogenic emissions of greenhouse gases (GHG) causing the global warming effect. Thus, the research efforts on different renewable fuels have grown in the last years [
2]. However, is widely accepted that this target will not be easy to implement, and it will require a major coordinated effort by many groups—political forces, scientific organizations, economic and social actors, and of course the citizens—to access and develop new economic and social scenarios where the energy and commodities will not be, as currently happens, completely dependent on fossil oil and consequently GHG producers [
3].
The term biodiesel is currently reserved for those biofuels made of a mixture of monoalkyl esters of long chain fatty acids, derived from renewable lipids such as vegetable oils or animal fats, that can be used in the current combustion engines without any modification, as they have physicochemical properties similar to conventional fuels. Besides, they offer additional advantages, like being renewable, biodegradable and nontoxic compounds, that are essentially free of sulfur and aromatics so that it represents an added value due to their environmental safety [
4,
5].
The benefits of biofuels over traditional fuels include also greater energy security, reduced environmental impact, foreign exchange savings, and positive socioeconomic issues related to the rural sector. The use of waste oils and fats in biodiesel production also reduces many environmental complications and could be friendly energy drivers for all countries [
6]. Thus, in recent years biodiesel production has become a very important potential alternative to partially fulfill the expected future energy demands in the transport sector [
7,
8]. In this connection, the most usual technology to process vegetable oils or animal fats is based on the conversion of the triglycerides (TG) to fatty acids methyl esters (FAME) by a transesterification reaction with methanol. However, there are also several alternative routes to use vegetable oils or animal fats like a biofuel, including direct use of vegetable oil, microemulsions and emulsifications [
9].
Regardless of the procedure used to obtain the so-called conventional biodiesel (homogeneous or heterogeneous, acid or basic catalysts, lipases, supercritical conditions,
etc.), in all cases the glycerol is formed as a by-product, representing a notable performance loss in the process, given that the market is already virtually flooded with the produced glycerine, obtained precisely as a by-product in the current manufacture of biodiesel [
10,
11]. Thus, this current method supposes a decreased yield of the process, always higher than nominal 12 wt%, due to the glycerol obtained as a reaction product, which in very small amount is also incorporated to the biodiesel (FAME, Fatty Acid Methyl Ester) phase. In this respect, the cleaning of glycerol is necessary, because of its ability to react with oxygen inside the engine at high temperature, where it could produce dehydration forming acrolein, that can be polymerised causing several problems, including coking of fuel. This coking can also generate deposits of carbonaceous compounds on the injector nozzles, pistons and valves in standard engines, thus reducing their efficiency and even their service life [
1,
8].
To avoid the problems associated with the generation of glycerol in the conventional process, a series of alternative methods are considered to get the highest atom efficiency by avoiding the production of glycerin. Thus, a target of great interest currently is the production, in only one reaction, of new biofuels that integrate the glycerol as a derivative product, miscible with the fatty acid methyl or ethyl esters (FAME or FAEE) obtained in the same transesterification process. Basically, this is possible by using some alternative esters, instead of the alcohol usually employed in the conventional process. Thus, if some glycerol derivative compound is obtained at the same time that FAME (or FAEE) in an interesterification process, a new biofuel is obtained in only one reaction avoiding the presence of glycerol, that is, instead of glycerol its corresponding ester is obtained, together to the FAME mixture. These new biofuels not only prevent the generation of waste glycerol, but also increase the yields of the process, always higher than nominal 12 wt%, by incorporating some glycerol derivatives into the reaction products. In this way, the highest atom efficiency, practically 100 wt%, is obtained. Novel methodologies to prepare esters from lipids using different acyl acceptors which directly afford alternative co-products are currently under development [
12].
The interesterification processes can be performed with the same catalysts applied in the standard transesterification processes (homogeneous or heterogeneous, acid or basic catalysts, lipases, supercritical conditions,
etc.), although at present most of these processes, when applied to the production of biofuels, are carried out using different lipases [
12], where instead of using methanol, the lipase-catalyzed synthesis of fatty acid alkyl esters can also be performed using alternative alcohol donors such as methyl or ethyl (alkyl) acetate and dimethyl or diethyl carbonate. These mixtures including glycerol derivative molecules have relevant physical properties to be employed as novel biofuels. The atom efficiency is also improved as all the atoms involved in the reaction become part of the final mixture. Even the reactants used remain together to the obtained reaction products to be directly used as biofuels [
12,
13,
14,
15,
16,
17,
18]. Thus, the transesterification reaction of triglycerides with dimethyl carbonate (DMC) [
13,
14] generates a mixture of FAME and glycerol carbonate (GC), which is soluble in FAME and has physical properties suitable for use as fuel, so it has been introduced as a new biofuel called DMC-BIOD [
15]. Similarly, the transesterification of methyl acetate with some vegetable oils generates Gliperol
® (Warsaw, Poland), a new patented biofuel [
16,
17,
18] constituted by three molecules of FAME and one molecule of triacetin or glycerol triacetate.
In recent studies an alternative methodology is implemented that avoids the generation of glycerol by developing a partial enzymatic ethanolysis of the triglyceride molecules, that generates two molecules of fatty acid ethyl esters (FAEE) and a molecule of monoacylglycerol (MG). This enzymatic method using pig pancreatic lipase (PPL) to cause the enantioselective ethanolysis of sunflower oil has shown promising results [
19,
20,
21,
22,
23,
24] in the preparation of a new biofuel patented by University of Cordoba, named Ecodiesel-100 [
22]. This method takes advantage of the 1.3-selective nature of many lipases, which allows stopping the process in the second step of the alcoholysis reaction to obtain a mixture of two moles of FAEE and one MG. In this respect it is interesting to point out in this respect that according to recent studies the presence of MG enhances the lubricating properties of the biofuel [
25,
26]. So far this process is being developed not only with PPL but also with some microbial lipases, both in free and immobilized forms [
19,
20,
21,
22,
23,
24].
The current existing limitations to the use of lipases are mainly associated with their high costs, so that in order to achieve an increase in viability and competitiveness respect to the enzymatic process, the present study aims to achieve the partial transesterification reaction, through the kinetic control of the chemical reaction, to obtain the same results previously described in stereoselective enzymatic processes. Thus, now is intended to obtain the same new biodiesel that contains monoacylglycerol, by using KF as alkaline heterogeneous catalyst, as an alternative to the more expensive lipases (
Figure 1).
Figure 1.
Representative scheme of the production of Ecodiesel, a biofuel, by partial methanolysis of sunflower oil.
Figure 1.
Representative scheme of the production of Ecodiesel, a biofuel, by partial methanolysis of sunflower oil.
In this respect, some promising results have been previously obtained by using CaO as an alkaline heterogeneous catalyst [
27]. In the present research, a series of supported KF catalysts, previously applied as basic catalysts in some organic synthetic processes [
28], are investigated in the methanolysis reaction of sunflower oil to obtain the desired selective transesterification, through the kinetic control of the consecutive chemical process, to get the same biodiesel without glycerol generation, as an alternative to the more expensive lipases.
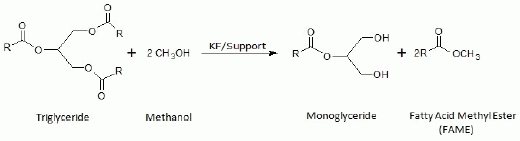
Taking into account that the transesterification reaction is a sequential process consisting in three successive methanolysis steps, where a molecule of TG (triglyceride) and three molecules of methanol lead to a molecule of glycerol and three of FAME, it is conceivable that some kinetic parameters like amount of catalyst, molar oil/methanol ratio, reaction temperature and the reaction time could be critical parameters to control in order to develop a selective process with only two successive methanolysis steps to keep the glycerol as a monoglyceride (
Figure 1).
On the other hand, heterogeneous catalysts, unlike to homogeneous ones, are environmentally benign and could operate in continuous processes. Moreover, they can be reused and regenerated. Among them, supported-KF catalysts seems to have a promising place according to the increasing number of research works devoted to its capability to catalyze the triglyceride methanolysis reaction [
29,
30,
31,
32,
33,
34,
35,
36]. In contrast, no reports have been found regarding the catalytic behaviour of supported CsF, NaF or LiF in transterification reactions of oils or fats to obtain biodiesel [
37].
However, despite the success obtained with supported KF catalysts [
29,
30,
31,
32,
33,
34,
35,
36,
37], one always gets a marked decrease in the catalytic activity, as compared to homogeneous catalysis, which requires operating at higher temperatures and pressures, with higher oil/methanol proportions, which so far has discouraged their implementation on an industrial scale for the conventional biodiesel production.
Nevertheless, this weaker basic character of supported KF catalysts could be an important advantage to achieve a selective methanolysis, where the process is stopped in the second step (like in
Figure 1), after optimizing the experimental conditions (catalyst weight, oil/methanol relative concentration, temperature, reaction time,
etc.) to achieve the kinetic control of the selective methanolysis process. In this way, the use of heterogeneous low cost catalysis to produce a biofuel without glycerol generation, could solve many of the problems of conventional homogeneous catalysts (NaOH or KOH), because the yields of the process are increased (10–12 wt%), the neutralization step of the reaction products is avoided, as well as the cleaning process after the synthesis of biodiesel and the waste management of glycerol generated.
2. Results and Discussion
2.4. Influence of Temperature on Process Performance
The optimized conditions (weight of catalyst, reaction time and molar oil/alcohol ratio) have been established according to an elevated temperature, 65 °C. However, the evaluation of the influence of reaction temperature on reaction performance may provide information of interest in relation to the catalysed reaction mechanism of the sunflower oil methanolysis process. Thus, under the experimental conditions of
Table 1 (time reaction 60 min, 0.8 g KF/Al
2O
3, 12 mL oil and 2.43 mL methanol) several reactions were carried out at different temperatures in the 45 to 65 °C range. Results shown in
Figure 4 indicate that Conversion and Selectivity reach the highest value, 100% and 99.9% respectively, at 50 °C, however viscosity values exhibit a continuous decrease with increasing temperature.
Recalling that the Selectivity is connected to those methanolysis products with similar RT (retention time) values to those hydrocarbons that comprise conventional fossil diesel, and in this interval not only the different FAMEs are present, but also the corresponding MGs. Besides, a 99% Selectivity with Conversion of 100% would indicate the presence of at least 1% of DG (diglyceride), so that the viscosity obtained is somewhat higher that corresponding to pure the FAME constituting conventional biodiesel. That is, it is perfectly possible for two reaction samples with similar Conversion and Selectivity values to differ in viscosity values. Thus, it is concluded the greater relevance of the viscosity with respect to the Conversion and Selectivity data, with respect to the information obtained on the consecutive reaction progress of sunflower oil methanolysis.
In this respect, the viscosity appears as the most sensitive parameter to the progress of the reaction where the Arrhenius equation that fit Equations (1) and (2) it can be applied as can be seen in
Figure 5, where the kinematic viscosity is a parameter directly related to the reaction rate constants:
Figure 4.
(a) Viscosity values and (b) Conversion and Selectivity values, obtained in the heterogeneous selective methanolysis of sunflower oil carried out under standard conditions, reaction time 60 min, 0.8 g of KF/Al2O3 catalyst, 12 mL of oil and 2.43 mL of methanol, under different reaction temperatures in the interval 45–65 °C.
Figure 4.
(a) Viscosity values and (b) Conversion and Selectivity values, obtained in the heterogeneous selective methanolysis of sunflower oil carried out under standard conditions, reaction time 60 min, 0.8 g of KF/Al2O3 catalyst, 12 mL of oil and 2.43 mL of methanol, under different reaction temperatures in the interval 45–65 °C.
Figure 5.
Arrhenius plot (Lnk vs. 1/T) obtained from the evolution of Lnk values with temperature, where k = viscosity−1; operating under standard conditions, with 12 mL of oil, 2.43 mL of methanol, 0.8 g of catalyst weight, 60 min reaction time and different reaction temperatures in the interval 25–65 °C.
Figure 5.
Arrhenius plot (Lnk vs. 1/T) obtained from the evolution of Lnk values with temperature, where k = viscosity−1; operating under standard conditions, with 12 mL of oil, 2.43 mL of methanol, 0.8 g of catalyst weight, 60 min reaction time and different reaction temperatures in the interval 25–65 °C.
Here we have that A is the Arrhenius pre exponential factor and Ea is the activation energy of the reaction. This equation is linear with respect to 1/T. If k is determined for varying temperatures, the plot of Ln k vs. 1/T should produce a straight line of slope −Ea/R. The inverse of kinematic viscosity is used because of this parameter inversely decreases with the progress of the reaction. In this way it is obtained that LnA = 5.37, where A is expressed in cSt−1. Activation energy value Ea = 1.16 Kcal/mol is a relative low value that indicates a low influence of temperature on the reaction rate. Thus, it manifests the higher catalytic activity of the KF/Al2O3 catalyst.
It is also important to note how the Selectivity, the Conversion and the inverse of the kinematic viscosity follow a parallel evolution among them, so that is perfectly demonstrated that with the transformation of TG into DG and this in MG, a gradual reduction in the values of kinematic viscosity is obtained, that is, the viscosity decreases more as the concentration of TG and DG is more reduced. The MG concentration does not appear to affect the viscosity of the mixture in a similar measure as those of TG or DG do. Presumably, the influence of these species in the viscosity of reaction mixture will be directly related to the relative values of their respective molecular weights, so that TG ≈ 3/2 DG ≈ 3 MG ≈ 3 FAME.
2.5. Influence of the Repeated Used of the Three Catalysts, on the Process Performance
Taking into account that a main advantage of heterogeneous catalysts is the possibility of their reuse, the catalytic behaviour of repeated uses of the three different KF/support catalysts was investigated, where as supports Al
2O
3 ZnO and MgO have been studied.
Figure 6,
Figure 7 and
Figure 8 show the variation of Conversion, Selectivity and viscosity over the five studied reuses.
To evaluate the reuses of the different catalysts, in the first reaction 12 mL sunflower oil and 2.43 mL methanol (corresponding to a 1/6 oil/methanol molar ratio), and 0.8 g catalyst (7 wt%) was used, operating at 65 °C for 60 min. In the next successive reactions the same amounts of sunflower oil and methanol were added after withdrawing the previous reaction products. Thus, after allowing the reaction product to be decanted for one hour, the supernatant liquid is removed with a pipette and the supported KF solid catalyst remains deposited in the bottom of the flask reactor, ready to be used again in the next reaction.
Figure 6.
(a) Viscosity values obtained in the successive reuses of heterogeneous selective methanolysis of sunflower oil under standard conditions, with 0.8 g of KF/Al2O3, 65 °C, 12 mL of oil and 2.43 mL methanol. (b) Conversion and Selectivity values obtained with identical conditions.
Figure 6.
(a) Viscosity values obtained in the successive reuses of heterogeneous selective methanolysis of sunflower oil under standard conditions, with 0.8 g of KF/Al2O3, 65 °C, 12 mL of oil and 2.43 mL methanol. (b) Conversion and Selectivity values obtained with identical conditions.
Figure 7.
(a) Viscosity values obtained in the successive reuses of heterogeneous selective methanolysis of sunflower oil (viscosity 32.0 cSt) under standard conditions, with 0.8 g of KF/ZnO, 65 °C, 12 mL of oil and 2.43 mL methanol. (b) Conversion and Selectivity values obtained with identical conditions.
Figure 7.
(a) Viscosity values obtained in the successive reuses of heterogeneous selective methanolysis of sunflower oil (viscosity 32.0 cSt) under standard conditions, with 0.8 g of KF/ZnO, 65 °C, 12 mL of oil and 2.43 mL methanol. (b) Conversion and Selectivity values obtained with identical conditions.
Figure 8.
(a) Viscosity values obtained in the successive reuses of heterogeneous selective methanolysis of sunflower oil (viscosity 32.0 cSt) under standard conditions, with 0.8 g of KF/MgO, 65 °C, 12 mL of oil and 2.43 mL methanol. (b) Conversion and Selectivity values obtained with identical conditions.
Figure 8.
(a) Viscosity values obtained in the successive reuses of heterogeneous selective methanolysis of sunflower oil (viscosity 32.0 cSt) under standard conditions, with 0.8 g of KF/MgO, 65 °C, 12 mL of oil and 2.43 mL methanol. (b) Conversion and Selectivity values obtained with identical conditions.
According to the results, with all three supported KF catalysts studied it is obtained that, after the first reaction, Conversion and Selectivity decrease continuously while the kinematic viscosity is increased from 5 to 7 cSt to values close to 20 cSt, corresponding to a lowering in the Conversions and Selectivity from 100% to values lower than 10%. In this respect, KF/Al
2O
3 exhibits the worst catalytic behaviour respect to reuse capability, despite of the fact of being the best heterogeneous KF catalyst studied in the first use. The catalysts KF/ZnO and KF/MgO allow a second reuse, with the same catalytic behavior seen in the first one, and a softer decline in Conversion and Performance that the Al
2O
3 catalyst. However, the CaO catalyst [
27] showed a better performance in regard to the reuse of the heterogeneous catalysts. Catalyst deactivation in no case could be associated to a leaching of the supported KF, according to results obtained after separation of the solid catalysts from the reaction when the reaction was started. These complementary studies, included in the Experimental section, indicate that reaction was always completely interrupted after the separation of supported KF catalyst. Thus, no KF was present in the medium reaction. In this way, deactivation ought to be adscribed to some kind of interaction of reaction products or to impurities, like free fatty acids presents in very little amounts in the sunflower oil.
4. Conclusions
In order to improve a new enzymatic methodology developed to obtain a second generation biodiesel that integrates glycerol as monoglyceride [
19,
20,
21,
22,
23,
24], partial transesterification reactions were achieved through the kinetic control of the chemical reaction. In this respect, supported KF catalysts were evaluated in this study as an inexpensive selective heterogeneous catalyst in the partial methanolysis of sunflower oil to produce an optimal mix with two FAME molecules and one MG molecule for every TG molecule. This biofuel, named Ecodiesel, is applicable to diesel engines because it exhibits similar properties to conventional biodiesel and does not produce glycerol as a by-product. Thus, according to the results the fact that in no case the slightest amount of glycerol was obtained according the GC analysis of the reaction products highlights the undoubted selective character of the consecutive methanolysis process.
We have therefore shown that with any of the three supported KF catalysts studied, the selected experimental conditions represent a significant optimization of the process to obtain in one step a biofuel with kinematic viscosity values around 4.5–8.5 cSt, very similar to conventional biodiesel, without generation of any type of residue, since only one phase is obtained, containing FAME, MG, and some little amount of diacylglycerol (DG) as well as the non-reacted methanol, unlike what happens when a larger amount of methanol is used, where two phases are produced. In such case, a conventional transesterification process is developed [
38] and variable amounts of glycerol are collected in the lower methanolic phase when a 12/1 molar methanol to oil ratio is used.
On the other hand, some studies [
39,
40,
41,
42] have proven that blends of diesel fuel and some short chain alcohols, like methanol, with biodiesel produced some less maximum power output than regular diesel. Besides, no significant difference in the emissions of CO
2, CO, and NO
x between regular diesel and biodiesel, methanol and diesel blends was observed. However, the use of these blends resulted in some reduction of particulate matter. Consequently, such blends can be used in a diesel engine without any modification, taking into account the limited changes obtained respect to the use of pure diesel.
On the other hand, the use of conventional biodiesel as a diesel fuel extender and lubricity improver is rapidly increasing. While most of the properties of biodiesel are comparable to those of petroleum-based diesel fuel, improvement of its low temperature flow characteristic still remains one of the major challenges when using biodiesel as an alternative fuel for diesel engines. However, a considerable reduction in pour point has been noticed by using some alcohol as cold flow improver [
41]. In this respect, Ecodiesel will display some higher cloud points and pour points than conventional biodiesel; however the presence of methanol as a surplus of the methanolysis reaction will compensate the low temperature flow characteristics. Besides, several additives may be used to maintain the basic chemical functions to improve ignition and combustion efficiency and to stabilize such fuel mixtures [
42]. Thus, it can be used directly after its production because it is obtained in only one phase and no purification step of residual glycerol or methanol is necessary.
Furthermore, to obtain this biofuel it is not necessary operate at high pressures and temperatures. On the contrary, this new biodiesel is obtained under mild reaction conditions, at atmospheric pressure, 6/1 methanol to oil molar ratio, 7 wt% catalyst respect to oil, 65 °C reaction temperature and 60 min reaction time. Besides, a comparatively higher yield is obtained respect to the conventional biodiesel reaction, because no glycerol is generated as byproduct.
Finally, although it was seen that after the second reuse a decrease in the catalytic activity occurs, this low cost heterogeneous process may be comparatively more profitable than the enzymatic process, due to the higher cost of commercial lipases.