Thousand Cankers Disease Complex: A Forest Health Issue that Threatens Juglans Species across the U.S.
Abstract
:1. Introduction
2. The Thousand Cankers Disease Complex
3. The Host Plant Species
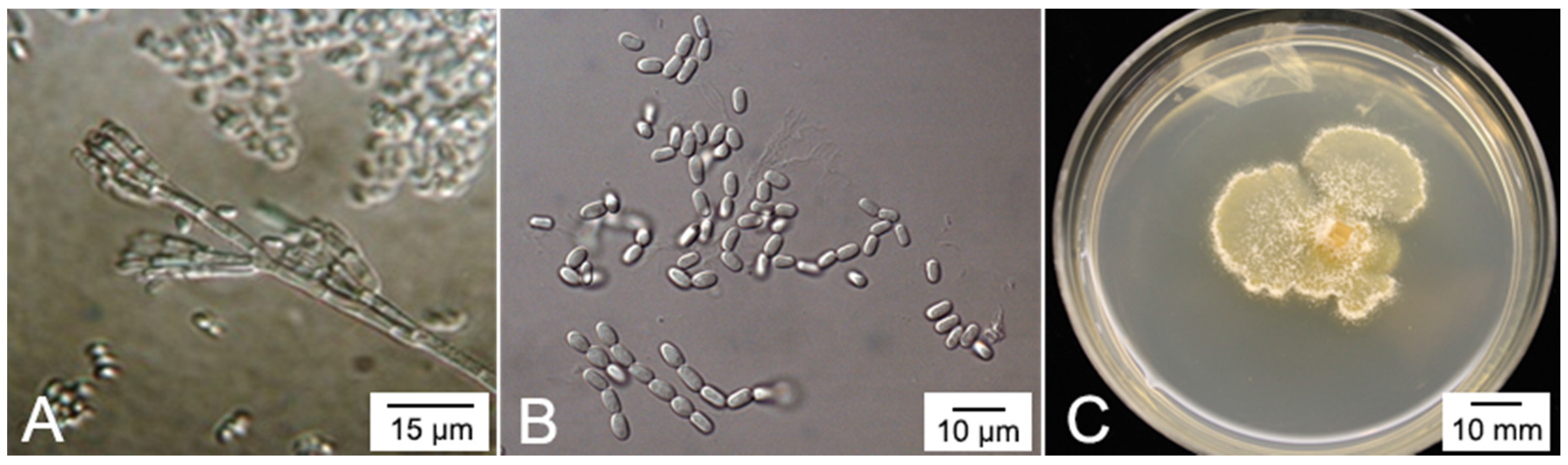
4. The Plant Pathogen
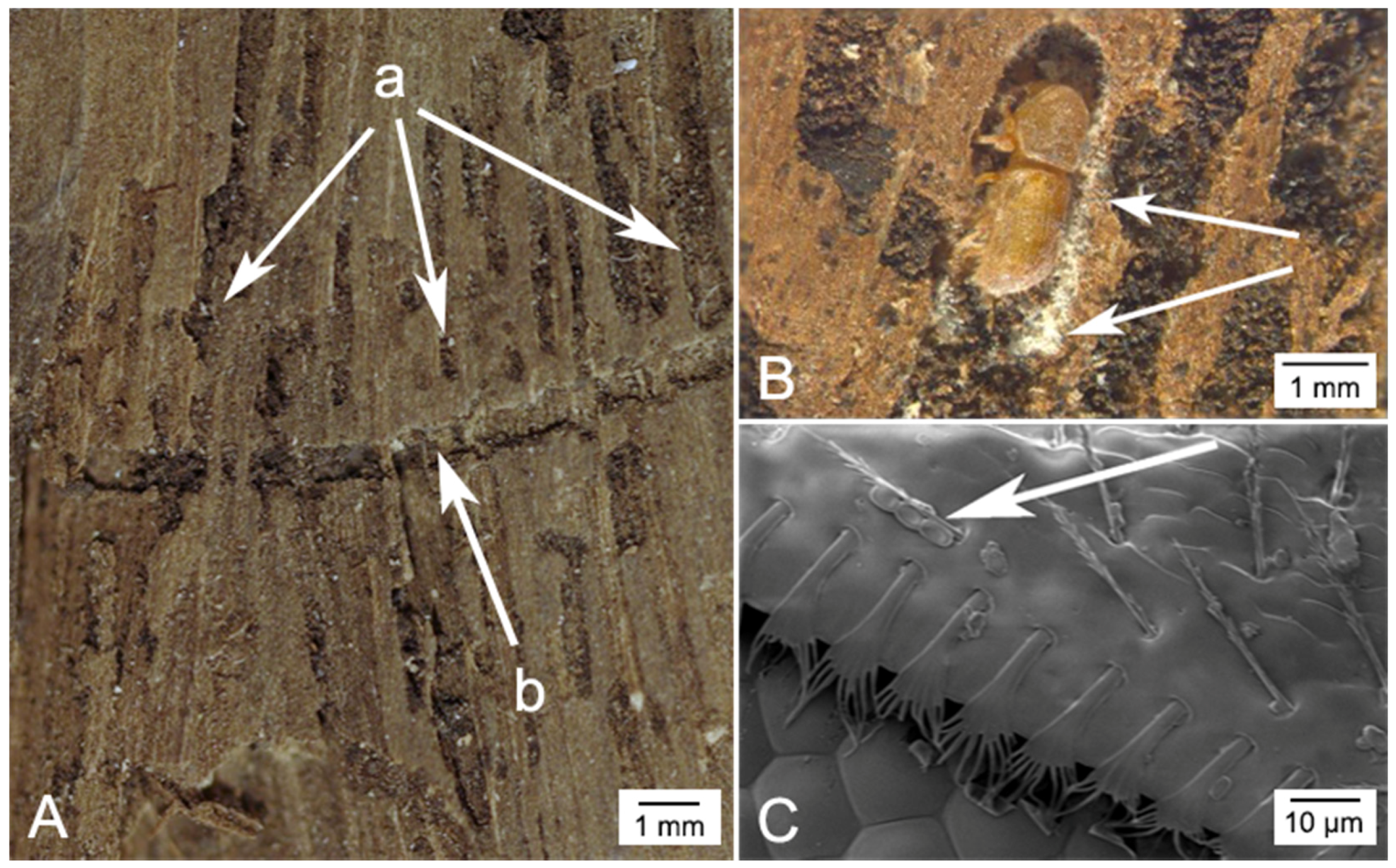
5. The Principle Vector
6. Alternative and Secondary Pathogens and Pathogen Vectors
7. Impact/Influence of Natural Enemies
8. Forest Health Implications
9. Control Measures
- Increased training, both for Extension agents who can inform and encourage citizen participation in eastern black walnut protection, and for professional foresters/arborists who can take steps to protect eastern black walnut in their managed forest communities;
- Increased industry and government oversight that includes directed enforcement of quarantines to limit walnut wood movement outside of containment areas;
- Additional research, with emphases focusing on optimized trapping protocols for P. juglandis and exploitation and enhancement of natural enemy predators and parasitoids across the range of J. nigra;
- Greater understanding of the synergistic pathosystem between F. solani and G. morbida elucidating host-pathogen interactions occurring via this disease complex;
- Genome-enabled research approaches focused on G. morbida that are expected to provide a better understanding of the biology of the pathogens involved in TCD and will help identify candidate genes and functions required in pathogenesis.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kolarík, M.; Freeland, E.; Utley, C.; Tisserat, N. Geosmithia morbida sp. nov., a new phytopathogenic species living in symbiosis with the walnut twig beetle (Pityophthorus juglandis) on Juglans in USA. Mycologia 2011, 103, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Tisserat, N.; Cranshaw, W.; Leatherman, D.; Utley, C.; Alexander, K. Black walnut mortality in Colorado caused by the walnut twig beetle and thousand cankers disease. Plant Health Prog. Publ. 2009. [Google Scholar] [CrossRef]
- Zerillo, M.M.; Caballero, J.I.; Woeste, K.; Graves, A.D.; Hartel, C.; Pscheidt, J.W.; Tonos, J.; Broders, K.; Cranshaw, W.; Seybold, S.J.; et al. Population Structure of Geosmithia morbida, the Causal Agent of Thousand Cankers Disease of Walnut Trees in the United States. PLoS ONE 2014, 9, e0112847. [Google Scholar] [CrossRef] [PubMed]
- Warmund, M.; van Sambeek, J. Thousand cankers disease: Geosmithia morbida spores isolated from a weevil. Mo. Environ. Gard. 2014, 20, 3. Available online: http://www.treesearch.fs.fed.us/pubs/46063 (accessed on 12 August 2016). [Google Scholar]
- Juzwik, J.; Banik, M.T.; Reed, S.E.; English, J.T.; Ginzel, M.D. Geosmithia morbida found on Weevil Species Stenomimus pallidus in Indiana. Plant Health Prog. 2015, 16, 7–10. [Google Scholar] [CrossRef]
- Tisserat, N.; Cranshaw, W.; Putnam, M.L.; Pscheidt, J.; Leslie, C.A.; Murray, M.; Hoffman, J.; Barkley, Y.; Alexander, K.; Seybold, S.J.; et al. Thousand Cankers Disease Is Widespread in Black Walnut in the Western United States. Plant Health Prog. 2011, 10, 1094. [Google Scholar] [CrossRef]
- Utley, C.; Nguyen, T.; Roubtsova, T.; Coggeshall, M.; Ford, T.M.; Grauke, L.J.; Graves, A.D.; Leslie, C.A.; McKenna, J.; Woeste, K.; et al. Susceptibility of Walnut and Hickory Species to Geosmithia morbida. Plant Dis. 2013, 97, 601–607. [Google Scholar] [CrossRef]
- Grant, J.F.; Windham, M.T.; Haun, W.G.; Wiggins, G.J.; Lambdin, P.L. Initial assessment of Thousand Cankers Disease on Black walnut, Juglans nigra, in eastern Tennessee. Forests 2011, 2, 741–748. [Google Scholar] [CrossRef]
- Thousand Cankers Disease. Available online: http://www.agriculture.pa.gov/Protect/PlantIndustry/TCD/Pages/default.aspx (accessed on 12 August 2016).
- Hansen, M.A.; Bush, E.; Day, E.; Griffin, G.; Dart, N. Walnut Thousand Cankers Disease Alert. Available online: http://www.vdacs.virginia.gov/pdf/techpestalert.pdf (accessed on 12 August 2016).
- Hadziabdic, D.; Windham, M.; Baird, R.; Vito, L.; Cheng, Q.; Grant, J.; Lambdin, P.; Wiggins, G.; Windham, A.; Merten, P.; et al. First Report of Geosmithia morbida in North Carolina: The Pathogen Involved in Thousand Cankers Disease of Black walnut. Plant Dis. 2013, 98, 992. [Google Scholar] [CrossRef]
- Fisher, J.R.; McCann, D.P.; Taylor, N.J. Geosmithia morbida, thousand cankers disease of black walnut pathogen, was found for the first time in southwestern Ohio. Plant Health Prog. 2013. [Google Scholar] [CrossRef]
- Juzwik, J.; McDermott-Kubeczko, M.; Stewart, T.J.; Ginzel, M.D. First Report of Geosmithia morbida on Ambrosia Beetles Emerged from Thousand Cankers-diseased Juglans nigra in Ohio. Plant Dis. 2016, 100, 1238. [Google Scholar] [CrossRef]
- Walnut Twig Beetle and Thousand Cankers Disease. Available online: http://mda.maryland.gov/plants-pests/Pages/tcd.aspx (accessed on 15 August 2016).
- Ginzel, M.; Juzwik, J. Geosmithia morbida, the Causal Agent of Thousand Cankers Disease, Found in Indiana. Available online: http://www.treesearch.fs.fed.us/pubs/46047 (accessed on 25 July 2016).
- Montecchio, L.; Faccoli, M. First record of Thousand Cankers Disease Geosmithia morbida and walnut twig beetle Pityophthorus juglandis on Juglans nigra in Europe. Plant Dis. 2014, 98, 696. [Google Scholar] [CrossRef]
- Montecchio, L.; Faccoli, M.; Short, D.; Fanchin, G.; Geiser, D.; Kasson, M.T. First Report of Fusarium solani phylogenetic species 25 associated with early stages of Thousand Cankers Disease on Juglans nigra and Juglans regia in Italy. Plant Dis. 2015, 99, 1183. [Google Scholar] [CrossRef]
- Cranshaw, W.; Tisserat, N. Pest Alert Walnut Twig Beetle and Thousand Cankers Disease of Black walnut. Colo. State Univ. 2010. Available online: http://extension.colostate.edu/docs/pubs/insect/1008_alert.pdf (accessed on 29 July 2016).
- Griffin, G.J. Status of thousand cankers disease on eastern black walnut in the eastern United States at two locations over 3 years. For. Pathol. 2015, 45, 203–214. [Google Scholar] [CrossRef]
- Hadziabdic, D.; Wadl, P.A.; Vito, L.M.; Boggess, S.L.; Scheffler, B.E.; Windham, M.T.; Trigiano, R.N. Development and characterization of sixteen microsatellite loci for Geosmithia morbida, the causal agent of thousand canker disease in black walnut (Juglans nigra). Conserv. Genet. Resour. 2012, 4, 287–289. [Google Scholar] [CrossRef]
- Hadziabdic, D.; Wadl, P.A.; Staton, M.E.; Klingeman, W.E.; Moulton, J.K.; Pscheidt, J.W.; Wiggins, G.J.; Grant, J.F.; Lambdin, P.L.; Windham, M.T.; et al. Development of microsatellite loci in Pityophthorus juglandis, a vector of thousand cankers disease in Juglans spp. Conserv. Genet. Resour. 2015, 7, 431–433. [Google Scholar] [CrossRef]
- Stone, D.E.; Oh, S.-H.; Tripp, E.A.; Rios G, L.E.; Manos, P.S. Natural history, distribution, phylogenetic relationships, and conservation of Central American black walnuts (Juglans sect. Rhysocaryon). J. Torrey Bot. Soc. 2009, 136, 1–25. [Google Scholar] [CrossRef]
- Rugman-Jones, P.F.; Seybold, S.J.; Graves, A.D.; Stouthamer, R. Phylogeography of the Walnut Twig Beetle, Pityophthorus juglandis, the Vector of Thousand Cankers Disease in North American Walnut Trees. PLoS ONE 2015, 10, e0118264. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.D. Juglans nigra L.; black walnut. In Silvics of North America, 2nd ed.Burns, R.M., Honkala, B.H., Eds.; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 2, pp. 391–399. [Google Scholar]
- Randolph, K.C.; Rose, A.K.; Oswalt, C.M.; Brown, M.J. Status of black walnut (Juglans nigra L.) in the eastern United States in light of the discovery of thousand cankers disease. Castanea 2013, 78, 2–14. [Google Scholar] [CrossRef]
- Victory, E.R.; Glaubitz, J.C.; Rhodes, O.E., Jr.; Woeste, K.E. Genetic homogeneity in Juglans nigra (Juglandaceae) at nuclear microsatellites. Am. J. Bot. 2006, 93, 118–126. [Google Scholar] [CrossRef]
- Boraks, A.; Broders, K.D. Population genetic diversity of the rare hardwood butternut (Juglans cinerea) in the northeastern USA. Tree Genet. Genomes 2016, 12, 43. [Google Scholar] [CrossRef]
- Parks, A.; Jenkins, M.; Ostry, M.; Zhao, P.; Woeste, K. Biotic and abiotic factors affecting the genetic structure and diversity of butternut in the southern Appalachian Mountains, USA. Tree Genet. Genomes 2014, 10, 541–554. [Google Scholar] [CrossRef]
- Frankham, R. Conservation genetics. Annu. Rev. Genet. 1995, 29, 305–327. [Google Scholar] [CrossRef] [PubMed]
- Hoban, S.M.; Borkowski, D.S.; Brosi, S.L.; McCleary, T.S.; Thompson, L.M.; McLachlan, J.S.; Pereira, M.A.; Schlarbaum, S.E.; Romero-Severson, J. Range-wide distribution of genetic diversity in the North American tree Juglans cinerea: A product of range shifts, not ecological marginality or recent population decline. Mol. Ecol. 2010, 19, 4876–4891. [Google Scholar] [CrossRef] [PubMed]
- Newton, L.; Fowler, G.; Neeley, A.; Schall, R.; Takeuchi, Y. Pathway Assessment: Geosmithia sp. and Pityophthorus juglandis Blackman movement from the western into the eastern United States. Available online: http://agriculture.mo.gov/plants/pdf/tc_pathwayanalysis.pdf (accessed on 29 July 2016).
- Voulgaridis, V.; Vassiliou, V.G. The Walnut Wood and its Utilisation to High Value Products. In V International Walnut Symposium 705; International Society for Horticultural Science: Leuven, Belgium, 2005; pp. 69–81. [Google Scholar] [CrossRef]
- Hishinuma, S.M.; Dallara, P.L.; Yaghmour, M.A.; Zerillo, M.M.; Parker, C.M.; Roubtsova, T.V.; Nguyen, T.L.; Tisserat, N.A.; Bostock, R.M.; Flint, M.L.; et al. Wingnut (Juglandaceae) as a new generic host for Pityophthorus juglandis (Coleoptera: Curculionidae) and the thousand cankers disease pathogen, Geosmithia morbida (Ascomycota: Hypocreales). Can. Entomol. 2016, 148, 83–91. [Google Scholar] [CrossRef]
- Lynch, S.C.; Wang, D.H.; Mayorquin, J.S.; Rugman-Jones, P.F.; Stouthamer, R.; Eskalen, A. First Report of Geosmithia pallida Causing Foamy Bark Canker, a New Disease on Coast Live Oak (Quercus agrifolia), in Association with Pseudopityophthorus pubipennis in California. Plant Dis. 2014, 98, 1276. [Google Scholar] [CrossRef]
- Schuelke, T.A.; Westbrook, A.; Broders, K.; Woeste, K.; MacManes, M.D. De novo genome assembly of Geosmithia morbida, the causal agent of thousand cankers disease. PeerJ 2016, 4, e1952. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, C.A.; Güldener, U.; Xu, J.-R.; Trail, F.; Turgeon, B.G.; Pietro, A.D.; Walton, J.D.; Ma, L.-J.; Baker, S.E.; Rep, M.; et al. The Fusarium graminearum Genome Reveals a Link Between Localized Polymorphism and Pathogen Specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Kämper, J.; Kahmann, R.; Bölker, M.; Ma, L.-J.; Brefort, T.; Saville, B.J.; Banuett, F.; Kronstad, J.W.; Gold, S.E.; Müller, O.; et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 2006, 444, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.M.; Tripathy, S.; Zhang, X.; Dehal, P.; Jiang, R.H.Y.; Aerts, A.; Arredondo, F.D.; Baxter, L.; Bensasson, D.; Beynon, J.L.; et al. Phytophthora Genome Sequences Uncover Evolutionary Origins and Mechanisms of Pathogenesis. Science 2006, 313, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Yaghmour, M.A.; Nguyen, T.L.; Roubtsova, T.V.; Hasey, J.K.; Fichtner, E.J.; DeBuse, C.; Seybold, S.J.; Bostock, R.M. First report of Geosmithia morbida on English walnut and its Paradox rootstock in California. Plant Dis. 2015, 99, 1183. [Google Scholar] [CrossRef]
- Hadziabdic, D.; Vito, L.M.; Windham, M.T.; Pscheidt, J.W.; Trigiano, R.N.; Kolarik, M. Genetic differentiation and spatial structure of Geosmithia morbida, the causal agent of thousand cankers disease in black walnut (Juglans nigra). Curr. Genet. 2014, 60, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Freeland, E. Intraspecific Variability of Geosmithia morbida the Causal Agent of Thousand Cankers Disease, and Effects of Temperature, Isolate and Host Family (Juglans nigra) on Canker Development. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2007. [Google Scholar]
- Anagnostakis, S.L. Chestnut Blight: The Classical Problem of an Introduced Pathogen. Mycologia 1987, 79, 23–37. [Google Scholar] [CrossRef]
- Brasier, C.M. Ophiostoma novo-ulmi sp. nov.; causative agent of current Dutch elm disease pandemics. Mycopathologia 1991, 115, 151–161. [Google Scholar] [CrossRef]
- Tsui, C.K.; Roe, A.D.; El-Kassaby, Y.A.; Rice, A.V.; Alamouti, S.M.; Sperling, F.A.; Cooke, J.E.; Bohlmann, J.; Hamelin, R.C. Population structure and migration pattern of a conifer pathogen, Grosmannia clavigera, as influenced by its symbiont, the mountain pine beetle. Mol. Ecol. 2012, 21, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Goss, E.M.; Larsen, M.; Vercauteren, A.; Werres, S.; Heungens, K.; Grünwald, N.J. Phytophthora ramorum in Canada: Evidence for migration within North America and from Europe. Phytopathology 2011, 101, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Blackman, M.W. The Genus Pityophthorus Eichh. in North America: A Revisional Study of the Pityophthori, with Descriptions of Two New Genera and Seventy-one Species; New York State College of Forestry at Syracuse University: Syracuse, NY, USA, 1928. [Google Scholar]
- Bright, D.E. Taxonomic Monograph of the Genus Pitypohthorus Eichhoff In North and Central America (Coleoptera: Scolytidae). Mem. Entomol. Soc. Can. 1981, 113, 1–378. [Google Scholar] [CrossRef]
- Wood, S.L.; Bright, D.E., Jr. A catalogue of Scolytidae and Platypodidae (Coleoptera). Part 2, Volumes A & B. Great. Basin Nat. Mem. 1992, 13, 1005. [Google Scholar]
- Seybold, S.J.; Coleman, T.W.; Dallara, P.L.; Dart, N.L.; Graves, A.D.; Pederson, L.A.; Spichiger, S.-E. Recent collecting reveals new state records and geographic extremes in the distribution of the walnut twig beetle, Pityophthorus juglandis Blackman (Coleoptera: Scolytidae), in the United States. Pan-Pac. Entomol. 2012, 88, 277–280. [Google Scholar] [CrossRef]
- Haun, G.; Powell, S.; Strohmeier, C.; Kirksey, J. State of Tennessee thousand cankers disease action plan. Tennessee Dep. Agric. 2010. Available online: http://www.protecttnforests.com/documents/TCD_ActionPlan.pdf (accessed on 18 August 2016).
- Cranshaw, W.; Tisserat, N. Questions and Answers about Thousand Cankers Disease of Walnut. Available online: http://www.thousandcankers.com/media/docs/CSU_TCD_FAQ_7_2012.pdf (accessed on 12 July 2016).
- Leslie, C.A.; Seybold, S.J.; Graves, A.D.; Cranshaw, W.; Tisserat, N. Potential impacts of thousand cankers disease on commercial walnut production and walnut germplasm conservation. In VI International Walnut Symposium 861; International Society for Horticultural Science: Leuven, Belgium, 2009; pp. 431–434. [Google Scholar] [CrossRef]
- Cranshaw, W. The walnut twig beetle and its association with 1000 cankers disease of walnut. In Metamorphosis—A New Beginning, Proceedings of the 56th Annual Meeting of the Entomological Society of America, Reno, NV, USA, 16–19 November 2008.
- Nix, K.A. The Life History and Control of Pityophthorus juglandis Blackman on Juglans nigra L. in Eastern Tennessee. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2013. [Google Scholar]
- Ginzel, M. WTB TCD. Available online: https://www.youtube.com/watch?v=Tta4SGdR_tM (accessed on 15 August 2016).
- Graves, A.D.; Coleman, T.W.; Flint, M.L.; Seybold, S.J. Walnut twig beetle and thousand cankers disease: Field identification guide. UC IPM Progr. Univ. Calif. Agric. Nat. Resour. 2009. Available online: http://www.ipm.ucdavis.edu/thousandcankers (accessed on 25 July 2016). [Google Scholar]
- Lambdin, P.; Nix, K.; Grant, J.; Pauslen, D.; Merten, P. Natural Enemies of the Walnut Twig Beetle in Eastern Tennessee. Int. J. Agric. For. 2015, 2, 31–39. [Google Scholar]
- Chen, Y.; Seybold, S.J. Crepuscular flight activity of an invasive insect governed by interacting abiotic factors. PLoS ONE 2014, 9, e105945. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.K.; Sitz, R.A.; Cranshaw, W.S.; Tisserat, N.A. The Effect of Temperature on Survival of Pityophthorus juglandis (Coleoptera: Curculionidae). Environ. Entomol. 2013, 42, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Klingeman, W.E.; Lambdin, P.L.; Wiggins, G.J. Independent Urban Landscape Collection and Field Monitoring of Pityophthoris juglandis Beetles in East Tennessee. University of Tennessee: Knoxville, TN, USA, 2016; Unpublished data. [Google Scholar]
- Tisserat, N. Stem Canker of Black-Walnut Caused by Fusarium solani in Kansas. Plant Dis. 1987, 71. [Google Scholar] [CrossRef]
- Toole, E.R. Cottonwood canker caused by Fusarium solani. Plant Rep. 1963, 47, 1032–1036. [Google Scholar]
- Vujanovic, V.; Cogliastro, A.; St-Arnaud, M.; Neumann, P.; Gagnon, D. First Report of Fusarium solani Canker and Wilt Symptoms on Red Oak (Quercus rubra) in Quebec, Canada. Plant Dis. 1999, 83, 78. [Google Scholar] [CrossRef]
- Chen, W.; Swart, W.J. First report of stem canker of English walnut caused by Fusarium solani in South Africa. Plant Dis. 2000, 84, 592. [Google Scholar] [CrossRef]
- Seybold, S.J.; Dallara, P.L.; Hishinuma, S.M.; Flint, M.L. Detecting and Identifying the Walnut Twig Beetle: Monitoring Guidelines for the Invasive Vector of Thousand Cankers Disease of Walnut. UC IPM Progr. Univ. Calif. Agric. Nat. Resour. 2012. Available online: http://www.ipm.ucdavis.edu/thousandcankers (accessed on 25 July 2016). [Google Scholar]
- Downie, N.M.; Arnett, R.H. The Beetles of Northeastern North America. 1. Introduction; Suborders Archostemata, Adephaga, and Polyphaga, thru Superfamily Cantharoidea; Sandhill Crane Press: Gainesville, FL, USA, 1996. [Google Scholar]
- Leavengood, J.M., Jr. The Checkered Beetles (Coleoptera: Cleridae) of Florida. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2008. [Google Scholar]
- Wiggins, G.J.; Grant, J.F.; Lambdin, P.L.; Merten, P.; Nix, K.A.; Hadziabdic, D.; Windham, M.T. Discovery of Walnut Twig Beetle, Pityophthorus juglandis, Associated with Forested Black walnut, Juglans nigra, in the Eastern U.S. Forests 2014, 5, 1185–1193. [Google Scholar] [CrossRef]
- Mayfield, A.E., III; Fraedrich, S.W.; Taylor, A.; Merten, P.; Myers, S.W. Efficacy of Heat Treatment for the Thousand Cankers Disease Vector and Pathogen in Small Black Walnut Logs. J. Econ. Entomol. 2014, 107, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Sitz, R. Management Options for the Walnut Twig Beetle, Pityophthorus juglandis Blackman, Vector of the Fungal Canker Pathogen Geosmithia morbida. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2007. [Google Scholar]
- Audley, J.; Mayfield, A.E.; Myers, S.W.; Taylor, A.; Klingeman, W.E. Phytosanitation methods influence posttreatment colonization of Juglans nigra logs by Pityophthorus juglandis (Coleoptera: Curculionidae: Scolytinae). J. Econ. Entomol. 2016, 109, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Audley, J.; Taylor, A.; Klingeman, W.E.; Mayfield, A.E.; Myers, S.W. Insecticide dip treatments to prevent walnut twig beetle colonization of black walnut logs. For. Prod. J. 2015. [Google Scholar] [CrossRef]
- Thousand Cankers Disease (TCD). Available online: https://www.tn.gov/agriculture/topic/ag-businesses-tcd (accessed on 12 August 2016).
- Walnut Twig Beetle. Available online: http://codes.ohio.gov/oac/901%3A5-58 (accessed on 12 August 2016).
- Virginia Department of Agriculture and Consumer Services. Diseases of Regulatory Concern. Available online: http://www.vdacs.virginia.gov/plant-industry-services-diseases-of-regulatory-concern.shtml (accessed on 12 August 2016).
- Wilson, P. News Release: Haywood County Wood Products under NCDA&CS Quarantine for Thousand Cankers Disease. Available online: http://www.ncagr.gov/paffairs/release/2013/1-13-Thousand-Cankers-Quarantine-Haywood-County.htm (accessed on 12 August 2016).
- Minnesota Department of Agriculture. Thousand Cankers Disease of Walnut. Available online: http://www.mda.state.mn.us/plants/plantdiseases/1000cankers.aspx (accessed on 12 August 2016).
- Conrad, A.O.; Taylor, N.J.; Bonello, P. Thousand Cankers Disease. Available online: http://ohioline.osu.edu/factsheet/plpath-tree-07-0 (accessed on 19 August 2016).
- Office of the Federal Register. Tolerances and Exemptions for Pesticide Chemicals Residues in Food—Specific Tolerances. Available online: https://www.law.cornell.edu/cfr/text/40/part-180/subpart-C (accessed on 1 November 2016).
- Nix, K.; Lambdin, P.; Grant, J.; Coots, C.; Merten, P. Concentration Levels of Imidacloprid and Dinotefuran in Five Tissue Types of Black walnut, Juglans nigra. Forests 2013, 4, 887–897. [Google Scholar] [CrossRef]
- Elbert, A.; Nauen, R.; Leicht, W. Imidacloprid, a novel chloronicotinyl insecticide: Biological activity and agricultural importance. In Insecticides with Novel Modes of Action; Ishaaya, I., Degheele, D., Eds.; Springer: Berlin, Germany, 1998; pp. 50–73. [Google Scholar] [CrossRef]
- Kodaka, K.; Kinoshita, K.; Wakita, T.; Yamada, E.; Kawahara, N.; Yasui, N. MTI-446: A Novel Systemic Insect Control Compound. In Proceedings of the Brighton Crop Protect Conference—Pests and Diseases; British Crop Protection Council: Farham, UK, 1998; pp. 616–632. [Google Scholar]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Carrillo, D.; Duncan, R.E.; Peña, J.E. Ambrosia Beetles (Coleoptera: Curculionidae: Scolytinae) that Breed in Avocado Wood in Florida. Fla. Entomol. 2012, 95, 573–579. [Google Scholar] [CrossRef]
- Fisher, J.J.; Hajek, A.E. The Effect of Maturation and Aging on Fungal Infection in the Asian Longhorned Beetle, Anoplophora glabripennis. In Proceedings of the 24th USDA Interagency Research Forum on Invasive Species, Annapolis, MD, USA, 8–11 January 2013; p. 68.
- Sweeney, J.; Silk, P.J.; Hughes, C.; Levallée, R.; Blais, M.; Guertin, C. Auto-Dissemination of Beauveria bassiana for Control of Brown Spruce Longhorned Beetle, Tetropium fuscum (F.), Coleoptera: Cerambycidae. In Proceedings of the 24th USDA Interagency Research Forum on Invasive Species, Annapolis, MD, USA, 8–11 January 2013; p. 98.
- Carroll, G. Fungal Endophytes in Stems and Leaves: From Latent Pathogen to Mutualistic Symbiont. Ecology 1988, 69, 2–9. [Google Scholar] [CrossRef]
- Jakobs-Schönwandt, D.; Lohse, R.; Patel, A.V. Cultivation and formulation of an endophytic Beauveria bassiana strain. In Proceedings of the 2011 American Phytopathological Society Annual Meeting, Honolulu, HI, USA, 6–10 August 2011.
- Lestari, A.; Rao, S. Isolation and pathogenicity of naturally-occurring entomopathogenic fungi to unique bark beetle field crop pest. In 2014 Annual Meeting of the Entomological Society of America: Science Impacting a Connected World, Proceedings of the 63rd Annual Meeting of the Entomological Society of America, Minneapolis, MN, USA, 10–13 November 2013.
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef] [PubMed]
- Ownley, B.H.; Dee, M.M.; Gwinn, K.D. Effect of conidial seed treatment rate of entomopathogenic Beauveria bassiana 11–98 on endophytic colonization of tomato seedlings and control of Rhizoctonia disease. In Proceedings of the 2008 American Phytopathological Society Annual Meeting, Minneapolis, MN, USA, 26–30 July 2008; p. 101.
- Rind, D.; Goldberg, R.; Hansen, J.; Rosenzweig, C.; Ruedy, R. Potential evapotranspiration and the likelihood of future drought. J. Geophys. Res. Atmos. 1990, 95, 9983–10004. [Google Scholar] [CrossRef]
- Wood, A.W.; Maurer, E.P.; Kumar, A.; Lettenmaier, D.P. Long-range experimental hydrologic forecasting for the eastern United States. J. Geophys. Res. Atmos. 2002, 107, 4429. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniels, D.A.; Nix, K.A.; Wadl, P.A.; Vito, L.M.; Wiggins, G.J.; Windham, M.T.; Ownley, B.H.; Lambdin, P.L.; Grant, J.F.; Merten, P.; et al. Thousand Cankers Disease Complex: A Forest Health Issue that Threatens Juglans Species across the U.S. Forests 2016, 7, 260. https://doi.org/10.3390/f7110260
Daniels DA, Nix KA, Wadl PA, Vito LM, Wiggins GJ, Windham MT, Ownley BH, Lambdin PL, Grant JF, Merten P, et al. Thousand Cankers Disease Complex: A Forest Health Issue that Threatens Juglans Species across the U.S. Forests. 2016; 7(11):260. https://doi.org/10.3390/f7110260
Chicago/Turabian StyleDaniels, Dixie A., Katheryne A. Nix, Phillip A. Wadl, Lisa M. Vito, Gregory J. Wiggins, Mark T. Windham, Bonnie H. Ownley, Paris L. Lambdin, Jerome F. Grant, Paul Merten, and et al. 2016. "Thousand Cankers Disease Complex: A Forest Health Issue that Threatens Juglans Species across the U.S." Forests 7, no. 11: 260. https://doi.org/10.3390/f7110260





