Feline Immunodeficiency Virus in South America
Abstract
:1. Introduction

| Reference | Year | Country / Geographical distribution | Technique | Felid species | No tested | % Positive |
|---|---|---|---|---|---|---|
| (43) | 1992 | Brazil/Chile | Western Blot | Puma concolor | 18 | 0 |
| (65) | 1994 | Argentina | Western Blot | Felis catus | 26 | 34.6 |
| (52) | 1997 | Brazil—São Paulo | ELISA | Felis catus | 401 | 11.7 |
| (9) | 2000 | Brazil—Rio Grande do Sul | PCR | Felis catus | 40 | 37.5 |
| (61) | 2002 | Brazil—Rio de Janeiro | ELISA | Felis catus | 126 | 16.6 |
| (18) | 2003 | Brazil—São Paulo | ELISA | Leopardus pardalis, L. tigrinus, L. wiedii, Herpaiturus yaguarondi, Oncifelis geoffroyi | 104 | 0 |
| (12) | 2003 | Brazil—Minas Gerais | PCR | Felis catus | 450 | 2.66 |
| (64) | 2007 | Brazil—Minas Gerais | PCR | Felis catus | 145 | 4.14 |
| (19) | 2006 | Brazil—Roraima; Acre; Mato Grosso; Mato Grosso do Sul; São Paulo; Rio de Janeiro | ELISA/Western Blot | Puma concolor; Leopardus pardalis; Leopardus tigrinus | 21 | 4.76/9.52 |
| (21) | 2007 | Bolivia—Chaco | ELISA | Leopardus pardalis, Oncifelis geoffroyi, Herpaiturus yaguarondi | 20 | 0 |
| (31) | 2008 | Brazil—São Paulo | PCR | Felis catus | 454 | 14.7 |
| (36) | 2008 | Ecuador—Galapagos | ELISA | Felis catus | 52 | 0 |
| (20) | 2011 | Brazil—São Paulo | ELISA/Western Blot | Different species of neotropic and exotic felids | 145 | 6.9 * |
2. Felis Catus
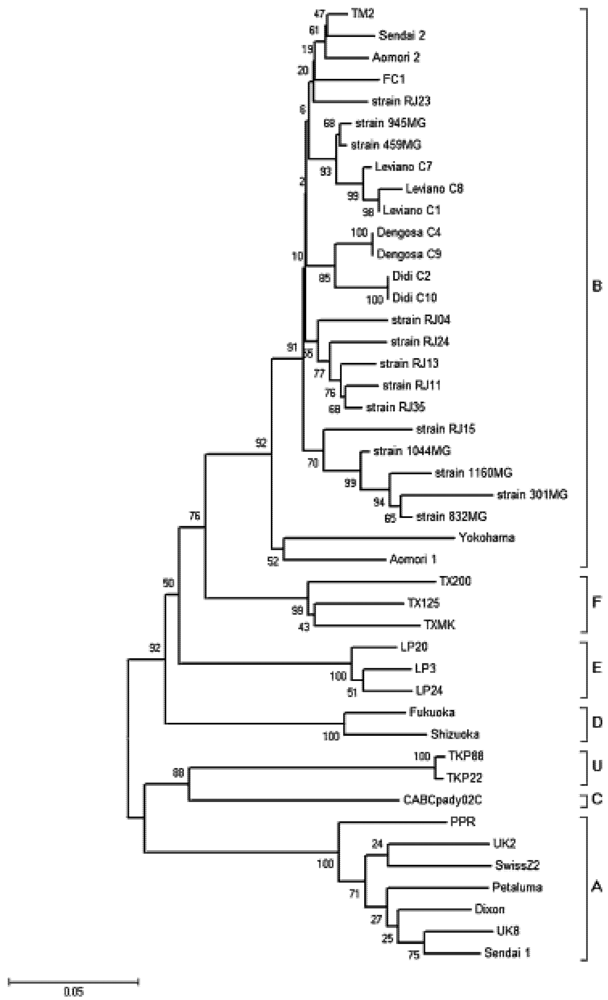
3. Nondomestic Felid Species
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Ackley, C.D.; Yamamoto, J.K.; Levy, N.; Pedersen, N.C.; Cooper, M.D. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J. Virol. 1990, 64, 5652–5655. [Google Scholar]
- Bachmann, M.H.; Mathiason-Dubard, C.; Learn, G.H.; Rodrigo, A.G.; Sodora, D.L.; Mazzetti, P.; Hoover, E.A.; Mullins, J.I. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J. Virol. 1997, 71, 4241–4253. [Google Scholar]
- Barr, M.C.; Zou, L.; Long, F.; Hoose, W.A.; Avery, R.J. Proviral organization and sequence analysis of feline immunodeficiency virus isolated from a Pallas' cat. Virology 1997, 228, 84–91. [Google Scholar]
- Beatty, J.A.; Lawrence, C.E.; Callanan, J.J.; Grant, C.K.; Gault, E.A.; Neil, J.C.; Jarrett, O. Feline immunodeficiency virus (FIV)-associated lymphoma: a potential role for immune dysfunction in tumourigenesis. Vet. Immunol. Immunop. 1998, 65, 309–322. [Google Scholar]
- Bendinelli, M.; Pistello, M.; Lombardi, S.; Poli, A.; Garzelli, C.; Matteucci, D.; Ceccherini-Nelli, L.; Malvaldi, G.; Tozzini, F. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin. Microbiol. Rev. 1995, 8, 87–112. [Google Scholar]
- Brown, E.W.; Miththapala, S.; O'Brien, S.J. Prevalence of exposure to feline immunodeficiency virus in exotic felid species. J. Zoo Wildlife Med. 1993, 24, 357–364. [Google Scholar]
- Brown, E.W.; Yuhki, N.; Packer, C.; O'Brien, S.J. A lion lentivirus related to feline immunodeficiency virus: epidemiologic and phylogenetic aspects. J. Virol. 1994, I, 5953–5968. [Google Scholar]
- Brown, M.A.; Munkhtsog, B.; Troyer, J.L.; Ross, S.; Sellers, R.; Fine, A.E.; Swanson, W.F.; Roelke, M.E.; O'Brien, S.J. Feline immunodeficiency virus (FIV) in wild Pallas' cats. Vet. Immunol. Immunop. 2010, 134, 90–95. [Google Scholar]
- Caldas, A.P.F.; Leal, E.S.; Silva, E.F.A.; Ravazzolo, A. Detection of feline immunodeficiency provirus in domestic cats by polymerase chain reaction. Pesquisa Vet. Brasil. 2000, 20, 20–25. [Google Scholar]
- Carpenter, M.A.; Brown, E.W.; Culver, M.; Johnson, W.E.; Pecon-Slattery, J.; Brousset, D.; O'Brien, S.J. Genetic and phylogenetic divergence of feline immunodeficiency virus in the puma (Puma concolor). J. Virol. 1996, 70, 6682–6693. [Google Scholar]
- Carpenter, M.A.; O'Brien, S.J. Coadaptation and immunodeficiency virus: lessons from the Felidae. Curr. Opin. Gen. Dev. 1995, 5, 739–745. [Google Scholar]
- Caxito, F.A.; Coelho, F.M.; Oliveira, M.E.; Resende, M. Feline immunodeficiency virus subtype B in domestic cats in Minas Gerais, Brazil. Vet. Res. Comm. 2006, 30, 953–956. [Google Scholar]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife--threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar]
- de Monte, M.; Nonnenmacher, H.; Brignon, N.; Ullmann, M.; Martin, J.P. A multivariate statistical analysis to follow the course of disease after infection of cats with different strains of the feline immunodeficiency virus (FIV). J. Virol. Methods 2002, 103, 157–170. [Google Scholar]
- Dean, G.A.; Reubel, G.H.; Moore, P.F.; Pedersen, N.C. Proviral burden and infection kinetics of feline immunodeficiency virus in lymphocyte subsets of blood and lymph node. J. Virol. 1996, 70, 5165–5169. [Google Scholar]
- Elder, J.H.; Lin, Y.C.; Fink, E.; Grant, C.K. Feline immunodeficiency virus (FIV) as a model for study of lentivirus infections: parallels with HIV. Curr. HIV Res. 2010, 8, 73–80. [Google Scholar]
- English, R.V.; Johnson, C.M.; Gebhard, D.H.; Tompkins, M.B. In vivo lymphocyte tropism of feline immunodeficiency virus. J. Virol. 1993, 67, 5175–5186. [Google Scholar]
- Filoni, C.; Adania, C.H.; Durigon, E.L.; Catão-Dias, J.L. Serosurvey for feline leukemia virus and lentiviruses in captive small neotropic felids in São Paulo state, Brazil. J. Zoo Wildlife Med. 2003, 34, 65–68. [Google Scholar]
- Filoni, C.; Catão-Dias, J.L.; Bay, G.; Durigon, E.L.; Jorge, R.S.; Lutz, H.; Hofmann-Lehmann, R. First evidence of feline herpesvirus, calicivirus, parvovirus, and Ehrlichia exposure in Brazilian free-ranging felids. J. Zoo Wildlife Med. 2006, 42, 470–477. [Google Scholar]
- Filoni, C.; Catão-Dias, J.L.; Cattori, V.; Willi, B.; Meli, M.L.; Ramiro Corrêa, S.H.; Cristina Marques, M.; Harumi Adania, C.; Ramos Silva, J.C.; Vianna Marvulo, M.F.; Ferreira Neto, J.S.; Luiz Durigon, E.; de Carvalho, V.M.; Dall'acqua Coutinho, S.; Lutz, H.; Hofmann-Lehmann, R. Surveillance using serological and molecular methods for the detection of infectious agents in captive Brazilian neotropic and exotic felids. J. Vet. Diagn. Invest. 2012, 24, 166–173. [Google Scholar]
- Fiorello, C.V.; Noss, A.J.; Deem, S.L.; Maffei, L.; Dubovi, E.J. Serosurvey of small carnivores in the Bolivian Chaco. J. Wildlife Dis. 2007, 43, 551–557. [Google Scholar]
- Franklin, S.P.; Troyer, J.L.; Terwee, J.A.; Lyren, L.M.; Boyce, W.M.; Riley, S.P.; Roelke, M.E.; Crooks, K.R.; Vandewoude, S. Frequent transmission of immunodeficiency viruses among bobcats and pumas. J. Virol. 2007, 81, 10961–10969. [Google Scholar]
- Hagiwara, M.K.; Reche Junior, A.; Dagli, M.L.Z. Feline immunodeficiency virus infection in cats from Sao Paulo, Brazil. Braz. J. Vet. Res. Anim. Sci. 1993, 30, 217–220. [Google Scholar]
- Hayward, J.J.; Taylor, J.; Rodrigo, A.G. Phylogenetic analysis of feline immunodeficiency virus in feral and companion domestic cats of New Zealand. J. Virol. 2007, 81, 2999–3004. [Google Scholar]
- Hofmann-Lehmann, R.; Fehr, D.; Grob, M.; Elgizoli, M.; Packer, C.; Martenson, J.S.; O'Brien, S.J.; Lutz, H. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free-ranging lions in east Africa. Clin. Diagn. Lab. Immun. 1996, 3, 554–562. [Google Scholar]
- Hosie, M.J.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Lloret, A.; Lutz, H.; Marsilio, F.; Pennisi, M.G.; Radford, A.D.; Thiry, E.; Truyen, U.; Horzinek, M.C. Feline immunodeficiency. ABCD guidelines on prevention and management. 2009, 11, 575–584. [Google Scholar]
- Hosie, M.J.; Beatty, J.A. Vaccine protection against feline immunodeficiency virus: setting the challenge. Aust. Vet. J. 2007, 85, 5–12. [Google Scholar]
- Hosie, M.J.; Robertson, C.; Jarrett, O. Prevalence of feline leukaemia virus and antibodies to feline immunodeficiency virus in cats in the United Kingdom. Vet. Rec. 1989, 125, 293–297. [Google Scholar]
- Joshi, A.; Garg, H.; Tompkins, M.B.; Tompkins, W.A. Preferential feline immunodeficiency virus (FIV) infection of CD4+ CD25+ T-regulatory cells correlates both with surface expression of CXCR4 and activation of FIV long terminal repeat binding cellular transcriptional factors. J. Virol. 2005, 79, 4965–4976. [Google Scholar]
- Kakinuma, S.; Motokawa, K.; Hohdatsu, T.; Yamamoto, J.K.; Koyama, H.; Hashimoto, H. Nucleotide sequence of feline immunodeficiency virus: classification of Japanese isolates into two subtypes which are distinct from non-Japanese subtypes. J. Virol. 1995, 69, 3639–3646. [Google Scholar]
- Lara, V.M.; Taniwaki, S.A.; Araujo Junior, J.P. Occurrence of feline immunodeficiency virus infection in cats. Cienc. Rural 2008, 38, 2245–2249. [Google Scholar]
- Lara, V.M.; TaniwakiII, S.A.; Araújo, J.P., Jr. Phylogenetic characterization of feline immunodeficiency virus (FIV) isolates from the state of São Paulo. Pesquisa Vet. Brasil. 2007, 27, 467–470. [Google Scholar]
- Leal, E.S.; Ravazzolo, A.P. Detecção do vírus da imunodeficiência felina (FIV) em felídeos selvagens pertencentes à região neotropical, através da técnica de reação em cadeia da polimerase (PCR). Hora Vet. 1998, 101, 57–60. [Google Scholar]
- Leutenegger, C.M.; Hofmann-Lehmann, R.; Riols, C.; Liberek, M.; Worel, G.; Lups, P.; Fehr, D.; Hartmann, M.; Weilenmann, P.; Lutz, H. Viral infections in free-living populations of the European wildcat. J. Wildl. Dis. 1999, 35, 678–686. [Google Scholar]
- Levy, J.; Crawford, C.; Hartmann, K.; Hofmann-Lehmann, R.; Little, S.; Sundahl, E.; Thayer, V. 2008 American Association of Feline Practitioners' feline retrovirus management guidelines. J. Fel. Med.Surg. 2008b, 10, 300–316. [Google Scholar]
- Levy, J.K.; Crawford, P.C.; Lappin, M.R.; Dubovi, E.J.; Levy, M.G.; Alleman, R.; Tucker, S.J.; Clifford, E.L. Infectious diseases of dogs and cats on Isabela Island, Galapagos. J. Vet. Intern. Med. 2008b, 22, 60–65. [Google Scholar]
- Lucas, S.R.R.; Hagiwara, M.K.; Reche, A.R., Jr.; Germano, P.M.L. Ocorrencia de anticorpos antitoxoplasma em gatos infectados naturalmente pelo virus da imunodeficiencia dos felinos. Braz. J. Vet. Res. Anim. Sci. 1998, 35, 41–45. [Google Scholar]
- Lutz, H.; Isenbügel, E.; Lehmann, R.; Sabapara, R.H.; Wolfensberger, C. Retrovirus infections in non-domestic felids: serological studies and attempts to isolate a lentivirus. Vet. Immunol. Immunop. 1992, 35, 215–224. [Google Scholar]
- Macieira, D.B.; de Menezes, RdeC.; Damico, C.B.; Almosny, N.R.; McLane, H.L.; Daggy, J.K.; Messick, J.B. Prevalence and risk factors for hemoplasmas in domestic cats naturally infected with feline immunodeficiency virus and/or feline leukemia virus in Rio de Janeiro-Brazil. J. Fel. Med. Surg. 2008, 10, 120–129. [Google Scholar] [CrossRef]
- Martins, A.N.; Medeiros, S.O.; Simonetti, J.P.; Schatzmayr, H.G.; Tanuri, A.; Brindeiro, R.M. Phylogenetic and genetic analysis of feline immunodeficiency virus gag, pol, and env genes from domestic cats undergoing nucleoside reverse transcriptase inhibitor treatment or treatment-naive cats in Rio de Janeiro, Brazil. J. Virol. 2008, 82, 7863–7874. [Google Scholar]
- McEwan, W.A.; McMonagle, E.L.; Logan, N.; Serra, R.C.; Kat, P.; Vandewoude, S.; Hosie, M.J.; Willett, B.J. Genetically divergent strains of feline immunodeficiency virus from the domestic cat (Felis catus) and the African lion (Panthera leo) share usage of CD134 and CXCR4 as entry receptors. J. Virol. 2008, 82, 10953–10958. [Google Scholar]
- Nishimura, Y.; Goto, Y.; Yoneda, K.; Endo, Y.; Mizuno, T.; Hamachi, M.; Maruyama, H.; Kinoshita, H.; Koga, S.; Komori, M.; Fushuku, S.; Ushinohama, K.; Akuzawa, M.; Watari, T.; Hasegawa, A.; Tsujimoto, H. Interspecies transmission of feline immunodeficiency virus from the domestic cat to the Tsushima cat (Felis bengalensis euptilura) in the wild. J. Virol. 1999, 73, 7916–7921. [Google Scholar]
- Olmsted, R.A.; Langley, R.; Roelke, M.E.; Goeken, R.M.; Adger-Johnson, D.; Goff, J.P.; Albert, J.P.; Packer, C.; Laurenson, M.K.; Caro, T.M. Worldwide prevalence of lentivirus infection in wild feline species: epidemiologic and phylogenetic aspects. J. Virol. 1992, 66, 6008–6018. [Google Scholar]
- Pancino, G.; Camoin, L.; Sonigo, P. Structural analysis of the principal immunodominant domain of the feline immunodeficiency virus transmembrane glycoprotein. J. Virol. 1995, 69, 2110–2118. [Google Scholar]
- Pecon-Slattery, J.; Troyer, J.L.; Johnson, W.E.; O'Brien, S.J. Evolution of feline immunodeficiency virus in Felidae: implications for human health and wildlife ecology. Vet. Immunol. Immunop. 2008, 123, 32–44. [Google Scholar]
- Pecoraro, M.R.; Tomonaga, K.; Miyazawa, T.; Kawaguchi, Y.; Sugita, S.; Tohya, Y.; Kai, C.; Etcheverrigaray, M.E.; Mikami, T. Genetic diversity of Argentine isolates of feline immunodeficiency virus. J. Gen. Virol. 1996a, 77, 2031–2035. [Google Scholar]
- Pecoraro, M.R.; Tomonaga, K.; Miyazawa, T.; Kawaguchi, Y.; Sugita, S.; Tohya, Y.; Kai, C.; Etcheverrigaray, M.E.; Mikami, T. Genetic diversity of Argentine isolates of feline immunodeficiency virus. J. Gen. Virol. 1996b, 77, 2031–2035. [Google Scholar]
- Pedersen, N.C.; Ho, E.W.; Brown, M.L.; Yamamoto, J.K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 1987, 235, 790–793. [Google Scholar]
- Pedersen, N.C.; Leutenegger, C.M.; Woo, J.; Higgins, J. Virulence differences between two field isolates of feline immunodeficiency virus (FIV-APetaluma and FIV-CPGammar) in young adult specific pathogen free cats. Vet. Immunol. Immunop. 2001, 79, 53–67. [Google Scholar]
- Pistello, M.; Cammarota, G.; Nicoletti, E.; Matteucci, D.; Curcio, M.; Del Mauro, D.; Bendinelli, M. Analysis of the genetic diversity and phylogenetic relationship of Italian isolates of feline immunodeficiency virus indicates a high prevalence and heterogeneity of subtype B. J. Gen. Virol. 1997, 78, 2247–2257. [Google Scholar]
- Reche, A.; Daniel, A.G.; Lazaro Strauss, T.C.; Taborda, C.P.; Vieira Marques, S.A.; Haipek, K.; Oliveira, L.J.; Monteiro, J.M.; Kfoury, J.R. Cutaneous mycoflora and CD4:CD8 ratio of cats infected with feline immunodeficiency virus. J. Fel. Med. Surg. 2010, 12, 355–358. [Google Scholar]
- Reche, A., Jr.; Hagiwara, M.K.; Lucas, S.R.R. Clinical study of acquired immunodeficiency syndrome in domestic cats in São Paulo. Braz. J. Vet. Res. Anim. Sci. 1997, 34, 152–155. [Google Scholar]
- Reggeti, F.; Ackerley, C.; Bienzle, D. CD134 and CXCR4 expression corresponds to feline immunodeficiency virus infection of lymphocytes, macrophages and dendritic cells. J. Gen. Virol. 2008, 89, 277–287. [Google Scholar]
- Reggeti, F.; Bienzle, D. Feline immunodeficiency virus subtypes A, B and C and intersubtype recombinants in Ontario, Canada. J. Gen. Virol. 2004, 85, 1843–1852. [Google Scholar] [CrossRef]
- Rivetti, A.V., Jr.; Caxito, F.A.; Resende, M.; Lobato, Z.I.P. Avaliação sorológica para Toxoplasma gondii pela imunofluorescência indireta e detecção do vírus da imunodeficiência felina pela nested PCR em felinos selvagens. Arq. Bras. Med. Vet. Zoo. 2008, 60, 1281–1283. [Google Scholar] [CrossRef]
- Roelke, M.E.; Brown, M.A.; Troyer, J.L.; Winterbach, H.; Winterbach, C.; Hemson, G.; Smith, D.; Johnson, R.C.; Pecon-Slattery, J.; Roca, A.L.; Alexander, K.A.; Klein, L.; Martelli, P.; Krishnasamy, K.; O'Brien, S.J. Pathological manifestations of feline immunodeficiency virus (FIV) infection in wild African lions. Virology 2009, 390, 1–12. [Google Scholar]
- Roelke, M.E.; Pecon-Slattery, J.; Taylor, S.; Citino, S.; Brown, E.; Packer, C.; Vandewoude, S.; O'Brien, S.J. T-lymphocyte profiles in FIV-infected wild lions and pumas reveal CD4 depletion. J. Wildl. Dis. 2006, 42, 234–248. [Google Scholar]
- Shimojima, M.; Miyazawa, T.; Ikeda, Y.; McMonagle, E.L.; Haining, H.; Akashi, H.; Takeuchi, Y.; Hosie, M.J.; Willett, B.J. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science 2004, 303, 1192–1195. [Google Scholar]
- Smirnova, N.; Troyer, J.L.; Schissler, J.; Terwee, J.; Poss, M.; VandeWoude, S. Feline lentiviruses demonstrate differences in receptor repertoire and envelope structural elements. Virology 2005, 342, 60–76. [Google Scholar]
- Sodora, D.L.; Shpaer, E.G.; Kitchell, B.E.; Dow, S.W.; Hoover, E.A.; Mullins, J.I. Identification of three feline immunodeficiency virus (FIV) env gene subtypes and comparison of the FIV and human immunodeficiency virus type 1 evolutionary patterns. J. Virol. 1994, 68, 2230–2238. [Google Scholar]
- Souza, H.J.M.; Teixeira, C.H.R.; Graça, R.F.S. Epidemiological study of feline leukaemia virus and feline immunodeficiency virus infections in domestic cats in the city of Rio de Janeiro. Clín. Vet. 2002, 36, 14–21. [Google Scholar]
- Teixeira, B.M.; Logan, N.; Cruz, J.C.; Reis, J.K.; Brandão, P.E.; Richtzenhain, L.J.; Hagiwara, M.K.; Willett, B.J.; Hosie, M.J. Genetic diversity of Brazilian isolates of feline immunodeficiency virus. Arch. Virology 2010, 155, 379–384. [Google Scholar]
- Teixeira, B.M.; Logan, N.; Samman, A.; Miyashiro, S.I.; Brandão, P.E.; Willett, B.J.; Hosie, M.J.; Hagiwara, M.K. Isolation and partial characterization of Brazilian samples of feline immunodeficiency virus. Vir. Res. 2011, 160, 59–65. [Google Scholar]
- Teixeira, B.M.; Rajão, D.S.; Haddad, J.P.A.; Leite, R.C.; Reis, J.K.P. Occurrence of feline immunodeficiency virus and feline leukemia virus in Sheltered domestic cats of Belo Horizonte. Arq. Bras. Med. Vet. Zoo. 2007, 59, 939–942. [Google Scholar]
- Tohya, Y.; Castellano, M.C.; Norimine, J.; Etcheverrigaray, M.E. Anticuerpos contra el virus da la inmunodeficiencia felina: Primeira comprobacion en Argentina. Rev. Med. Vet. 1994, 75, 242–246. [Google Scholar]
- Troyer, J.L.; Pecon-Slattery, J.; Roelke, M.E.; Johnson, W.; VandeWoude, S.; Vazquez-Salat, N.; Brown, M.; Frank, L.; Woodroffe, R.; Winterbach, C.; Winterbach, H.; Hemson, G.; Bush, M.; Alexander, K.A.; Revilla, E.; O'Brien, S.J. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J. Virol. 2005, 79, 8282–8294. [Google Scholar]
- Troyer, J.L.; Vandewoude, S.; Pecon-Slattery, J.; McIntosh, C.; Franklin, S.; Antunes, A.; Johnson, W.; O'Brien, S.J. FIV cross-species transmission: an evolutionary prospective. Vet. Immun. Immunop. 2008, 123, 159–166. [Google Scholar]
- Weaver, E.A. A detailed phylogenetic analysis of FIV in the United States. PLoS One 2010, 5, e12004. [Google Scholar]
- Weaver, E.A.; Collisson, E.W.; Slater, M.; Zhu, G. Phylogenetic analyses of Texas isolates indicate an evolving subtype of the clade B feline immunodeficiency viruses. J. Virol. 2004, 78, 2158–2163. [Google Scholar]
- White, J.; Stickney, A.; Norris, J.M. Feline immunodeficiency virus: disease association versus causation in domestic and nondomestic felids. Vet. Clin. North Am. Small Anim. Pract. 2011, 41, 1197–1208. [Google Scholar]
- Willett, B.J.; Hosie, M.J. Chemokine receptors and co-stimulatory molecules: unravelling feline immunodeficiency virus infection. Vet. Immun. Immunop. 2008, 123, 56–64. [Google Scholar]
- Willett, B.J.; McMonagle, E.L.; Bonci, F.; Pistello, M.; Hosie, M.J. Mapping the domains of CD134 as a functional receptor for feline immunodeficiency virus. J. Virol. 2006a, 80, 7744–7747. [Google Scholar]
- Willett, B.J.; McMonagle, E.L.; Ridha, S.; Hosie, M.J. Differential utilization of CD134 as a functional receptor by diverse strains of feline immunodeficiency virus. J. Virol. 2006b, 80, 3386–3394. [Google Scholar]
- Wozencraft, W.C. Order Carnivora. In Mammal species of the world: a taxonomic and geographic reference, 3nd; Wilson, D.E., Reeder, D.M., Eds.; Johns Hopkins University Press: Baltimore, USA, 2005; Volume 1, pp. 532–628. [Google Scholar]
- Yamada, H.; Miyazawa, T.; Tomonaga, K.; Kawaguchi, Y.; Maeda, K.; Castellano, M.C.; Kai, C.; Tohya, Y.; Mikami, T. Phylogenetic analysis of the long terminal repeat of feline immunodeficiency viruses from Japan, Argentina and Australia. Arch. Virology 1995, 140, 41–52. [Google Scholar]
- Yamamoto, J.K.; Hansen, H.; Ho, E.W.; Morishita, T.Y.; Okuda, T.; Sawa, T.R.; Nakamura, R.M.; Pedersen, N.C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J. Am. Vet. Med. Assoc. 1989, 194, 213–220. [Google Scholar]
- Yamamoto, J.K.; Pu, R.; Sato, E.; Hohdatsu, T. Feline immunodeficiency virus pathogenesis and development of a dual-subtype feline-immunodeficiency-virus vaccine. AIDS 2007, 21, 547–563. [Google Scholar]
- Zanuto, M.S.; Froes, T.R.; Teixeira, A.L.; Hagiwara, M.K. Caracteristicas clinicas da fase aguda da infeccao experimental de felinos pelo virus da imunodeficiencia felina. Pesq. Vet. Brasil. 2011, 31, 255–260. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Teixeira, B.M.; Hagiwara, M.K.; Cruz, J.C.M.; Hosie, M.J. Feline Immunodeficiency Virus in South America. Viruses 2012, 4, 383-396. https://doi.org/10.3390/v4030383
Teixeira BM, Hagiwara MK, Cruz JCM, Hosie MJ. Feline Immunodeficiency Virus in South America. Viruses. 2012; 4(3):383-396. https://doi.org/10.3390/v4030383
Chicago/Turabian StyleTeixeira, Bruno M., Mitika K. Hagiwara, Juliano C. M. Cruz, and Margaret J. Hosie. 2012. "Feline Immunodeficiency Virus in South America" Viruses 4, no. 3: 383-396. https://doi.org/10.3390/v4030383





