1. Introduction
Alloy membranes with hydrogen selective properties have broad technological applications in the field of hydrogen production and purification [
1,
2,
3]. Usually, thin, crystalline, Pd-based membranes are used for the purification of hydrogen due to their high permeability to hydrogen (~2.0 10
−8 mol m
−1 s
−1 Pa
−0.5 for Pd
75Ag
25) and selectivity [
4]. The operating temperatures of these membranes are higher than 573 K. Palladium, however, is an expensive and scarce element, therefore inexpensive and abundantly available alloys are being investigated as a replacement. Amorphous alloy membranes show promising properties [
5], such as excellent mechanical strength, high thermal stability and soft magnetic properties [
6,
7,
8].
A promising class of amorphous alloy membranes are the Ni-Zr or Ni-Nb-Zr ones. Hara
et al. reported that Ni-Zr based alloys are stable, and do not become brittle in the temperature range between 473 K to 623 K while the hydrogen permeability of the Ni
64Zr
36 sample is of the order of 10
−9 mol m
−1 s
−1 Pa
−0.5 [
9,
10,
11]. Later studies showed that the hydrogen permeability of Ni-Nb-Zr amorphous samples is significantly enhanced by the addition of Zr to the alloys, reaching a permeability comparable to that of the Pd-based alloys, on the order of 10
−9–10
−8 mol m
−1 s
−1 Pa
−0.5 [
12,
13,
14,
15,
16].
The Ni-Nb-Zr membranes can be produced in the amorphous state by various techniques, such as planar flow cast or melt spinning. These amorphous alloys are stable at operating temperatures up to 623 K, but devitrify at temperatures higher than 780 K. In the Ni-Zr and Ni-Nb-Zr systems the crystallization temperature (T
x) decreases as the Zr content increases [
15,
17]; for example the T
x ~843 K for Ni
63.7Zr
36.3, that decreases to T
x ~653 K for Ni
30Zr
70. Another example is that of Ni
60Nb
40 with a T
x ~943 K which decreases to T
x ~843 K with the addition of 30 at.% Zr (
i.e., in the alloy (Ni
0.6Nb
0.4)
70Zr
30). Permeation tests on Ni-Nb-Zr amorphous alloys are usually conducted in the temperature range of 673–723 K [
12,
13,
14,
15,
16]; the permeability of Pd (1.8–2.0 10
−8 mol m
−1 s
−1 Pa
−0.5) [
14] is slightly higher than that of our alloys (Ni
0.6Nb
0.4)
70Zr
30 (1.4 × 10
−8 mol m
−1 s
−1 Pa
−0.5) at 723 K and of (Ni
0.6Nb
0.4)
80Zr
20 (8 × 10
−9 mol m
−1 s
−1 Pa
−0.5) at 673 K [
14]. Typically, in a laboratory these experiments take one to two weeks during which the membranes are at elevated temperatures and are exposed to hydrogen. To terminate the experiment, it is important that all the hydrogen be desorbed from the membrane before cooling down to room temperature. If all the hydrogen is not removed, these membranes have a propensity to embrittle. The embrittlement of the samples with or without hydrogen is heavily influenced by their elastic properties, therefore, in this paper we investigate the changes of the elastic modulus of (Ni
0.6Nb
0.4)
1−xZr
x membranes induced by thermal treatments and hydrogenation. One of the property we are interested is in the amorphous to amorphous phase transitions as a function of temperature.
2. Results
Two pristine membranes were investigated in the present study: (1) (Ni
0.6Nb
0.4)
70Zr
30, thereafter referred to as Zr
30, and (Ni
0.6Nb
0.4)
80Zr
20 as Zr
20. The effect of thermal treatments and/or hydrogenation on these two specimens will be determined. X-ray diffraction (XRD) data of the membranes are reported in
Figure 1. The starting materials display a broad peak centered around 39.9° in Zr20 and 39.3° in Zr30, which confirms the manly amorphous nature of the pristine membranes.
Figure 1.
X-ray diffraction (XRD) spectra of all samples with attribution to crystalline phases (magenta diamonds: ZrO
2; green solid circles: Ni
10Zr
7; orange squares: Ni
48Zr
52) [
18,
19,
20,
21,
22].
Figure 1.
X-ray diffraction (XRD) spectra of all samples with attribution to crystalline phases (magenta diamonds: ZrO
2; green solid circles: Ni
10Zr
7; orange squares: Ni
48Zr
52) [
18,
19,
20,
21,
22].
Differential thermal analysis (DTA) measurements (in
Figure 2) show the crystallization temperature. Two sharp peaks at 816 and 836 K, were observed for the Zr30 alloy, whereas Zr20 sample displayed one peak at ~868 K. We attribute these peaks to the crystallization process of the amorphous alloys, in agreement with previous reports [
14,
15,
17]. The crystallization of Zr30 sample is a multistep process, as suggested by the weak structures (peaks) present in the DTA signal. A multistep crystallization is very common in amorphous alloys for hydrogen storage. It is worth noting that the temperatures at which our samples display the highest peaks of the DTA signals well agree with the known crystallization temperatures of the two alloys: T
x ~876 K for the Zr20 and 808 K for the Zr30 alloy [
15].
The occurrence of the crystallization process at high temperatures is confirmed by XRD data collected on samples Zr30 and Zr20 heated to 973 K in an argon environment (
Figure 1). In Zr30 sample Bragg peaks are observed after such thermal treatments at ~38.4, 40.2, 42.1 and 55.5° 2θ that have been ascribed to Ni
48Zr
52 phase [
21], and Bragg pea K located at 39.2° 2θ, that may possibly due to the presence of Ni
10Zr
7 phase [
22]. Note that the Bragg peaks of ZrO
2 are due to the exposure of the sample to air for XRD experiments [
18,
19,
20]. The Zr20 sample displays similar characteristics when heated to 973 K. For the sake of completeness we also heated a Zr30 sample only up to 673 K for 8 h in an argon atmosphere. In the case of the Zr30 sample heated to 673 K, (much lower than the T
x~816 K for Zr30), the XRD pattern shows the characteristic broad amorphous hump at ~40° 2θ, and a minor peak at 30.2°, ascribed to ZrO
2.
Figure 2.
Differential thermal analysis (DTA) measurements on the two amorphous membranes conducted with a temperature rate of 4 K/min after subtraction of the background records with empty crucibles.
Figure 2.
Differential thermal analysis (DTA) measurements on the two amorphous membranes conducted with a temperature rate of 4 K/min after subtraction of the background records with empty crucibles.
In order to check whether the hydrogenation process can induce crystallization, the membranes with the two different content of Zr were hydrogenated at 673 K by using a home-made Sieverts apparatus, starting with an initial hydrogen pressure of ~0.55 MPa. The hydrogenation process for Zr30 lasted 4 h, and 22 h for Zr20; with a final hydrogen content of ~0.4 wt%H for Zr20 and ~0.8 wt% H for Zr30, after which the experiments were terminated. In
Figure 1 the high intensity Bragg peaks around 40° 2θ are retained in the hydrogenated samples, and therefore after hydrogenation the specimens remain mainly amorphous (see
Figure 1). Only the hydrogenated Zr30 sample displays two Bragg peaks at 28.3 and 29.9° 2θ due to the formation of ZrO
2 [
18,
19,
20,
21,
22].
Dynamical Mechanical analysis (DMA) results yielded interesting results; the tensile modulus values, M, measured from the room temperature to 673 K are shown in
Figure 3. At room temperature the tensile modulus of Zr20 (M = 1.0 × 10
10 Pa) is higher than that of Zr30 (M = 6.5 × 10
9 Pa). The modulus decreases almost linearly up to 503 K for the Zr20 and up to 543 K for the Zr30 sample, showing a strong decrease of M by a factor of ~2 which is clearly visible. In another region of the spectrum above ~633 K one can observe, again, a much smoother linear decrease of the modulus. An almost linear decrease of the elastic modulus with increasing temperature is usually observed in many solids and can be explained by the Debye theory [
23].
We could not measure the tensile modulus of samples in which a full crystallization was induced by a thermal treatment in argon at 923 K in the thermobalance, because the membranes become so fragile that they break as soon as they are mounted in the clamps of the Dynamic Mechanical Analyzer (DMA) used for the elastic modulus measurements. Therefore a direct comparison of the elastic properties of the crystallized samples could not be performed.
Figure 3.
Dynamic Mechanical Analyzer (DMA) measurements of the membranes.
Figure 3.
Dynamic Mechanical Analyzer (DMA) measurements of the membranes.
DMA measurements were repeated on the hydrogenated membranes. They were more fragile than the starting materials and in some cases small pieces of the hydrogenated samples broke up before measurements. In order to be sure that the hydrogenated membranes do not break during measurements we carefully measured the elastic moduli both on heating and cooling, comparing the values of M along the thermal cycle and checking that no sudden and irreversible change of the elastic modulus occurred. These DMA measurements of the hydrogenated membranes are reported in
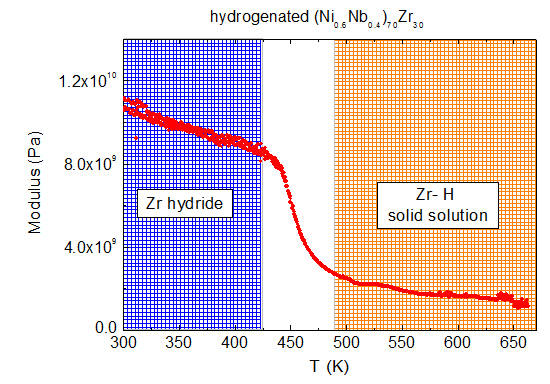
Figure 3. One can note that for both Zr concentrations the elastic modulus at room temperature and below 423 K is higher in hydrogenated samples than in the starting materials. Upon heating above the room temperature, M decreases almost linearly up to ~393 K for Zr30, and up to ~423 K for Zr20. Above such temperatures, M displays a strong and sharp decrease by a factor 3–4. For both samples such variation occurs in a very limited temperature range, 423 K to 673 K for the Zr30, and 443 K to 523 K for the Zr20 sample. For both specimens a smooth linear decrease of M is observed above 523 K.
The appearance of an abrupt decrease of M is strictly due to the presence of hydrogen in the hydrogenated samples and cannot be explained as an effect of the thermal cycle used during hydrogenation (in our case, heating at 673 K for at least 4 h). The proof of this comes from a measurement performed on a piece of the Zr30 membrane heated at 673 K for 8 h (sample Zr30_TT) in an argon atmosphere (a longer thermal treatment than that used for the hydrogenation process at the same temperature). Zr30_TT shows a smooth increase of the modulus, without the abrupt change presented by the hydrogenated membrane (see
Figure 3). Indeed, the temperature dependence of M for sample Zr30_TT strictly resembles that of the pristine membrane, except that the absolute value is slightly higher. This increase of the absolute value of M in the thermally treated specimen can be possibly due the formation of nanometric crystallites, not detected by X-ray diffraction because of their small dimensions, whose presence increases the elastic stiffness of the sample.
The tensile modulus of the hydrogenated samples measured on subsequent cooling, after heating to 673 K, (in the
Figure 3) again shows strong variations between 423 K and 523 K for the Zr30, and between 443 K to 523 K for the Zr20 sample. In the case of the Zr20 sample this feature stretches to a broader temperature range than the Zr30 sample with the modulus jump clearly visible.
To confirm if the hydrogenated sample are not completely desorbed, thermogravimetric analyses (TGA) measurements were performed on the hydrogenated Zr30 samples. Results show that hydrogen was not completely released by heating at 673 K as shown in
Figure 4. The hydrogen gas starts to release above 473 K but there is incomplete dehydrogenation below ~873 K. One can note that above 873 K (
Figure 4) the sample starts to oxidize, and the mass increases. For both the Zr20 and the Zr30 samples half of the initial hydrogen content is retained after thermal treatments up to ~673 K.
Figure 4.
TGA scan of the hydrogenated Zr30 sample.
Figure 4.
TGA scan of the hydrogenated Zr30 sample.
3. Discussion
A striking difference between the elastic moduli variation of the pristine (unhydrogenated) and the hydrogenated membranes is evident in
Figure 3. In the hydrogenated Zr30 membrane we observe an abrupt decrease of the elastic modulus in an extremely limited range of 423 K to 473 K, and 443 K to 523 K for the Zr20 membrane sample. Such variations are completely absent in the pristine materials and they resemble the modulus changes occurring concomitantly with a phase transition. Indeed, no change in the shape and position of the modulus drop are visible when measurements are conducted with different frequencies (results not shown). Conversely, if the modulus drop was due to a thermally activated process, one would observe a shift of the modulus variations toward higher temperatures when M was measured at higher frequency [
23,
24].
Usually, these materials display a gradual decrease of the elastic modulus with increasing temperature [
23]. However, materials undergoing phase transitions display strong deviations from such a trend and present sharp changes of M as a function of temperature around the phase transition temperature. Elastic moduli typically display remarkable variations in correspondence of phase transitions, independently of their order [
24]. It should be noted that the DSC or DTA can easily detect first order phase transitions and measure latent heats of phase transitions, but are not well suited to detect phase transitions of the second or higher order; for these DMA measurements are critical.
The XRD measurements indicate that both the pristine and the hydrogenated (Ni
0.6Nb
0.4)
1−xZr
x membranes investigated in the present study are mainly amorphous. However, some studies of similar samples indicate that at the microscopic level they are not completely amorphous: they actually possess some nano-crystalline inclusions [
16,
25,
26]. Such nano-crystals are too small and diluted to cause a detectable diffraction signal in standard diffractometers [
16,
25,
26]. Indeed, TEM analysis of Ni
64Zr
36 samples pointed out that after thermal treatments the surface shows the presence of fine precipitates, 500 nm in diameter, with a high concentration of Ni [
16]. Moreover, some blistering possibly due to the precipitation of γ-ZrH was observed [
16]. Also Jayala Kshmi
et al. reported that nano-crystallization takes place in hydrogenated Zr- and Ni- based glasses, even though XRD is unable to detect crystalline precipitates [
25,
26]. Indeed, electron diffraction patterns show the formation of nanocrystals of γ-ZrH after hydrogenation the Zr
50Ni
27Nb
18Co
5 alloy, with dimensions of the order of ~2 nm and the formation of the Ni
2H phase in Ni
59Zr
16Ti
13Nb
7Sn
3Si
2 [
26].
In view of this framework, we suggest that the modulus variation in the hydrogenated samples around 473 K is due to the formation on cooling of nanometric Zr hydride or its dissolution on heating. Indeed, Zr is an energy favorable site for the absorption of hydrogen, as pointed out for example by XAFS measurements and molecular dynamics simulations [
27]. We suggest that in the Zr rich zones of the samples, hydrogen can form a solid solution with the Zr atoms. However, as temperature is decreases the solid solution undergoes a phase transition to a new stable hydride phase. On heating, the process is reversible. The Zr-H phase diagram [
28,
29] presents two regions with phase transitions in the temperature range around 473 K: the two lines defining the separation between the α and δ phases and the coexistence region between the δ hydride phase and α-Zr phase. We suggest that the modulus variations reported in
Figure 3 correspond to the transition from the solid solution to the hydride on cooling and from the stable hydride to the solid solution on heating. Some modulus variations due to hydride formation and dissolution have also been previously observed in other Zr alloys, such as Zircaloy-4 or Zr-2.5Nb [
30,
31].
TGA measurements indicate that at least half of the hydrogen initially present in the samples is liberated after a thermal treatment at 673 K. On the other side DMA measurements find a reasonable recovery of the elastic modulus after the same thermal treatment. These results are not in contrast in view of the attribution of the modulus drop to the formation and dissolution of Zr hydride. Indeed Zr hydride is only a minority phase, whose presence is detected by DMA measurements. As Zr atoms are the energy preferred sites for absorption of hydrogen, Zr-H is the most stable of the hydride phases in the sample and therefore it will be the least affected by dehydrogenation. Therefore a partial release of hydrogen from the samples does not affect the presence of the Zr hydride.
In summary, we suggest that the present measurements of the elastic modulus of hydrogenated membranes strongly indicate that nanometer size Zr hydride can form or dissolve as a function of temperature and therefore the elastic properties of the amorphous materials display a dramatic variation around 473 K. In this way the modulus measurements become an easily way to detect the presence of nanocrystalline Zr hydrides.
4. Experimental Section
Crystalline (Ni
0.6Nb
0.4)
100−xZr
x (x = 20 and 30) alloy ingots were prepared by arc melting in a purified Ar atmosphere. Amorphous ribbons ~100 μm thick and 30 mm wide have been prepared using the melt-spinning method [
15].
Simultaneous TGA-DTA measurements were conducted by means of a Setaram Setsys Evolution 1200 TGA system (Caluire, France) [
32]. The furnace was continuously flooded with high purity argon (60 ml/min). For each experiment a sample mass of ~10 mg was used.
XRD have been recorded at room temperature by using an X-Pert PANalytical theta-theta diffractometer (Almelo, The Netherlands) in the range 10–70° with a step of 0.025°, time/step = 3 s. Millimetric pieces of the membranes have been spread on the planar glass holder and then measured in the XRD chamber.
Dynamic Mechanical Analysis (DMA) was carried out on small membrane pieces 4–6 mm wide and 10–12 mm long by using a DMA 8000 (Perkin Elmer, Waltham, MA, USA) in the so-called “tension configuration” [
33,
34,
35]. The storage modulus,
M, was measured at frequencies of 1 and 10 Hz between room temperature and 673 K, that is the maximum temperature allowed by the system. All measurements were carried out in a flux of pure argon to prevent oxidation of samples. In order to directly compare DTA and DMA measurements, we conducted the two experiments at the same temperature rate (4 K/min).
Hydrogenation of the samples was obtained by means of a home-made Sieverts apparatus working up to a 20 MPa pressure and a 773 K temperature [
36]. The amount of hydrogen exchanged between the gas atmosphere and the solid samples is measured by the pressure variation in calibrated cylinders connected by Swagelok tubes to the reaction chamber where the sample is placed. A homemade computer program developed in the Labview language is used to acquire the time evolution of the pressure detected by two micro-Baratron transducers working between 0 and 0.7 MPa and between 0 and 20 MPa, respectively, and of the temperature of the five cylinders and of the tubes connecting the cylinders and the reaction chamber. The real gas state equation is used to calculate the exchanged hydrogen moles. For all samples we chose to conduct hydrogenation processes at T = 673 K and p ≈ 0.55 MPa, because these are the temperature and pressure at which the membranes have practical application in gas reactors in order to separate H
2 from other gases. We extended the hydrogenation procedure until a steady state was obtained and no more hydrogen was absorbed by the membranes. The Zr30 sample, exposed to p ≈ 0.55 MPa, absorbs more hydrogen and in a shorter time than the Zr20 specimen. After only 4 h, Zr30 reaches a hydrogen concentration value of 0.8 wt%. On the contrary, in the same conditions, Zr20 absorbs only 0.4 wt% even after 22 h.