Divergence of Primary Cognate B- and T-Cell Proliferative Responses to Subcutaneous and Intravenous Immunization with Virus-Like Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cell Lines, Plasmids, VLP Production and Characterization
2.3. Cell Isolation, Purification and CFSE Labeling
2.4 CD4 Proliferation Assay in Vivo
2.5. CD4 Proliferation Assay in Vitro
2.6. B-Cell Proliferation and Expansion Assay in Vivo
2.7. B-Cell Proliferation in Vitro
3. Results
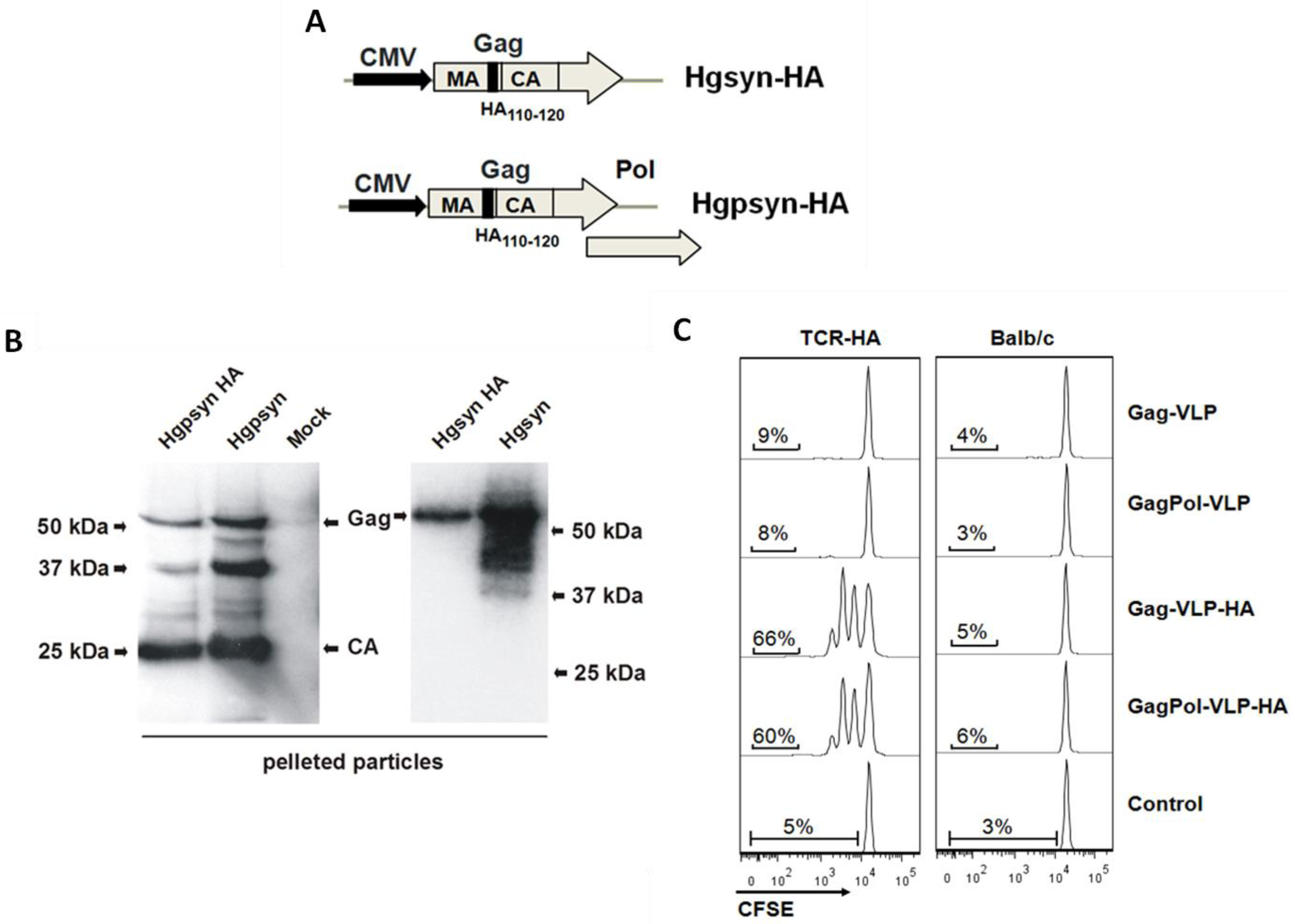
3.1. Adaptation of a TCR-Transgenic Mouse Model for the Activation of CD4+ T-Helper Cells by VLP-Derived Epitopes
| Abbreviations | Envelope Proteins | Core Proteins |
|---|---|---|
| Gag-VLP | HIV-Env | HIV-Gag |
| GagPol-VLP | HIV-Env | HIV-Gag/Pol |
| Gag-VLP-HA | HIV-Env | HIV-Gag with HA110-120 |
| GagPol-VLP-HA | HIV-Env | HIV-Gag/Pol with HA110-120 |
| HEL-VLP | Membrane-anchored HEL | HIV-Gag/Pol |
3.2. Proliferative Response of Naive CD4+ T-Cells to VLP-Derived Epitopes in Vivo

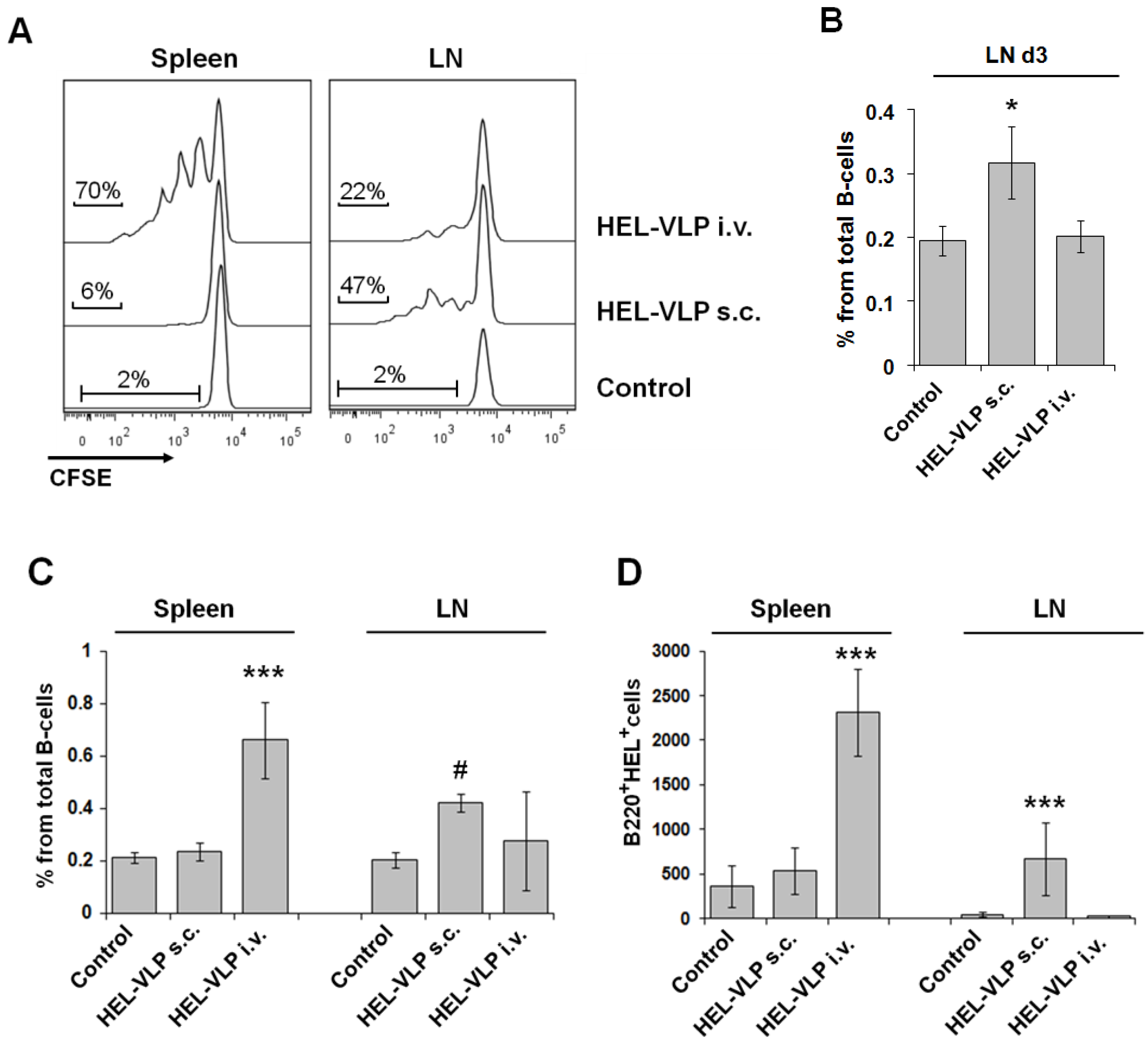
3.3. Proliferation and Expansion of Naive Cognate B-Cells after VLP Immunization
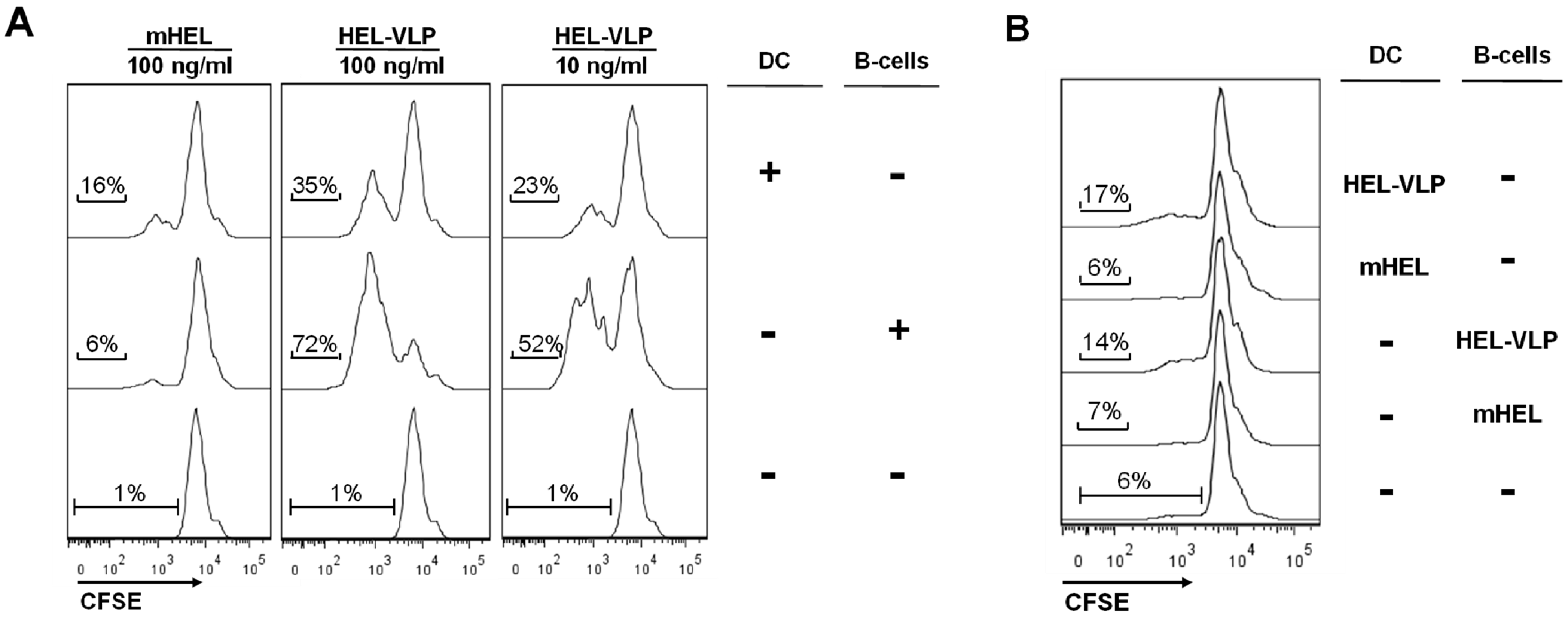
3.4. Efficacy of B-Cell Activation by DC Mediated Transfer of VLP-Associated Surface Antigen in Vitro
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Walker, L.M.; Burton, D.R. Rational antibody-based HIV-1 vaccine design: Current approaches and future directions. Curr. Opin. Immunol. 2010, 22, 358–366. [Google Scholar] [CrossRef]
- McBurney, S.P.; Young, K.R.; Ross, T.M. Membrane embedded HIV-1 envelope on the surface of a virus-like particle elicits broader immune responses than soluble envelopes. Virology 2007, 358, 334–346. [Google Scholar] [CrossRef]
- Nabi, G.; Temchura, V.; Grossmann, C.; Kuate, S.; Tenbusch, M.; Uberla, K. T cell independent secondary antibody responses to the envelope protein of simian immunodeficiency virus. Retrovirology 2012, 9, 42. [Google Scholar] [CrossRef]
- Tong, T.; Crooks, E.T.; Osawa, K.; Robinson, J.E.; Barnes, M.; Apetrei, C.; Binley, J.M. Multi-Parameter Exploration of HIV-1 Virus-Like Particles as Neutralizing Antibody Immunogens in Guinea Pigs, Rabbits and Macaques. Virology 2014, 456–457, 55–69. [Google Scholar] [CrossRef]
- Cubas, R.; Zhang, S.; Kwon, S.; Sevick-Muraca, E.M.; Li, M.; Chen, C.; Yao, Q. Virus-like particle (VLP) Lymphatic Trafficking and Immune Response Generation after Immunization by Different Routes. J. Immunother. 2009, 32, 118–128. [Google Scholar] [CrossRef]
- Batista, F.D.; Harwood, N.E. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 2009, 9, 15–27. [Google Scholar]
- Cyster, J.G. B cell follicles and antigen encounters of the third kind. Nat. Immunol. 2010, 11, 989–996. [Google Scholar] [CrossRef]
- Gonzalez, S.F.; Degn, S.E.; Pitcher, L.A.; Woodruff, M.; Heesters, B.A.; Carroll, M.C. Trafficking of B cell antigen in lymph nodes. Annu. Rev. Immunol. 2011, 29, 215–233. [Google Scholar] [CrossRef]
- McHeyzer-Williams, L.J.; McHeyzer-Williams, M.G. Antigen-specific memory B cell development. Annu. Rev. Immunol. 2005, 23, 487–513. [Google Scholar] [CrossRef]
- Nabi, G.; Genannt Bonsmann, M.S.; Tenbusch, M.; Gardt, O.; Barouch, D.H.; Temchura, V.; Uberla, K. GagPol-specific CD4+ T-cells increase the antibody response to Env by intrastructural help. Retrovirology 2013, 10, 117. [Google Scholar] [CrossRef]
- Wagner, R.; Graf, M.; Bieler, K.; Wolf, H.; Grunwald, T.; Foley, P.; Überla, K. Rev-independent expression of synthetic gag-pol genes of human immunodeficiency virus type 1 and simian immunodeficiency virus: Implications for the safety of lentiviral vectors. Hum. Gene Ther. 2000, 11, 2403–2413. [Google Scholar] [CrossRef]
- Gardt, O.; Grewe, B.; Tippler, B.G.; Überla, K.; Temchura, V.V. HIV-derived lentiviral particles promote T-cell independent activation and differentiation of naïve cognate conventional B2-cells in vitro. Vaccine 2013, 31, 5088–5098. [Google Scholar]
- Grewe, B.; Hoffmann, B.; Ohs, I.; Blissenbach, M.; Brandt, S.; Tippler, B.; Grunwald, T.; Uberla, K. Cytoplasmic utilization of human immunodeficiency virus type 1 genomic RNA is not dependent on a nuclear interaction with gag. J. Virol. 2012, 86, 2990–3002. [Google Scholar] [CrossRef]
- Kirberg, J.; Baron, A.; Jakob, S.; Rolink, A.; Karjalainen, K.; von Boehmer, H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J. Exp. Med. 1994, 180, 25–34. [Google Scholar] [CrossRef]
- Rainone, V.; Dubois, G.; Temchura, V.; Überla, K.; Clivio, A.; Nebuloni, M.; Lauri, E.; Trabattoni, D.; Veas, F.; Clerici, M. CCL28 Induces Mucosal Homing of HIV-1-Specific IgA-Secreting Plasma Cells in Mice Immunized with HIV-1 Virus-Like Particles. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Phan, T.G.; Amesbury, M.; Gardam, S.; Crosbie, J.; Hasbold, J.; Hodgkin, P.D.; Basten, A.; Brink, R. B Cell Receptor—Independent Stimuli Trigger Immunoglobulin (Ig) Class Switch Recombination and Production of IgG Autoantibodies by Anergic Self-Reactive B Cells. J. Exp. Med. 2003, 197, 845–860. [Google Scholar] [CrossRef]
- Wykes, M.; Pombo, A.; Jenkins, C.; MacPherson, G.G. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 1998, 161, 1313–1319. [Google Scholar]
- Wykes, M.; MacPherson, G. Dendritic cell-B-cell interaction: Dendritic cells provide B cells with CD40-independent proliferation signals and CD40-dependent survival signals. Immunology 2000, 100, 1–3. [Google Scholar] [CrossRef]
- Balázs, M.; Martin, F.; Zhou, T.; Kearney, J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 2002, 17, 341–352. [Google Scholar] [CrossRef]
- Tsunetsugu-Yokota, Y.; Morikawa, Y.; Isogai, M.; Kawana-Tachikawa, A.; Odawara, T.; Nakamura, T.; Grassi, F.; Autran, B.; Iwamoto, A. Yeast-derived human immunodeficiency virus type 1 p55(gag) virus-like particles activate dendritic cells (DCs) and induce perforin expression in Gag-specific CD8(+) T cells by cross-presentation of DCs. J. Virol. 2003, 77, 10250–10259. [Google Scholar] [CrossRef]
- Buonaguro, L.; Tornesello, M.L.; Tagliamonte, M.; Gallo, R.C.; Wang, L.X.; Kamin-Lewis, R.; Abdelwahab, S.; Lewis, G.K.; Buonaguro, F.M. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J. Virol. 2006, 80, 9134–9143. [Google Scholar] [CrossRef]
- Deml, L.; Speth, C.; Dierich, M.P.; Wolf, H.; Wagner, R. Recombinant HIV-1 Pr55gag virus-like particles: Potent stimulators of innate and acquired immune responses. Mol. Immunol. 2005, 42, 259–277. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Temchura, V.; Kalinin, S.; Nabi, G.; Tippler, B.; Niezold, T.; Überla, K. Divergence of Primary Cognate B- and T-Cell Proliferative Responses to Subcutaneous and Intravenous Immunization with Virus-Like Particles. Viruses 2014, 6, 3334-3347. https://doi.org/10.3390/v6083334
Temchura V, Kalinin S, Nabi G, Tippler B, Niezold T, Überla K. Divergence of Primary Cognate B- and T-Cell Proliferative Responses to Subcutaneous and Intravenous Immunization with Virus-Like Particles. Viruses. 2014; 6(8):3334-3347. https://doi.org/10.3390/v6083334
Chicago/Turabian StyleTemchura, Vladimir, Svetlana Kalinin, Ghulam Nabi, Bettina Tippler, Thomas Niezold, and Klaus Überla. 2014. "Divergence of Primary Cognate B- and T-Cell Proliferative Responses to Subcutaneous and Intravenous Immunization with Virus-Like Particles" Viruses 6, no. 8: 3334-3347. https://doi.org/10.3390/v6083334





