Semiconductor Nanomaterials-Based Fluorescence Spectroscopic and Matrix-Assisted Laser Desorption/Ionization (MALDI) Mass Spectrometric Approaches to Proteome Analysis
Abstract
:1. Introduction
2. Overview of Colloidal Semiconductor QDs
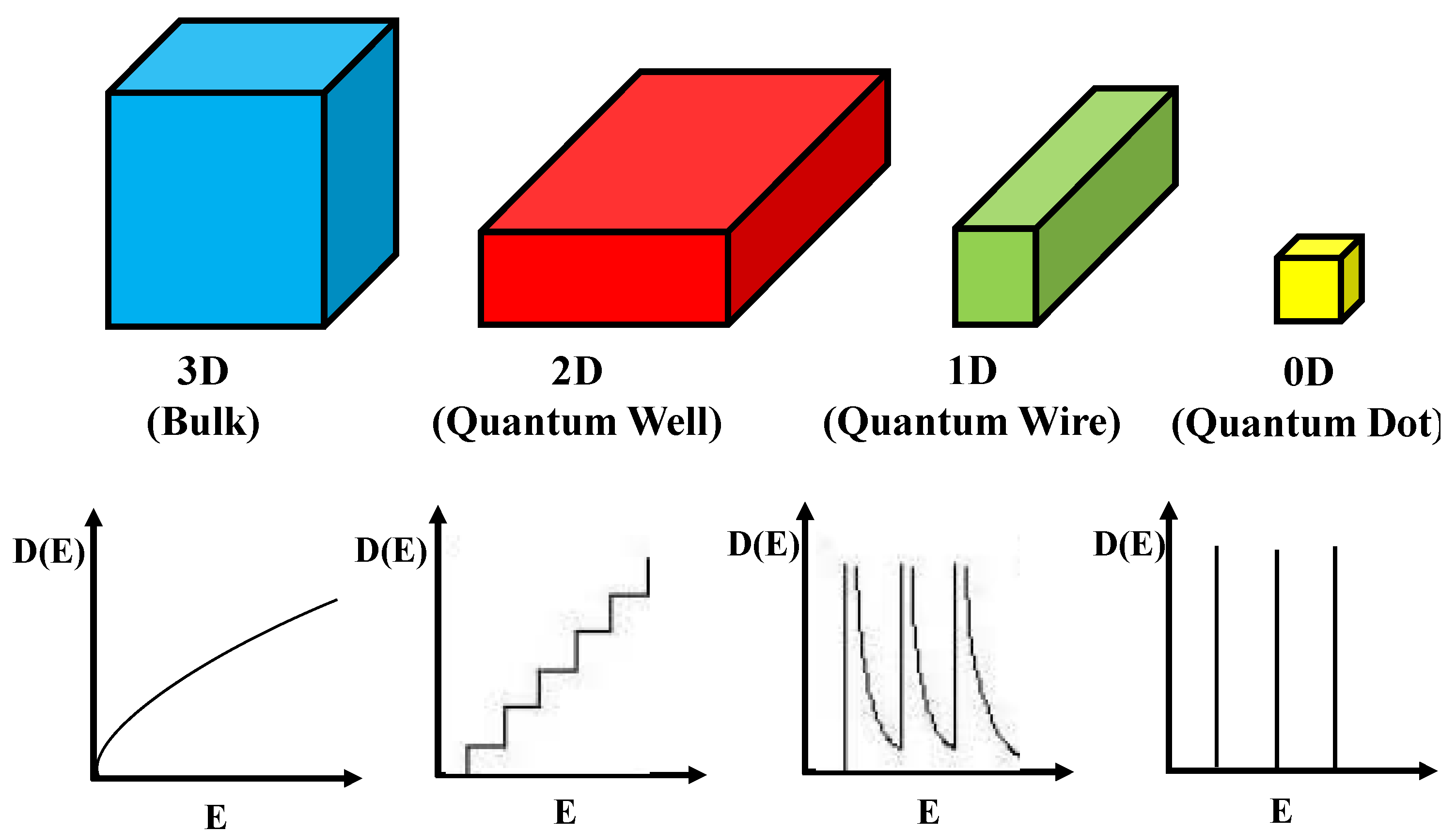
Types of Semiconductor QDs



3. Semiconductor QDs-Based Fluorescence Approaches for Biomolecules Assays
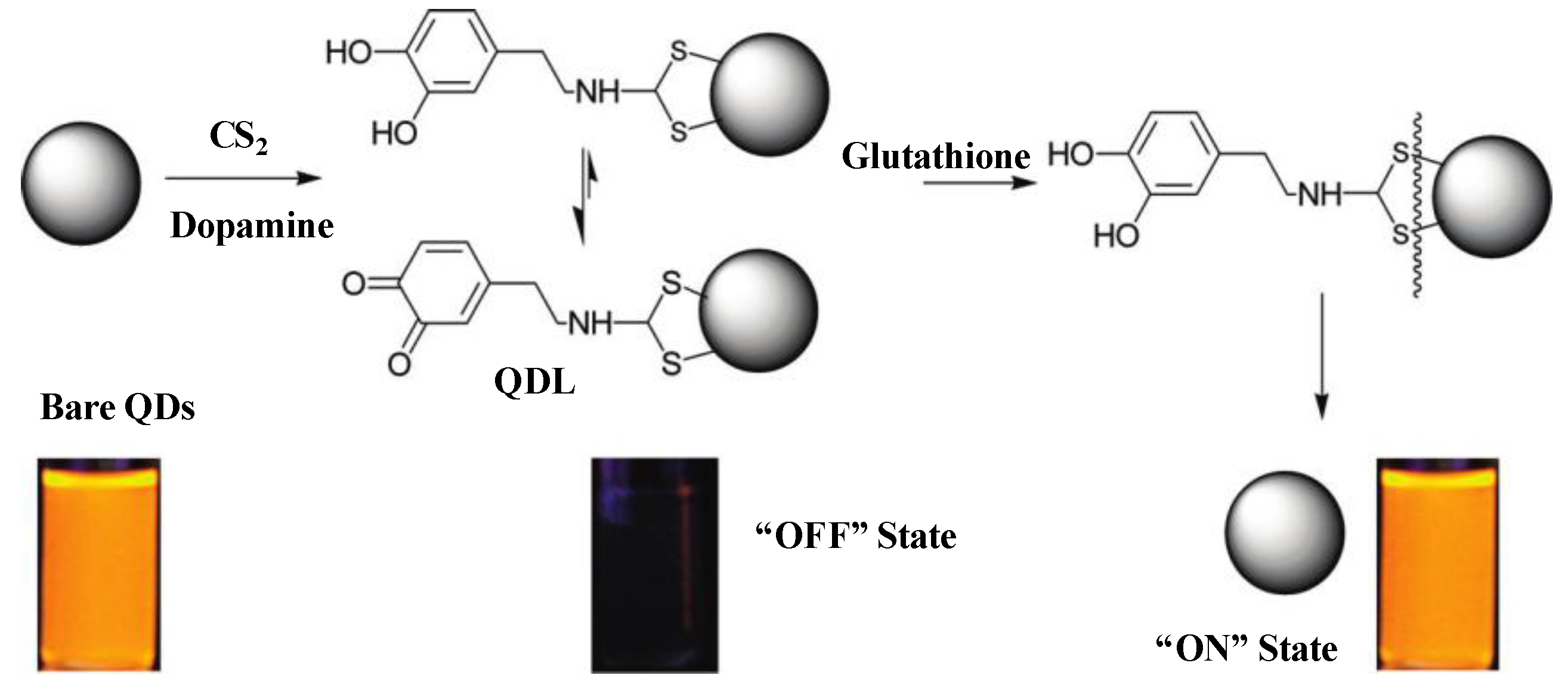
3.1. Semiconductor QDs-Based FRET and BRET Approaches


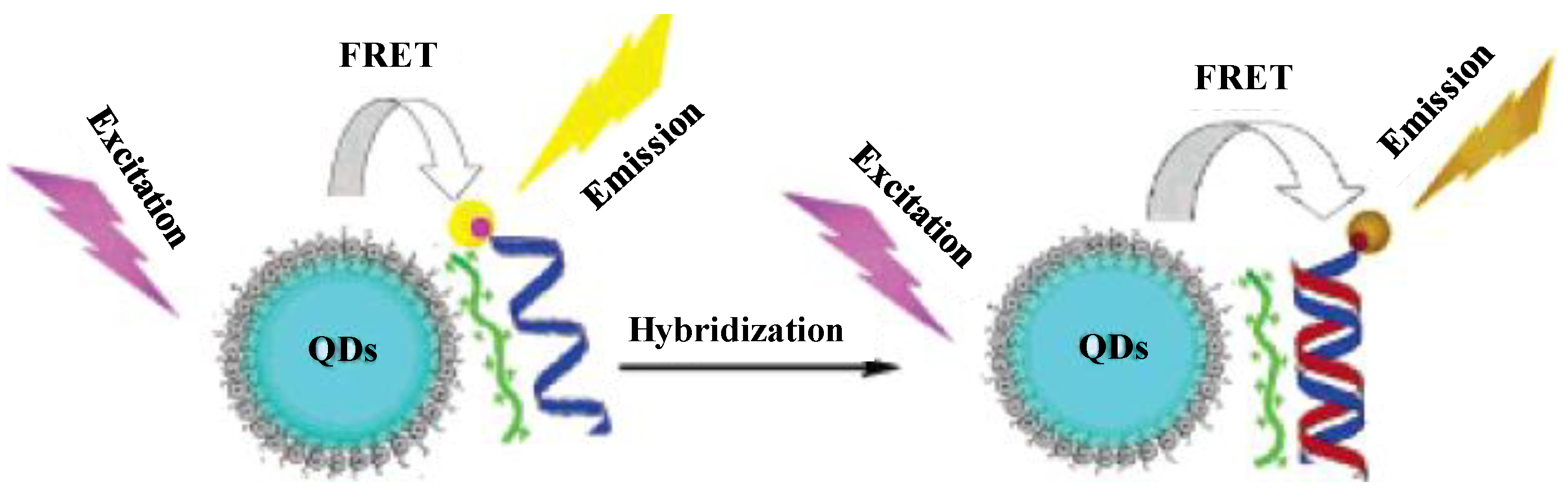
3.2. Semiconductor QDs as Probes for Analysis of DNA, Bacteria and Cancer Cells
3.3. Semiconductor QDs as Probes for Analysis of Peptides and Proteins

| Name of QDs | Name of the ligand | Diameter (nm) | Analytes | Detection limit (nM) | Reference |
|---|---|---|---|---|---|
| CdS:Mn/ZnS | DDTC | 5 | GSH | - | [30] |
| CdS:Mn/ZnS | Amine group | 3.1 | TAT-peptide | - | [31] |
| CdSe/ZnS | TGA | - | GSH | - | [32] |
| CdS | MPA | 2–5 | Salmonella typhimurium cells | - | [33] |
| Mn2+ZnS | Chitosan | 3–5 | E. coli | - | [34] |
| Mn2+ZnS and ZnS | Chitosan | 4.5 | PANC-1 cell | - | [35] |
| CdS | TGA | 5 | DNA | - | [36] |
| CdSe/ZnS | Peptides (GFE, KDE, LyP-1) | <10 | Tumors | - | [40] |
| CdSe/ZnS | PBA, PEA, PMMA | 2.5–5.0 | Cancer cell | 10–100 | [42] |
| CdSe | TOPO-sugars | 5–50 | Hela cell | - | [44] |
| CdSe/ZnS | FA-PEA-PEG-750 | 13 | KB vs. A549 cells | - | [66] |
| CdSe/ZnS | Amino, carobxy and hydroxy groups PEG | - | Hela cell | - | [67] |
| CdSe/ZnS | PEG- D-mannose, D-galactose, and D-galactosamine | 15–20 | Hepatocellular carcinoma cell line HepG2 | - | [68] |
| QDs | Streptavidin | - | DNA | 4.8 × 10−15 | [69] |
| CdTe | PDADMAC | - | DNA | - | [70] |
| CdSe/ZnS | TOPO-DDA, TOPO-HDA | 3.3 | DNA | <1.0 × 10−9 | [71] |
| CdSe/ZnS and iron oxide NPs | Streptavidin | 30 | DNA | 5.0 × 10−6 | [72] |
| CdSe | TGA | 3–4 | E. coli and S. aureus | 102 CFU/mL | [73] |
| CdSe | BSA | 5 | E. coli (HB101) | - | [74] |
| CdSe/ZnS | TGA-IgG | 6 | Salmonella typhi | 102 cells/mL | [75] |
| CdTe | MAA | ~3.4 | rPrP, E. Coli | 3.0 | [76] |
| CdSe and CdSe/CdS | Citrate | - | cytochrome c, hemoglobin and myoglobin | - | [77] |
| CdSe | Diethanolamine | 20 | BSA | - | [78] |
| CdSe/ZnS | DSPE-PEG-2000 | 3.5–4 | BSA | - | [79] |
| CdTe | TGA-neutral red-BSA | 3.1 | BSA | 1.97 | [80] |
| CdTe | TGA | 2–3 | Chymotrypsin | - | [81] |
| CdTe | MEA | - | HSA | 4.2 | [82] |
| CdTe | MPA, NAC, GSH | - | HSA | - | [83] |
| CdTe | MPA | 2–4.8 | HSA | - | [85] |
| CdTe | MPA, L-Cys, GSH | 3.5 | BSA | - | [86] |
| ZnS | L-Cys | 17 | BSA, HSA, γ-globulin, ovalbumin | 0.06–0.56 | [87] |
| ZnSe | MAA | 25 | BSA | 30.3 | [88] |
| CdSe | SFCA ( n = 4, 6) | - | Methionine and phenylalanine | 3000–4000 | [89] |
4. Semiconductor Nanomaterials-Based MALDI-MS for Biomolecules Analysis
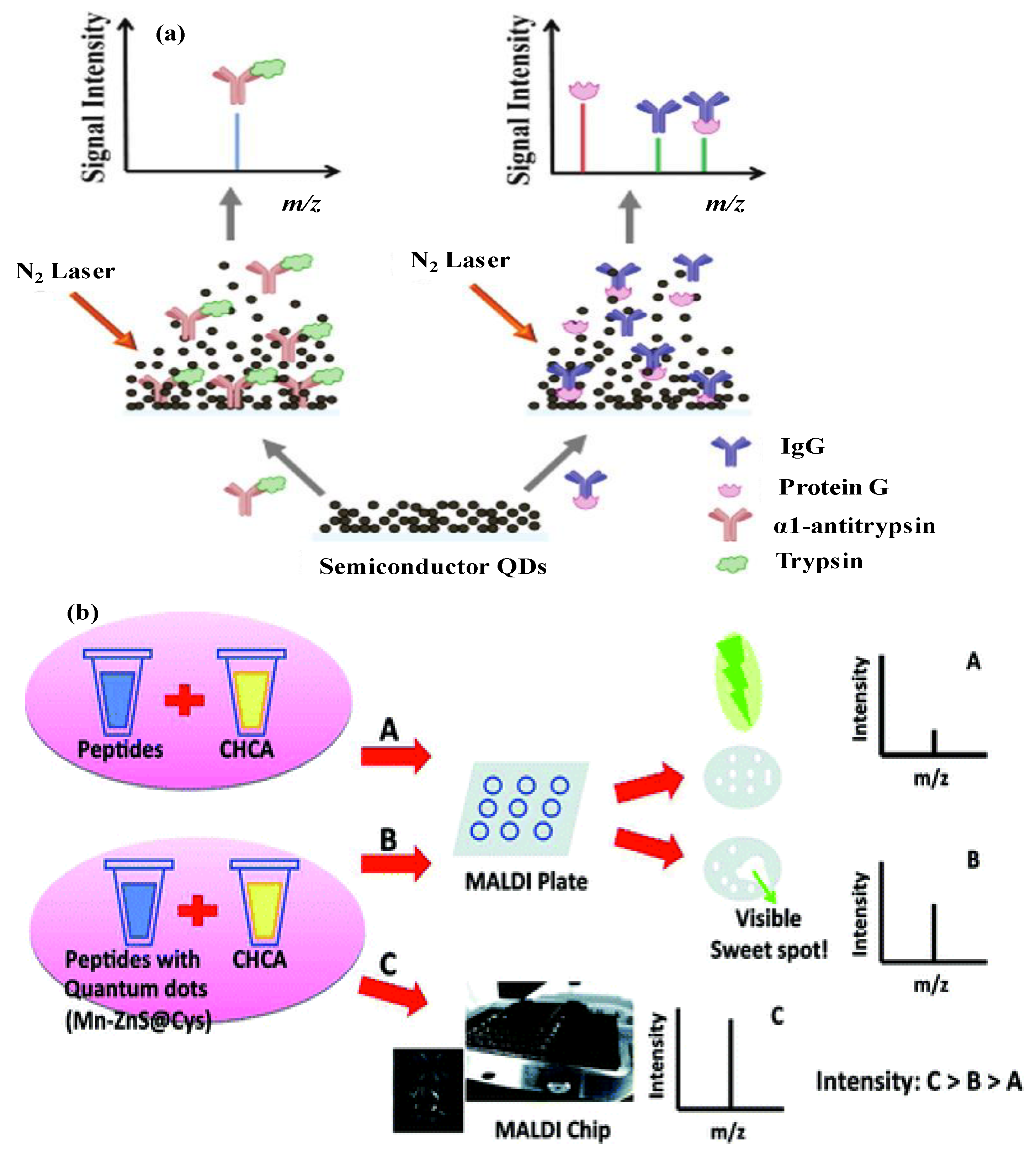
4.1. Metal Sulphide Semiconductor NMs-Based MALDI-MS
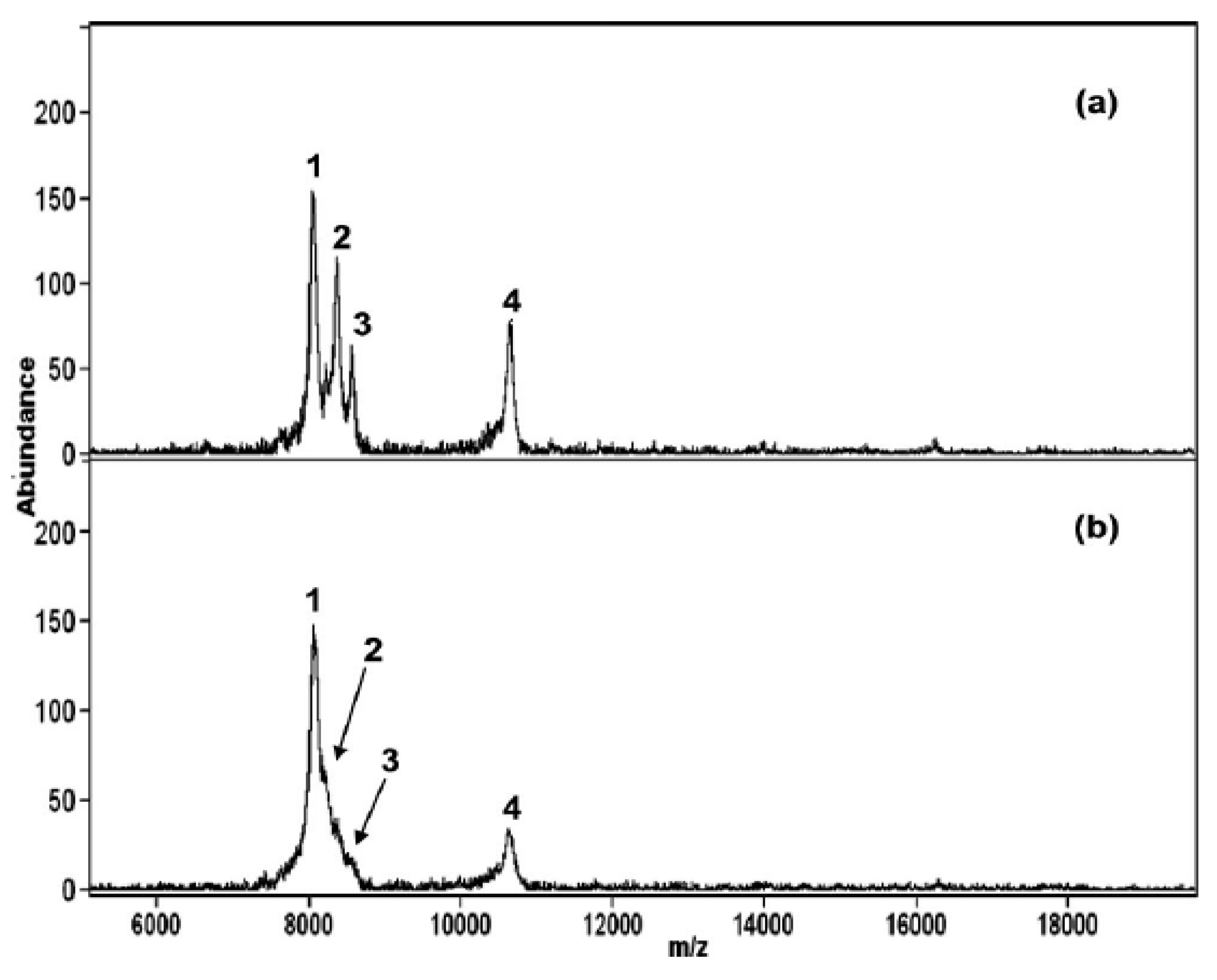

4.2. Metal Selenide, Telluride and Oxide Semiconductor NMs-Based MALDI-MS

4.3. Semiconductor Nanomaterials-Based MALDI-MS for Bacteria Analysis
| Name of semiconductor NPs | Capping ligand | Analytes | Size (nm) | Detection limit | Technique | Reference |
|---|---|---|---|---|---|---|
| ZnS | MPA | Insulin, ubiquitin | - | 85–91 nM | MALDI-MS | [96] |
| ZnS | N3 | Milk and ubiquitin-like proteins | 15 | - | MALDI-MS | [97] |
| CdS | MPA | Peptides and proteins | 5 | - | LDI-MS | [98] |
| CdS | MPA | Digested proteins | 5 | - | ESI-MS | [100] |
| CdS | ATP, MUA | Peptides and proteins | 15–30 | 0.01–63 nM | MALDI-MS | [101] |
| CdSe | MUA | Peptides and proteins | <10 | - | LDI-MS | [102] |
| Cd2+-doped CNTs- CdS NPs | - | Cytochrome c and lysozyme | - | 1–7 nM | AP-MALDI-MS and MALDI-MS | [103] |
| ZnSe | MPA | Leu-enk, Met-enk, HW6, substance P and Angio-II, and proteins (cytochrome c, myoglobin and lysozyme) | <5 | - | MALDI-MS | [104] |
| ZnSe | MPA | Insulin, ubiquitin, cytochrome c, myoglobin and lysozyme | <10 | - | MALDI-MS | [105] |
| Mn2+-ZnS | Cysteine | Peptides and proteins | 5.1 | ~1.0 pM | MALDI MS | [91] |
| HgTe | MPA | Angio- I, Insulin, cytochrome c, BSA, IgG and E. coli | 20 | 0.2–450 nM | SALDI- and MALDI-MS | [90] |
| HgTe | MPA | α1-antitrypsin−trypsin and IgG−protein G complexes, | - | 0.5–3.0 µM | MALDI-MS | [106] |
| CdS:Mn/ZnS | DDTC | Tryptic digests of cytochrome c, lysozyme and BSA | 6 ± 2 | - | MALDI-MS | [107] |
| BaTiO3 | - | Tryptic digests of α- and β- casein and milk proteins | 30 | - | MALDI-MS | [108] |
| SnO2 and TiO2 | PMMA | Myoglobin | 2–8 | 1 nM | SALDI-MS | [109] |
| Au, TiO2, Se, CdTe QDs, Fe3O4, and Pt | - | Glutathione, Angio-I, insulin, cytochrome c and chymotrypsin | - | 140–4400 fM | SALDI-MS | [110] |
| Mg(OH)2 | Oleic acid | Gramicidin D, valinomycin, E. coli | <35 | - | MALDI-MS | [111] |
| ZnO | - | E. coli | - | - | MALDI-MS | [112] |
| ZnO | - | Staphylococcus aureus | - | - | MALDI-MS | [113] |
| CdS | MPA | Extracellular polysaccharides in E. coli | <5 | - | MALDI-MS | [114] |
| TiO2 | - | Staphylococcus aureus | - | - | MALDI-MS | [115] |
| TiO2 | bacteria | Staphylococcus aureus subsp. aureus and Pseudomonas aeruginosa | - | - | MALDI-MS | [116] |
| ZrO2 and ZrO2-SiO2 | - | Leu-enk, Met-enk, HW6, and milk proteins | 20–30 | 75–105 fM | AP-MALDI-MS | [117] |
| TiO2-dopamine and TiO2-CdS | - | Gramicidin D, myoglobin, cytochrome c, α- and β-caseins | 5–20 | 1 nM | MALDI-MS | [118] |
| BaTiO3 | HOA | PLs and Hydrophobic proteins in E. coli | 30–40 | 0.20–0.40 µM | MALDI-MS | [119] |
5. Conclusions and Future Perspectives
Acknowledgements
Conflicts of Interest
References
- Alivisatos, A.P.; Gu, W.; Larabell, C. Quantum dots as cellular probes. Annu. Rev. Biomed. Eng. 2005, 7, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2007, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, B.; Zhang, H.; Wang, Y. The fluorescence bioassay platforms on quantum dots nanoparticles. J. Fluoresc. 2005, 15, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Willard, D.M. Nanoparticles in bioanalytics. Anal. Bioanal. Chem. 2003, 376, 284–286. [Google Scholar] [PubMed]
- Mirzaei, J.; Reznikov, M.; Hegmann, T. Quantum dots as liquid crystal dopants. J. Mater. Chem. 2012, 22, 22350–22365. [Google Scholar] [CrossRef]
- Dorfs, D.; Krahne, R.; Falqui, A.; Manna, L.; Giannini, C.; Zanchet, D. Synthesis and Characterization of Quantum Dots; Andrews, D., Scholes, G., Wiederrecht, G., Eds.; Comprehensive Nanoscience and Technology Elsevier, Academic Press: San Diego, CA, USA, 2011; pp. 219–270. [Google Scholar]
- Rogach, A.L. Fluorescence energy transfer in hybrid structures of semiconductor nanocrystals. Nano Today 2011, 6, 355–365. [Google Scholar] [CrossRef]
- Wise, F.W. Lead salt quantum dots: The limit of strong quantum confinement. Acc. Chem. Res. 2000, 33, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Somers, R.C.; Bawendi, M.G.; Nocera, D.G. CdSe nanocrystal based chem-/bio-sensors. Chem. Soc. Rev. 2007, 36, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Wu, H.F.; Mehta, V. Prospects of engineering quantum dots applications in ultrasensitive assays. In Quantum Dots: Applications, Synthesis and Characterization; Orion, C., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2012; Chapter 5; pp. 69–108. [Google Scholar]
- Henglein, A. Small-particle research—Physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem. Rev. 1989, 89, 1861–1873. [Google Scholar] [CrossRef]
- Wang, Y.; Herron, N. Nanometer-sized semiconductor clusters-materials synthesis, quantum size effects, and photophysical properties. J. Phys. Chem. 1991, 95, 525–532. [Google Scholar] [CrossRef]
- Miniaturization; Gilbert, H.D. (Ed.) Reinhold: New York, NY, USA, 1961; pp. 282–296.
- Talapin, D.V.; Rogach, A.L.; Kornowski, A.; Haase, M.; Weller, H. Highly luminescent monodisperse CdSe and CdSe/ZnS nanocrystals synthesized in a hexadecylamine–trioctylphosphine oxide–trioctylphospine mixture. Nano Lett. 2001, 1, 207–211. [Google Scholar] [CrossRef]
- Franzl, T.; Muller, J.; Klar, T.A.; Rogach, A.L.; Feldmann, J.; Talapin, D.V.; Weller, H. CdSe:Te nanocrystals: Band-edge vs. Te-Related emission. J. Phys. Chem. C 2007, 111, 2974–2979. [Google Scholar] [CrossRef]
- Brus, L.E. Electron–electron and electron‐hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Bawendi, M.G.; Steigerwald, M.L.; Brus, L.E. The quantum mechanics of larger semiconductor clusters (“Quantum Dots”). Annu. Rev. Phys. Chem. 1990, 41, 477–496. [Google Scholar] [CrossRef]
- Brus, L.E. A simple model for the ionization potential, electron affinity, and aqueous redox potentials of small semiconductor crystallites. J. Chem. Phys. 1983, 79, 5566–5571. [Google Scholar] [CrossRef]
- Gaponenko, S.V. Optical Properties of Semiconductor Nanocrystalsl; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Poole, C.P.; Owens, F.J. Introduction to Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Kittel, C. Introduction to Solid State Physics; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Harrison, P. Quantum Well, Wires, and Dots; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Leatherdale, C.A.; Woo, W.K.; Mikulec, F.V.; Bawendi, M.G. On the absorption cross section of CdSe nanocrystal quantum dots. J. Phys. Chem. B 2002, 106, 7619–7622. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Semiconductor nanocrystals: Structure, properties, and band gap engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Pokrant, S.; Whaley, K.B. Tight-binding studies of surface effects on electronic structure of CdSe nanocrystals: The role of organic ligands, surface reconstruction, and inorganic capping shells. Eur. Phys. J. D 1999, 6, 255–267. [Google Scholar] [CrossRef]
- Underwood, D.F.; Kippeny, T.; Rosenthal, S.J. Ultrafast carrier dynamics in CdSe nanocrystals determined by femtosecond fluorescence upconversion spectroscopy. J. Phys. Chem. B 2001, 105, 436–443. [Google Scholar] [CrossRef]
- Murphy, C.J. Peer reviewed: Optical sensing with quantum dots. Anal. Chem. 2002, 74, 520A–526A. [Google Scholar] [CrossRef] [PubMed]
- Clapp, A.R.; Medintz, I.L.; Mattoussi, H. Förster resonance energy transfer investigations using quantum-dot fluorophores. Chem. Phys. Chem. 2006, 7, 47–57. [Google Scholar] [PubMed]
- Tian, Z.; Wu, W.; Li, A.D.Q. Photoswitchable nanoprobes for biological imaging applications. In Trace Analysis with Nanomaterials; Pierce, D.T., Zhao, J.X., Eds.; Springer: Berlin, Germany, 2007; Chapter 1; pp. 3–8. [Google Scholar]
- Banerjee, S.; Kar, S.; Perez, J.M.; Santra, S. Quantum dot-based OFF/ON probe for detection of glutathione. J. Phys. Chem. C 2009, 113, 9659–9666. [Google Scholar] [CrossRef]
- Santra, S.; Yang, H.; Holloway, P.H.; Stanley, J.T.; Mericle, R.A. Synthesis of water-dispersible fluorescent, radio-opaque, and paramagnetic CdS:Mn/ZnS quantum dots: A multifunctional probe for bioimaging. J. Am. Chem. Soc. 2005, 127, 1656–1657. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bao, C.; Zhong, X.; Zhao, C.; Zhu, L. Highly selective detection of glutathione using a quantum-dot-based OFF-ON fluorescent probe. Chem. Commun. 2010, 46, 2971–2973. [Google Scholar] [CrossRef]
- Li, H.; Shih, W.H.; Shih, W.Y. Synthesis and characterization of aqueous carboxyl-capped CdS quantum dots for bioapplications. Ind. Eng. Chem. Res. 2007, 46, 2013–2019. [Google Scholar] [CrossRef]
- Baruah, S.; Ortinero, C.; Shipin, O.V.; Dutta, J. Manganese doped zinc sulfide quantum dots for detection of Escherichia coli. J. Fluoresc. 2012, 22, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Q.; Kang, B.; Dai, Y.D.; Zhang, H.X.; Chen, D. One-step fabrication of biocompatible chitosan-coated ZnS and ZnS:Mn2+ quantum dots via a γ-radiation route. Nanoscale Res. Lett. 2011, 6, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ma, X. The detection application of CdS quantum dots in labeling DNA molecules. Biomed. Mater. 2006, 1, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ma, X. Labeling patterned DNA molecules using CdS fluorescence nanoparticles. J. Nanosci. Nanotechnol. 2009, 9, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Mallapragada, S.K.; Clapp, A.R. Sensing polymer/DNA polyplex dissociation using quantum dot fluorophores. ACS Nano 2011, 25, 129–138. [Google Scholar] [CrossRef]
- Ho, Y.P.; Kung, M.C.; Yang, S.; Wang, T.H. Multiplexed hybridization detection with multicolor colocalization of quantum dot nanoprobes. Nano Lett. 2005, 5, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Akerman, M.E.; Chan, W.C.; Laakkonen, P.; Bhatia, S.N.; Ruoslahti, E. Nanocrystal targeting in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 12617–12621. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.R.; Zipfel, W.R.; Williams, R.M.; Clark, S.W.; Bruchez, M.P.; Wise, F.W.; Webb, W.W. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 2003, 300, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, Y.; Wang, M.; Wu, Z.G.; Huang, B.H. Rapid identification of Staphylococcus aureus directly from positive blood culture media using quantum dots as fluorescence probes. APMIS 201 2013, 121, 348–352. [Google Scholar] [CrossRef]
- Osaki, F.; Kanamori, T.; Sando, S.; Sera, T.; Aoyagi, Y. A quantum dot conjugated sugar ball and its cellular uptake. On the size effects of endocytosis in the subviral region. J. Am. Chem. Soc. 2004, 126, 6520–6521. [Google Scholar] [CrossRef] [PubMed]
- Kaul, Z.; Yaguchi, T.; Kaul, S.C.; Hirano, T.; Wadhwa, R.; Taira, K. Mortalin imaging in normal and cancer cells with quantum dot immuno-conjugates. Cell Res. 2003, 13, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Frasco, M.F.; Chaniotakis, N. Semiconductor quantum dots in chemical sensors and biosensors. Sensors 2009, 9, 7266–7286. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, H. Host-molecule-coated quantum dots as fluorescent sensors. Anal. Bioanal. Chem. 2010, 397, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Mattoussi, H.; Mauro, J.M.; Goldman, E.R.; Anderson, G.P.; Sundar, V.C.; Mikulec, F.V.; Bawendi, M.G. Self-assembly of CdSe-ZnS quantum dot bioconjugates using an engineered recombinant protein. J. Am. Chem. Soc. 2000, 122, 12142–12150. [Google Scholar] [CrossRef]
- Goldman, E.R.; Mattoussi, H.; Anderson, G.P.; Medintz, I.L.; Mauro, J.M. Fluoroimmunoassays using antibody-conjugated quantum dots. Methods Mol. Biol. 2005, 303, 19–34. [Google Scholar] [PubMed]
- Goldman, E.R.; Clapp, A.R.; Anderson, G.P.; Uyeda, H.T.; Mauro, J.M.; Medintz, I.L.; Mattoussi, H. Multiplexed toxin analysis using four colors of quantum dot fluororeagents. Anal. Chem. 2004, 76, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Lao, U.L.; Mulchandani, A.; Chen, W. Simple conjugation and purification of quantum dot-antibody complexes using a thermally responsive elastin-protein L scaffold as immunofluorescent agents. J. Am. Chem. Soc. 2006, 128, 14756–14757. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xiao, Q.; He, Z.K.; Liu, Y.; Tinnefeld, P.; Su, X.R.; Peng, X.N. A high sensitive and specific QDs FRET bioprobe for MNase. Chem. Commun. 2008, 5990–5992. [Google Scholar]
- Medintz, I.L.; Clapp, A.R.; Brunel, F.M.; Tiefenbrunn, T.; Uyeda, H.T.; Chang, E.L.; Deschamps, J.R.; Dawson, P.E.; Mattoussi, H. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nat. Mater. 2006, 5, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Prasuhn, D.E.; Feltz, A.; Blanco-Canosa, J.B.; Susumu, K.; Stewart, M.H.; Mei, B.C.; Yakovlev, A.V.; Loukov, C.; Mallet, J.M.; Oheim, M.; et al. Quantum dot peptide biosensors for monitoring caspase 3 proteolysis and calcium ions. ACS Nano 2010, 4, 5487–5497. [Google Scholar] [CrossRef] [PubMed]
- Boeneman, K.; Mei, B.C.; Dennis, A.M.; Bao, G.; Deschamps, J.R.; Mattoussi, H.; Medintz, I.L. Sensing caspase 3 activity with quantum dot-fluorescent protein assemblies. J. Am. Chem. Soc. 2009, 131, 3828–3829. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, N.; Geissler, D. Semiconductor quantum dots as FRET acceptors for multiplexed diagnostics and molecular ruler application. Adv. Exp. Med. Biol. 2012, 733, 75–86. [Google Scholar] [PubMed]
- Algar, W.R.; Wegner, D.; Huston, A.L.; Blanco-Canosa, J.B.; Stewart, M.H.; Armstrong, A.; Dawson, P.E.; Hildebrandt, N.; Medintz, I.L. Quantum dots as simultaneous acceptors and donors in time-gated Förster resonance energy transfer relays: Characterization and biosensing. J. Am. Chem. Soc. 2012, 134, 1876–1891. [Google Scholar] [CrossRef] [PubMed]
- Morgner, F.; Geissler, D.; Stufler, S.; Butlin, N.G.; Löhmannsröben, H.G.; Hildebrandt, N. A quantum-dot-based molecular ruler for multiplexed optical analysis. Angew. Chem. Int. Ed. Engl. 2010, 49, 7570–7574. [Google Scholar] [CrossRef] [PubMed]
- Morgner, F.; Stufler, S.; Geissler, D.; Medintz, I.L.; Algar, W.R.; Susumu, K.; Stewart, M.H.; Blanco-Canosa, J.B.; Dawson, P.E.; Hildebrandt, N. Terbium to quantum dot FRET bioconjugates for clinical diagnostics: Influence of human plasma on optical and assembly properties. Sensors 2011, 11, 9667–9684. [Google Scholar] [CrossRef] [PubMed]
- Geissler, D.; Charbonnière, L.J.; Ziessel, R.F.; Butlin, N.G.; Löhmannsröben, H.G.; Hildebrandt, N. Quantum dot biosensors for ultrasensitive multiplexed diagnostics. Angew. Chem. Int. Ed. Engl. 2010, 49, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Chen, K.H.; Strano, M.S. Aptamer-capped nanocrystal quantum dots: A new method for label-free protein detection. J. Am. Chem. Soc. 2006, 128, 15584–15585. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Cater, S.F.; Ellington, A.D. Quantum-dot aptamer beacons for the detection of proteins. ChemBioChem 2005, 6, 2163–2166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; So, M.-K.; Loening, A.M.; Yao, H.; Gambhir, S.S.; Rao, J. HaloTag protein-mediated site-specific conjugation of bioluminescent proteins to quantum dots. Angew. Chem. Int. Ed. 2006, 45, 4936–4940. [Google Scholar] [CrossRef]
- Xia, Z.; Xing, Y.; So, M.-K.; Koh, A.L.; Sinclair, R.; Rao, J. Multiplex detection of protease activity with quantum dot nanosensors prepared by intein-mediated specific bioconjugation. Anal. Chem. 2008, 80, 8649–8655. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, X.; Li, Y.; Shi, Y.; Su, X. Multicolor quantum dot-encoded microspheres for the detection of biomolecules. Talanta 2007, 72, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Song, E.Q.; Zhang, Z.L.; Luo, Q.Y.; Lu, W.; Shi, Y.B.; Pang, D.W. Tumor cell targeting using folate-conjugated fluorescent quantum dots and receptor-mediated endocytosis. Clin. Chem. 2009, 55, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Howarth, M.; Greytak, A.B.; Zheng, Y.; Nocera, D.G.; Ting, A.Y.; Bawendi, M.G. Compact biocompatible quantum dots functionalized for cellular imaging. J. Am. Chem. Soc. 2008, 130, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Kikkeri, R.; Lepenies, B.; Adibekian, A.; Laurino, P.; Seeberger, P.H. In vitro imaging and in vivo liver targeting with carbohydrate capped quantum dots. J. Am. Chem. Soc. 2009, 131, 2110–2112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Yeh, C.H.; Kuroki, M.; Wang, T.H. Single-quantum-dot-based DNA nanosensor. Nat. Mater. 2005, 4, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, L.; Kjallman, T.H.M.; Soeller, C.; Sejdic, J.T. DNA hybridization detection with blue luminescent quantum dots and dye-labeled single-stranded DNA. J. Am. Chem. Soc. 2007, 129, 3048–3049. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Cupps, J.M.; Fan, X.D. Compact quantum dot probes for rapid and sensitive DNA detection using highly efficient fluorescence resonant energy transfer. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, B.C.; Lee, J.H.; Kim, J.B.; Gu, M.B. Specific detection of DNA using quantum dots and magnetic beads for large volume samples. Biotechnol. Bioprocess Eng. 2006, 11, 449–454. [Google Scholar] [CrossRef]
- Xue, X.; Pan, J.; Xie, H.; Wang, J.; Zhang, S. Fluorescence detection of total count of Escherichia coli and Staphylococcus aureus on water-soluble CdSe quantum dots coupled with bacteria. Talanta 2009, 77, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ye, F.; Fang, T.; Niu, W.; Liu, P.; Min, X.; Li, X. Bovine serum albumin-directed synthesis of biocompatible CdSe quantum dots and bacteria labeling. J. Colloid Interface Sci. 2011, 355, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Jackeray, R.; Abid, C.K.V.Z.; Singh, G.; Jain, S.; Chattopadhyaya, S.; Sapra, S.; Shrivastav, T.G.; Singh, H. Selective capturing and detection of Salmonella typhi on polycarbonate membrane using bioconjugated quantum dots. Talanta 2011, 84, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Zheng, H.Z.; Long, Y.J.; Huang, C.Z.; Hao, J.Y.; Zhou, D.B. CdTe quantum dots as a highly selective probe for prion protein detection: Colorimetric qualitative, semi-quantitative and quantitative detection. Talanta 2011, 83, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bai, H.; Yang, C.; Yang, X. A sensitive method for the detection of proteins by high-efficiency fluorescence quenching. Analyst 2005, 130, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Ju, P.; Fan, H.; Liu, T.; Cui, L.; Ai, S. Probing the interaction of flower-like CdSe nanostructure particles targeted to bovine serum albumin using spectroscopic techniques. J. Lumin. 2011, 131, 1724–1730. [Google Scholar] [CrossRef]
- Depalo, N.; Mallardi, A.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Curri, M.L. Bovine serum albumin-directed synthesis of biocompatible CdSe quantum dots and bacteria labeling. J. Colloid Interface Sci. 2008, 325, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Lu, J.; Yan, M.; Yu, F.; Yu, J.; Sun, X. Fluorescence resonance energy transfer sensor between quantum dot donors and neutral red acceptors and its detection of BSA in micelles. Dye. Pigment. 2011, 91, 304–308. [Google Scholar] [CrossRef]
- Peng, J.; Liu, S.; Yan, S.; Fan, X.; He, Y. A study on the interaction between CdTe quantum dots and chymotrypsin using optical spectroscopy. Colloids Surf. A 2010, 359, 13–17. [Google Scholar] [CrossRef]
- He, Y.; Yin, P.; Gong, H.; Peng, J.; Liu, S.; Fan, X.; Yan, S. Characterization of the interaction between mercaptoethylamine capped CdTe quantum dots with human serum albumin and its analytical application. Sens. Actuators B 2011, 157, 8–13. [Google Scholar] [CrossRef]
- Lai, L.; Lin, C.; Xu, Z.Q.; Han, X.L.; Tian, F.F.; Mei, P.; Li, D.W.; Ge, Y.S.; Jiang, F.L.; Zhang, Y.Z.; et al. Spectroscopic studies on the interactions between CdTe quantum dots coated with different ligands and human serum albumin. Spectrochim. Acta A 2012, 97, 366–376. [Google Scholar] [CrossRef]
- Xiao, J.; Bai, Y.; Wang, Y.; Chen, J.; Wei, X. Systematic investigation of the influence of CdTe QDs size on the toxic interaction with human serum albumin by fluorescence quenching method. Spectrochim. Acta A 2010, 76, 93–97. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, S.; Su, W.; Li, P.; Ma, J.; Luo, F.; Chen, J.; Liu, Y. Systematically investigations of conformation and thermodynamics of HSA adsorbed to different sizes of CdTe quantum dots. Colloids Surf. B 2013, 102, 76–82. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Zhou, X.; Fang, T.; Liu, P.; Liu, P.; Min, X.; Li, X. Interaction of different thiol-capped CdTe quantum dots with bovine serum albumin. J. Lumin. 2012, 132, 1695–1700. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Zhao, D.H.; Chen, J.L.; Li, Y.X.; Wang, L.Y.; Wang, L.; Zhou, Y.Y.; Zhuo, S.J.; Wu, Y.Q. Application of L-cysteine-capped nano-ZnS as a fluorescence probe for the determination of proteins. Anal. Bioanal. Chem. 2004, 378, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, F.; Ding, Y.; Wang, Y.; Zhang, L. Synthesis of functionalized ZnSe nanoparticles and their applications in the determination of bovine serum albumin. J. Fluoresc. 2009, 19, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, J.; Li, F.; Li, H. Synthesis of water-soluble CdSe quantum dots by ligand exchange with p-sulfonatocalix(n)arene (n = 4, 6) as fluorescent probes for amino acids. Nanotechnology 2008, 19, 205501–205508. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Yang, Z.; Lin, Y.W.; Chen, W.T.; Lin, H.J.; Chang, H.T. Detection of proteins and protein-ligand complexes using HgTe nanostructure matrixes in surface-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2010, 82, 4543–4550. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Chien, M.W.; Chen, G.F.; Chen, S.Y.; Yu, C.S.; Liao, M.Y.; Lai, C.C.C. Quantum dots enhance peptide detection by matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2011, 83, 6593–6600. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Waki, H.; Ido, Y.; Akita, S.; Yoshida, Y.; Yoshida, T. Protein and polymer analyses up to m/z 100 000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1988, 2, 151–153. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Rotello, V.M.; Vachet, R.W. Engineered nanoparticle surfaces for improved mass spectrometric analyses. Analyst 2009, 134, 2183–2188. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Chen, W.T.; Chang, H.T. Nanoparticle-based mass spectrometry for the analysis of biomolecules. Chem. Soc. Rev. 2011, 40, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.F.; Gopal, J.; Abdelhamid, H.N.; Hasan, N. Quantum dot applications endowing novelty to analytical proteomics. Proteomics 2012, 19–20, 2949–2961. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Kiran, K.; Wu, H.F. Comparison of ZnS semiconductor nanoparticles capped with various functional groups as the matrix and affinity probes for rapid analysis of cyclodextrins and proteins in surface-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 2008, 80, 9681–9688. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.F.; Kailasa, S.K.; Shastri, L. Electrostatically self-assembled azides on zinc sulfide nanoparticles as multifunctional nanoprobes for peptide and protein analysis in MALDI-TOF MS. Talanta 2010, 82, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Wu, H.F. Interference free detection for small molecules: Probing the Mn2+-doped effect and cysteine capped effect on the ZnS nanoparticles for coccidiostats and peptide analysis in SALDI-TOF MS. Analyst 2010, 135, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Kailasa, S.K.; Wu, H.F.; Chen, Z.Y. High resolution detection of high mass proteins up to 80,000 Da via multifunctional CdS quantum dots in laser desorption/ionization mass spectrometry. Talanta 2010, 83, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Kailasa, S.K.; Wu, H.F. Quantum dots—Electrospray ionization mass spectrometry: 3-Mercaptopropanic acid capped CdS quantum dots as accelerating and enrichment probes for microwave tryptic digestion of proteins. Rapid Commun. Mass Spectrom. 2009, 23, 3603–3607. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Wu, H.F. Semiconductor cadmium sulphide nanoparticles as matrices for peptides and as co-matrices for the analysis of large proteins in matrix-assisted laser desorption/ionization reflectron and linear time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Kailasa, S.K.; Wu, H.F. Quantum dots laser desorption/ionization MS: Multifunctional CdSe quantum dots as the matrix, concentrating probes and acceleration for microwave enzymatic digestion for peptide analysis and high resolution detection of proteins in a linear MALDI-TOF MS. Proteomics 2009, 9, 2656–2667. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Wu, H.F. Multifunctional nanoparticles composite for MALDI-MS: Cd2+-doped carbon nanotubes with CdS nanoparticles as the matrix, preconcentrating and accelerating probes of microwave enzymatic digestion of peptides and proteins for direct MALDI-MS analysis. J. Mass Spectrom. 2010, 45, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.F.; Chung, F.T. 3-Mercaptopropionic acid modified ZnSe quantum dots as the matrix for direct surface-assisted laser desorption/ionization mass spectrometric analysis of peptides/proteins from sodium salt solution. Rapid Commun. Mass Spectrom. 2011, 25, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Shastri, L.; Kailasa, S.K.; Wu, H.F. Cysteine-capped ZnSe quantum dots as affinity and accelerating probes for microwave enzymatic digestion of proteins via direct matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis. Rapid Commun. Mass Spectrom. 2009, 23, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Chiang, C.K.; Lee, C.H.; Chang, H.T. Using surface-assisted laser desorption/ionization mass spectrometry to detect proteins and protein–protein complexes. Anal. Chem. 2012, 84, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Wu, H.F. Functionalized quantum dots modified with dopamine dithiocarbamate as the matrix for quantification of efavirenz in human plasma and as affinity probes for rapid identification of microwave tryptic digested proteins in MALDI-TOF-MS. J. Proteomics 2012, 75, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Wu, H.F. Rapid enrichment of phosphopeptides by BaTiO3 nanoparticles after microwave-assisted tryptic digest of phosphoproteins, and their identification by MALDI-MS. Microchim. Acta 2012, 179, 83–90. [Google Scholar] [CrossRef]
- Xiong, H.M.; Guan, X.Y.; Jin, L.H.; Shen, W.W.; Lu, H.J.; Xia, Y.Y. Surfactant-free synthesis of SnO2@PMMA and TiO2@PMMA core–shell nanobeads designed for peptide/protein enrichment and MALDI-TOF MS analysis. Angew. Chem. Int. Ed. 2008, 47, 4204–4207. [Google Scholar] [CrossRef]
- Chiang, C.K.; Chiang, N.C.; Lin, Z.H.; Lan, G.Y.; Lin, Y.W.; Chang, H.T. Nanomaterial-based surface-assisted laser desorption/ionization mass spectrometry of peptides and proteins. J. Am. Soc. Mass Spectrom. 2010, 21, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Wu, H.F. Dispersive liquid-liquid microextraction using functionalized Mg(OH)2 NPs with oleic acid as hydrophobic affinity probes for the analysis of hydrophobic proteins in bacteria by MALDI-MS. Analyst 2012, 137, 4490–4496. [Google Scholar] [CrossRef] [PubMed]
- Gopal, J.; Wu, H.F.; Lee, C.H. Matrix assisted laser desorption ionization time of flight mass spectrometry as a rapid and reliable technique for directly evaluating bactericidal activity: Probing the critical concentration of ZnO nanoparticles as affinity probes. Anal. Chem. 2010, 82, 9617–9621. [Google Scholar] [CrossRef] [PubMed]
- Gopal, J.; Wu, H.F.; Lee, C.H.; Manikandan, M. Tracing the pathogen staphylococcus aureus on laboratory ants using physical preconcentration coupled ZnO nanoparticle assisted MALDI-TOF MS. Analyst 2012, 137, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Gopal, J.; Wu, H.F.; Gedda, G. Quantifying the degradation of extracellular polysaccharides of Escherichia coli by CdS quantum dots. J. Mater. Chem. 2011, 21, 13445–13451. [Google Scholar] [CrossRef]
- Gopal, J.; Narayana, J.; Wu, H.F. TiO2 nanoparticle assisted mass spectrometry as biosensor of Staphylococcus aureus, key pathogen in nosocomial infections from air, skin surface and human nasal passage. Biosens. Bioelectron. 2011, 27, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Gopal, J.; Wu, H.F. Fabrication of titanium based MALDI bacterial chip for rapid, sensitive and direct analysis of pathogenic bacteria. Biosens. Bioelectron. 2013, 39, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Wu, H.F. Multifunctional ZrO2 nanoparticles and ZrO2@SiO2 nanorods for improved MALDI-MS analysis of cyclodextrin, peptide and phosphoproteins. Anal. Bioanal. Chem. 2010, 396, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Kailasa, S.K.; Wu, H.F.; Nawaz, M. Surface-modified TiO2 nanoparticles as affinity probes and as matrices for the rapid analysis of phosphopeptides and proteins in MALDI-TOF-MS. J. Sep. Sci. 2010, 33, 3400–3408. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Wu, H.F. Surface modified BaTiO3 nanoparticles as the matrix for phospholipids and as extracting probes for LLME of hydrophobic proteins in Escherichia coli by MALDI-MS. Talanta 2013, 114, 283–290. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kailasa, S.K.; Cheng, K.-H.; Wu, H.-F. Semiconductor Nanomaterials-Based Fluorescence Spectroscopic and Matrix-Assisted Laser Desorption/Ionization (MALDI) Mass Spectrometric Approaches to Proteome Analysis. Materials 2013, 6, 5763-5795. https://doi.org/10.3390/ma6125763
Kailasa SK, Cheng K-H, Wu H-F. Semiconductor Nanomaterials-Based Fluorescence Spectroscopic and Matrix-Assisted Laser Desorption/Ionization (MALDI) Mass Spectrometric Approaches to Proteome Analysis. Materials. 2013; 6(12):5763-5795. https://doi.org/10.3390/ma6125763
Chicago/Turabian StyleKailasa, Suresh Kumar, Kuang-Hung Cheng, and Hui-Fen Wu. 2013. "Semiconductor Nanomaterials-Based Fluorescence Spectroscopic and Matrix-Assisted Laser Desorption/Ionization (MALDI) Mass Spectrometric Approaches to Proteome Analysis" Materials 6, no. 12: 5763-5795. https://doi.org/10.3390/ma6125763




