Vaccinia Virus Natural Infections in Brazil: The Good, the Bad, and the Ugly
Abstract
:1. Introduction
1.1. A Little Bit of History
1.2. The Disease Named Bovine Vaccinia
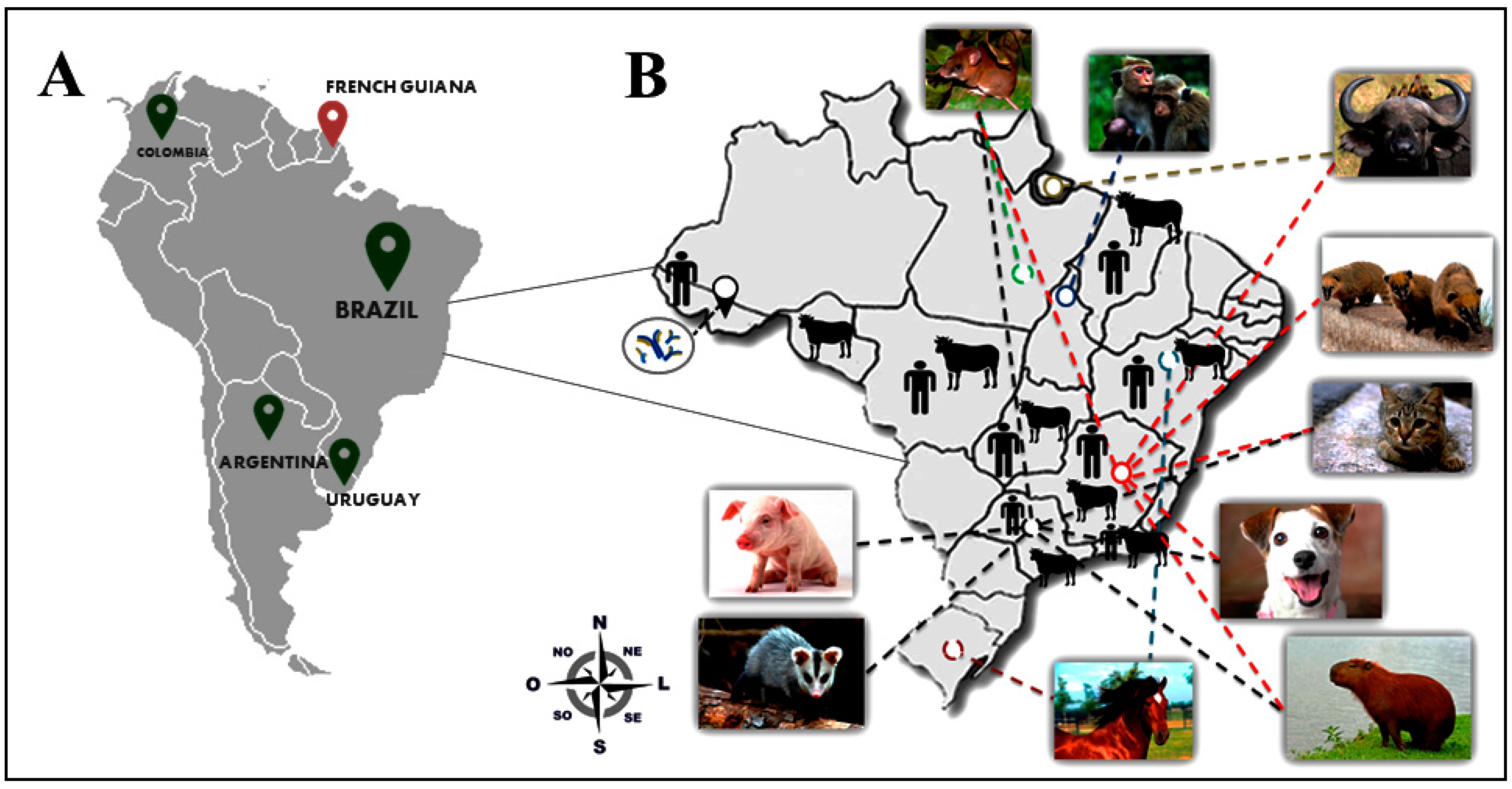
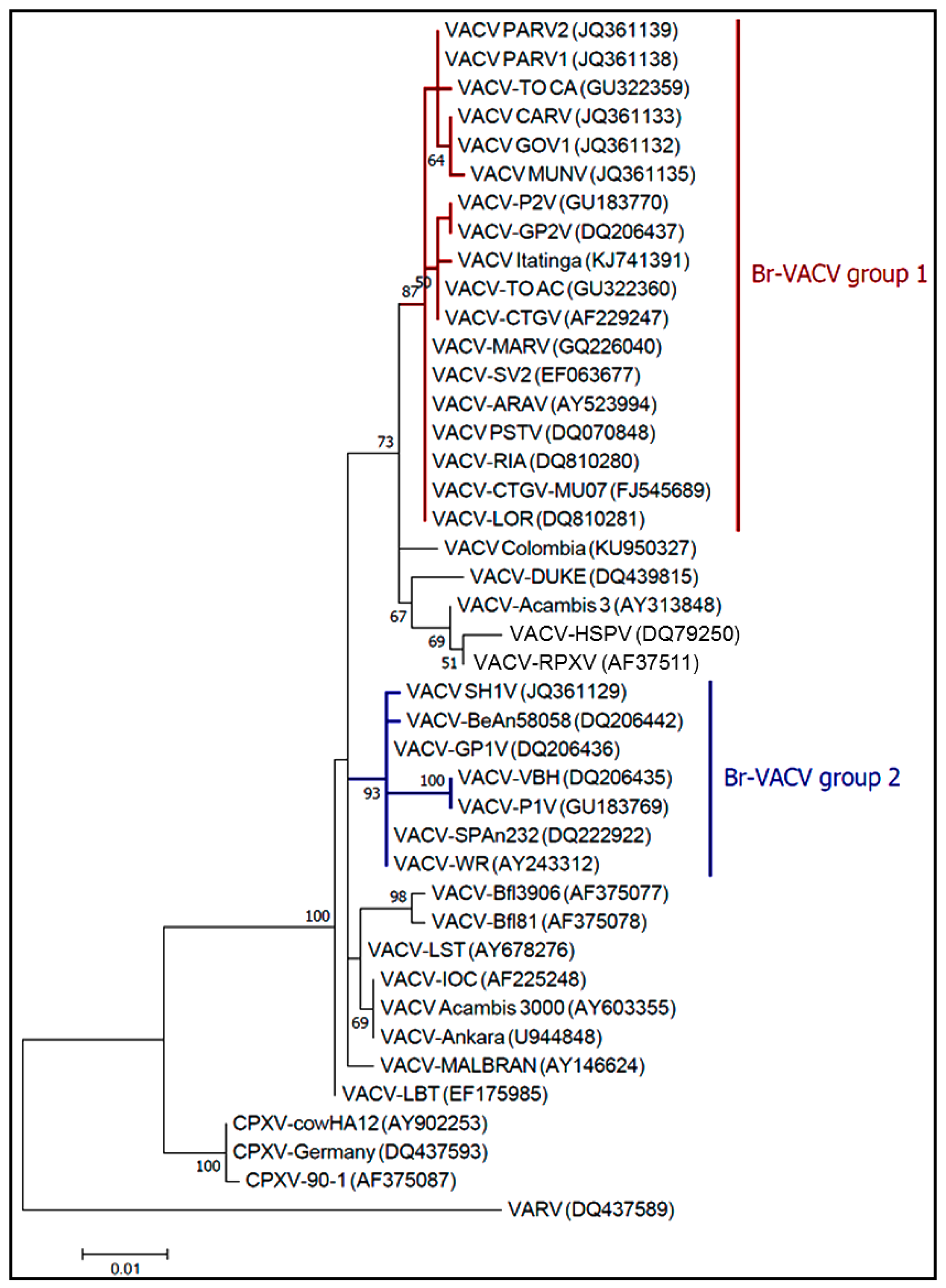
2. The Good: Uncovering the Eco-Epidemiological and Evolutionary Aspects of Natural Infections with Vaccinia Virus
3. The Bad: The Economic and Public Health Burden Associated with Bovine Vaccinia and the Alternative Routes of Zoonotic VACV Transmission in Brazil
3.1. The Burden of Bovine Vaccinia for Agricultural Industry in Brazil
3.2. Bovine Vaccinia: A Neglected Public Health Concern
4. Alternative Routes of Zoonotic VACV Infections
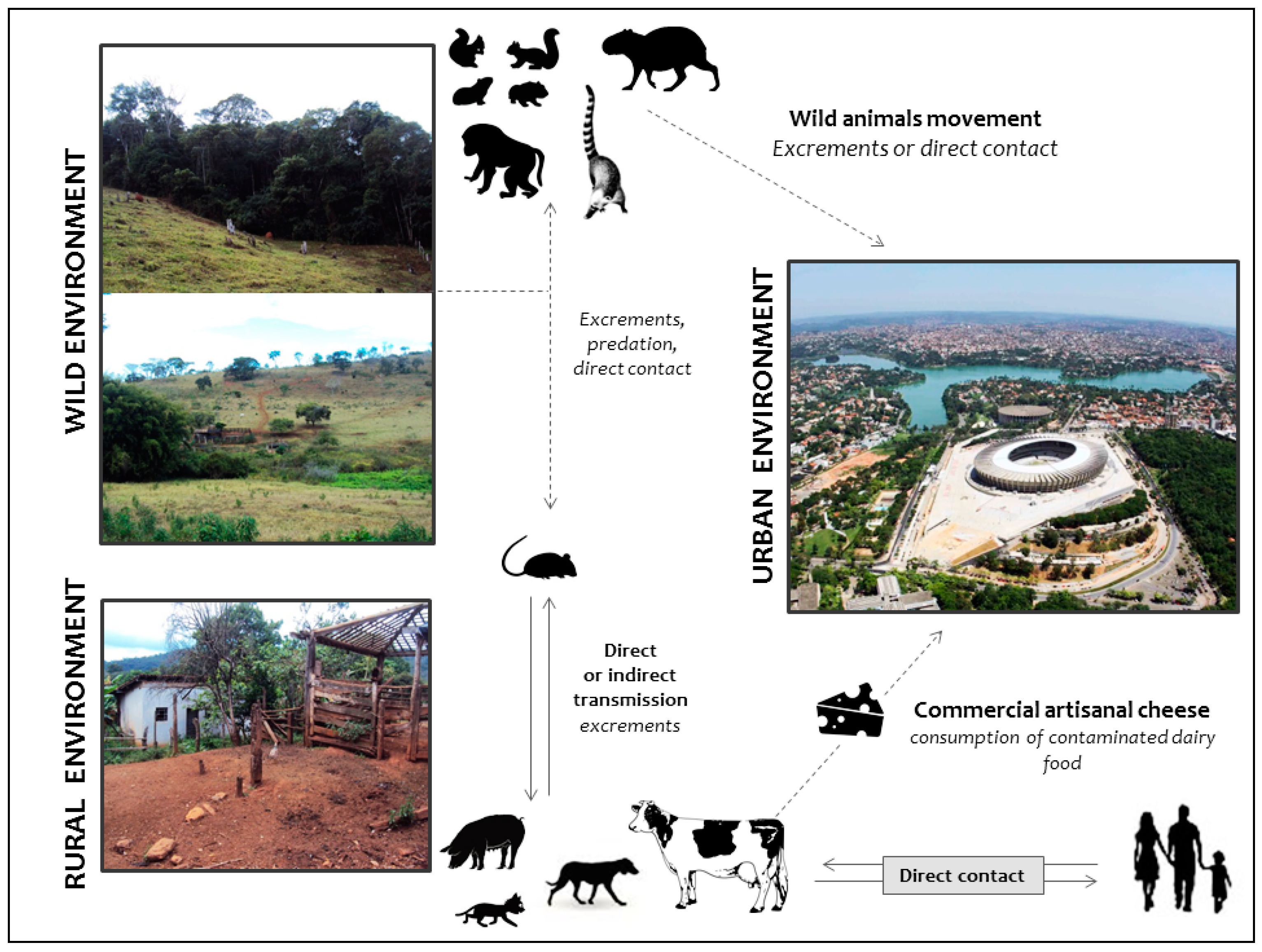
5. The Ugly: Spreading of VACV to Urban Environments
6. Concluding Remarks
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Damon, I. Poxviruses; Lippincott-Raven: Philadelphia, PA, USA, 2013; pp. 2160–2184. [Google Scholar]
- Duggan, A.T.; Perdomo, M.F.; Piombino-Mascali, D.; Marciniak, S.; Poinar, D.; Emery, M.V.; Buchmann, J.P.; Duchêne, S.; Jankauskas, R.; Humphreys, M.; et al. 17th century variola virus reveals the recent history of smallpox. Curr. Biol. 2016, 26, 3407–3412. [Google Scholar] [CrossRef] [PubMed]
- Fenner, F.; Henderson, D. Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Henderson, D.; Preston, R. Smallpox: The Death of a Disease—The Inside Story of Eradicating a Worldwide Killer, 1st ed.; Prometheus Books: Amherst, NY, USA, 2009. [Google Scholar]
- Thèves, C.; Biagini, P.; Crubézy, E. The rediscovery of smallpox. Clin. Microbiol. Infect. 2014, 20, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N. Emergence and reemergence of smallpox: The need for development of a new generation smallpox vaccine. Vaccine 2011, 29, D49–D53. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.A.; Klepac, P. Lessons from the eradication of smallpox: An interview with D.A. Henderson. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog. 2013, 9, e1003756. [Google Scholar] [CrossRef] [PubMed]
- Baxby, D. Jenner’s Smallpox Vaccine; Heinemann Educational Books Ltd.: London, UK, 1981. [Google Scholar]
- Sánchez-Sampedro, L.; Perdiguero, B.; Mejias-Perez, E.; Garcia-Arriaza, J.; di Pilato, M.; Esteban, M. The evolution of poxvirus vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef] [PubMed]
- Downie, A.W. A Study of the Lesions Produced Experimentally by Cowpox Virus; The Pathological Society of Great Britain and Ireland: London, UK, 1939. [Google Scholar]
- Downie, A.W. The Immunological Relationship of the Virus of Spontaneous Cowpox to Vaccinia Virus; The Bacteriological Department of the London Hospital and Medical College: London, UK, 1939. [Google Scholar]
- Damaso, C.R. Revisiting Jenner’s mysteries, the role of the Beaugency lymph in the evolutionary path of ancient smallpox vaccines. Lancet Infect. Dis. 2017. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Archives of the Smallpox Eradication Programme; World Health Organization (WHO): Geneva, Switzerland, 1982. [Google Scholar]
- Trindade, G.S.; Emerson, G.L.; Carroll, D.S.; Kroon, E.G.; Damon, I.K. Brazilian vaccinia viruses and their origins. Emerg. Infect. Dis. 2007, 13, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Moussatché, N.; Damaso, C.R.; McFadden, G. When good vaccines go wild: Feral Orthopoxvirus in developing countries and beyond. J. Infect. Dev. Ctries 2008, 2, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Medaglia, M.L.; Moussatché, N.; Nitsche, A.; Dabrowski, P.W.; Li, Y.; Damon, I.K.; Lucas, C.G.; Arruda, L.B.; Damaso, C.R. Genomic analysis, phenotype, and virulence of the historical brazilian smallpox vaccine strain IOC: Implications for the origins and evolutionary relationships of vaccinia virus. J. Virol. 2015, 89, 11909–11925. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.B.; Borges, I.A.; Campos, S.P.; Vieira, F.N.; de Ázara, T.M.; Marques, F.A.; Costa, G.B.; Luis, A.P.M.; de Oliveira, J.S.; Ferreira, P.C.P.; et al. Serologic and molecular evidence of vaccinia virus circulation among small mammals from different biomes, Brazil. Emerg. Infect. Dis. 2017, 23, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, G.; Balamurugan, V.; Prabhu, M.; Yogisharadhya, R.; Bora, D.P.; Gandhale, P.N.; Sankar, M.S.; Kulkarni, A.M.; Singh, R.K.; Bhanuprakash, V. Emerging and re-emerging zoonotic buffalopox infection: A severe outbreak in Kolhapur (Maharashtra), India. Vet. Ital. 2010, 46, 439–448. [Google Scholar] [PubMed]
- Singh, R.K.; Balamurugan, V.; Bhanuprakash, V.; Venkatesan, G.; Hosamani, M. Emergence and reemergence of vaccinia-like viruses: Global scenario and perspectives. Indian J. Virol. 2012, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Goyal, T.; Varshney, A.; Bakshi, S.K.; Barua, S.; Bera, B.C.; Singh, R.K. Buffalo pox outbreak with atypical features: A word of caution and need for early intervention! Int. J. Dermatol. 2013, 52, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Baxby, D.; Hill, B.J. Characteristics of a new poxvirus isolated from Indian buffaloes. Arch. Gesamte Virusforsch. 1971, 35, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Singh, I.P.; Garg, S.K.; Varshney, K.C. Experimental pathogenesis of buffalo pox virus in rabbits: Clinico-pathological studies. Acta Virol. 1986, 30, 390–396. [Google Scholar] [PubMed]
- Dumbell, K.; Richardson, M. Virological investigations of specimens from buffaloes affected by buffalopox in Maharashtra State, India between 1985 and 1987. Arch. Virol. 1993, 128, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Nedunchelliyan, S.; Reddy, D.S.; Venkataraman, K.S. Buffalo pox infection in man. Indian J. Public Health 1992, 36, 57. [Google Scholar] [PubMed]
- Kolhapure, R.M.; Deolankar, R.P.; Tupe, C.D.; Raut, C.G.; Basu, A.; Dama, B.M.; Pawar, S.D.; Joshi, M.V.; Padbidri, V.S.; Goverdhan, M.K.; et al. Investigation of buffalopox outbreaks in Maharashtra State during 1992–1996. Indian J. Med. Res. 1997, 106, 441–446. [Google Scholar] [PubMed]
- Bhanuprakash, V.; Venkatesan, G.; Balamurugan, V.; Hosamani, M.; Yogisharadhya, R.; Chauhan, R.S.; Pande, A.; Mondal, B.; Singh, R.K. Pox outbreaks in sheep and goats at Makhdoom (Uttar Pradesh), India: Evidence of sheeppox virus infection in goats. Transbound. Emerg. Dis. 2010, 57, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Hosamani, M.; Balamurugan, V.; Satheesh, C.C.; Shingal, K.R.; Tatwarti, S.B.; Bambal, R.G.; Ramteke, V.; Yadav, M.P. An outbreak of buffalopox in buffalo (Bubalus bubalis) dairy herds in Aurangabad, India. Rev. Sci. Tech. 2006, 25, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Fenner, F. The biological characters of several strains of vaccinia, cowpox and rabbitpox viruses. Virology 1958, 5, 502–529. [Google Scholar] [CrossRef]
- DiGiacomo, R.F.; Maré, C. The Biology of the Laboratory Rabbit, 2nd ed.; Academic Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Franco-Luiz, A.P.; Fagundes-Pereira, A.; Costa, G.B.; Alves, P.A.; Oliveira, D.B.; Bonjardim, C.A.; Ferreira, P.C.P.; de Souza Trindade, G.; Panei, C.J.; Galosi, C.M.; et al. Spread of vaccinia virus to cattle herds, Argentina, 2011. Emerg. Infect. Dis. 2014, 20, 1576–1578. [Google Scholar] [CrossRef] [PubMed]
- Franco-Luiz, A.P.; Oliveira, D.B.; Pereira, A.F.; Gasparini, M.C.S.; Bonjardim, C.A.; Ferreira, P.C.P.; de Souza Trindade, G.; Puentes, R.; Furtado, A.; Abrahão, J.S.; et al. Detection of vaccinia virus in dairy cattle serum samples from 2009, uruguay. Emerg. Infect. Dis. 2016, 22, 2174–2177. [Google Scholar] [CrossRef] [PubMed]
- Usme-Ciro, J.A.; Paredes, A.; Walteros, D.M.; Tolosa-Pérez, E.N.; Laiton-Donato, K.; del Carmen Pinzón, M.; Petersen, B.W.; Gallardo-Romero, N.F.; Li, Y.; Wilkins, K.; et al. Detection and molecular characterization of zoonotic poxviruses circulating in the amazon region of Colombia, 2014. Emerg. Infect. Dis. 2017, 23, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Kroon, E.G.; Mota, B.E.F.; Abrahão, J.S.; da Fonseca, F.G.; de Souza Trindade, G. Zoonotic Brazilian Vaccinia virus: From field to therapy. Antivir. Res. 2011, 92, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Damaso, C.R.; Esposito, J.J.; Condit, R.C.; Moussatché, N. An emergent poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virology 2000, 277, 439–449. [Google Scholar] [CrossRef] [PubMed]
- De Souza Trindade, G.; da Fonseca, F.G.; Marques, J.T.; Nogueira, M.L.; Mendes, L.C.N.; Borges, A.S.; Peiró, J.R.; Pituco, E.M.; Bonjardim, C.A.; Ferreira, P.C.P.; et al. Araçatuba virus: A vaccinialike virus associated with infection in humans and cattle. Emerg. Infect. Dis. 2003, 9, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Nagasse-Sugahara, T.K.; Kisielius, J.J.; Ueda-Ito, M.; Curti, S.P.; Figueiredo, C.A.; Cruz, Á.S.; Silva, M.M.J.; Ramos, C.H.; Silva, M.C.C.; Sakurai, T.; et al. Human vaccinia-like virus outbreaks in São Paulo and Goiás States, Brazil: Virus detection, isolation and identification. Rev. Inst. Med. Trop. Sao Paulo 2004, 46, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.; Drumond, B.P.; Trindade, G.S.; Lobato, Z.I.; da Fonseca, F.G.; dos Santos, J.R.; Madureira, M.C.; Guedes, M.I.; Ferreira, J.M.; Bonjardim, C.A.; et al. Passatempo virus, a vaccinia virus strain, Brazil. Emerg. Infect. Dis. 2005, 11, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- De Souza Trindade, G.; Drumond, B.P.; Guedes, M.I.M.C.; Leite, J.A.; Mota, B.E.F.; Campos, M.A.; da Fonseca, F.G.; Nogueira, M.L.; Lobato, Z.I.P.; Bonjardim, C.A.; et al. Zoonotic vaccinia virus infection in Brazil: Clinical description and implications for health professionals. J. Clin. Microbiol. 2007, 45, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Angerami, R. Viroses Emergentes No Brasil; Editora Fiocruz: Rio de Janeiro, Brazil, 2008; p. 132. [Google Scholar]
- Megid, J.; Appolinário, C.M.; Langoni, H.; Pituco, E.M.; Okuda, L.H. Vaccinia virus in humans and cattle in southwest region of Sao Paulo state, Brazil. Am. J. Trop. Med. Hyg. 2008, 79, 647–651. [Google Scholar] [PubMed]
- Silva-Fernandes, A.T.; Travassos, C.E.P.F.; Ferreira, J.M.S.; Abrahão, J.S.; de Oliveira Rocha, E.S.; Viana-Ferreira, F.; dos Santos, J.R.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Natural human infections with Vaccinia virus during bovine vaccinia outbreaks. J. Clin. Virol. 2009, 44, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Trindade, G.S.; Guedes, M.I.; Drumond, B.P.; Mota, B.E.; Abrahão, J.S.; Lobato, Z.I.; Gomes, J.A.; Corrêa-Oliveira, R.; Nogueira, M.L.; Kroon, E.G.; et al. Zoonotic vaccinia virus: Clinical and immunological characteristics in a naturally infected patient. Clin. Infect. Dis. 2009, 48, e37–e40. [Google Scholar] [CrossRef] [PubMed]
- Megid, J.; Borges, I.A.; Abrahão, J.S.; Trindade, G.S.; Appolinário, C.M.; Ribeiro, M.G.; Allendorf, S.D.; Antunes, J.M.A.; Silva-Fernandes, A.T.; Kroon, E.G. Vaccinia virus zoonotic infection, São Paulo State, Brazil. Emerg. Infect. Dis. 2012, 18, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Sant’ana, F.F.; Leal, A.D.A.; Rabelo, R.E.; Vulcani, V.A.; Junior, F.; Jair, A.; Cargnelutti, J.F.; Flores, E.F. Outbreaks of vesicular diseases caused by Vaccinia virus in dairy cattle from Goiás State, Brazil (2010–2012). Pesqui. Vet. Bras. 2013, 860–866. [Google Scholar] [CrossRef]
- Schatzmayr, G.H.; Romijn, P.C.; Barreto, D.F.; Silva, E.E.; da Costa Farias Filho, J.; Tavares, A.F.D.A.; Barth, O.M. An outbreak of vesicopustular disease in humans and dairy cattle in the state of Rio de Janeiro in 2006. Virus Rev. Res. 2005, 10. [Google Scholar] [CrossRef]
- Rivetti, A.V.; Guedes, M.I.M.; Rehfeld, I.S.; Oliveira, T.M.; Matos, A.C.D.; Abrahão, J.S.; Kroon, E.G.; Lobato, Z.I. Bovine vaccinia, a systemic infection: Evidence of fecal shedding, viremia and detection in lymphoid organs. Vet. Microbiol. 2013, 162, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Guedes, M.I.; Rehfeld, I.S.; Oliveira, T.M.L.; Assis, F.L.; Matos, A.C.D.; Abrahão, J.S.; Kroon, E.G.; Lobato, Z.I.P. Detection of Vaccinia virus in blood and faeces of experimentally infected cows. Transbound. Emerg. Dis. 2013, 60, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, I.S.; Guedes, M.I.M.; Matos, A.C.D.; de Oliveira, T.M.; Junior, A.V.R.; Moura, A.C.J.; Paes, P.R.O.; do Lago, L.A.; Kroon, E.G.; Lobato, Z.I.P. Clinical, hematological and biochemical parameters of dairy cows experimentally infected with Vaccinia virus. Res. Vet. Sci. 2013, 95, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, I.S.; Fraiha, A.L.S.; Matos, A.C.D.; Guedes, M.I.M.; Costa, E.A.; de Souza, M.R.; Cavalcante, L.F.; Lobato, Z.I. Short communication: Survival of Vaccinia virus in inoculated cheeses during 60-day ripening. J. Dairy Sci. 2017, 100, 7051–7054. [Google Scholar] [CrossRef] [PubMed]
- De Souza Trindade, G.; Li, Y.; Olson, V.A.; Emerson, G.; Regnery, R.L.; da Fonseca, F.G.; Kroon, E.G.; Damon, I. Real-time PCR assay to identify variants of Vaccinia virus: Implications for the diagnosis of bovine vaccinia in Brazil. J. Virol. Methods 2008, 152, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, I.S.; Matos, A.C.D.; Guedes, M.I.M.C.; Costa, A.G.; Fraiha, A.L.S.; Lobato, Z.I.P. Subclinical bovine vaccinia: An important risk factor in the epidemiology of this zoonosis in cattle. Res. Vet. Sci. 2017, 114, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Lobato, Z.; Trindade, G.S.; Frois, M.C.M.; Ribeiro, E.B.T.; Dias, G.R.C.; Teixeira, B.M.; Lima, F.A.; Almeida, G.M.F.; Kroon, E.G. Outbreak of exantemal disease caused by Vaccinia virus in human and cattle in Zona da Mata region, Minas Gerais. Arq. Bras. Med. Vet. Zootec. 2005, 57. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Silva-Fernandes, A.T.; Assis, F.L.; Guedes, M.I.; Drumond, B.P.; Leite, J.A.; Coelho, L.F.; Turrini, F.; Fonseca, F.G.; Lobato, Z.I.; et al. Human Vaccinia virus and Pseudocowpox virus co-infection: Clinical description and phylogenetic characterization. J. Clin. Virol. 2010, 48, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Trindade, G.; Guedes, M.I.M.C.; Costa, G.B.; Figueiredo, P.O.; Abrahão, J.S.; Kroon, E.G.; Foseca, F.G. A 31 year-old Brazilian man with exanthematous lesions. J. Vaccines Vaccin. 2014, 5. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Guedes, M.I.M.; Trindade, G.S.; Fonseca, F.G.; Campos, R.K.; Mota, B.F.; Lobato, Z.I.; Silva-Fernandes, A.T.; Rodrigues, G.O.; Lima, L.S.; et al. One more piece in the VACV ecological puzzle: Could peridomestic rodents be the link between wildlife and bovine vaccinia outbreaks in Brazil? PLoS ONE 2009, 4, e7428. [Google Scholar] [CrossRef] [PubMed]
- Schatzmayr, H.G.; Costa, R.V.C.; Gonçalves, M.C.R.; Barreto, D.F.; Batista, V.H.; Silva, M.E.V.; Brust, L.A.C.; Barth, O.M. Human infections caused by vaccinia-like poxviruses in Brazil. Rev. Soc. Bras. Med. Trop. 2009, 42, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.L.; Borges, I.A.; Mesquita, V.S.; Ferreira, P.C.; Trindade, G.S.; Kroon, E.G.; Abrahão, J.S. Vaccinia virus in household environment during bovine vaccinia outbreak, Brazil. Emerg. Infect. Dis. 2013, 19, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Campos, R.K.; de Souza Trindade, G.; da Fonseca, F.G.; Ferreira, P.C.P.; Kroon, E.G. Outbreak of severe zoonotic vaccinia virus infection, Southeastern Brazil. Emerg. Infect. Dis. 2015, 21, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.B.; Assis, F.L.; Ferreira, P.C.P.; Bonjardim, C.A.; de Souza Trindade, G.; Kroon, E.G.; Abrahão, J.S. Group 1 Vaccinia virus zoonotic outbreak in Maranhao State, Brazil. Am. J. Trop. Med. Hyg. 2013, 89, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Franco-Luiz, A.P.; Pereira, A.F.; de Oliveira, C.H.S.; Barbosa, J.D.; Oliveira, D.B.; Bonjardim, C.A.; Ferreira, P.C.P.; de Souza Trindade, G.; Abrahão, J.S.; Kroon, E.G. The detection of Vaccinia virus confirms the high circulation of Orthopoxvirus in buffaloes living in geographical isolation, Marajó Island, Brazilian Amazon. Comp. Immunol. Microbiol. Infect. Dis. 2016, 46, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.G.; Lanna, M.C.S.; Campos, M.A.S.; Kitajima, E.W.; Peres, J.N.; Golgher, R.R.; Ferreira, P.C.P.; Kroon, E.G. Morphological and molecular characterization of the poxvirus BeAn 58058. Arch. Virol. 1998, 143, 1171–1186. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, F.G.; Trindade, G.S.; Silva, R.L.; Bonjardim, C.A.; Ferreira, P.C.; Kroon, E.G. Characterization of a vaccinia-like virus isolated in a Brazilian forest. J. Gen. Virol. 2002, 83, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.T.; de Souza Trindade, G.; Da Fonseca, F.G.; Dos Santos, J.R.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Characterization of ATI, TK and IFN-α/βR genes in the genome of the BeAn 58058 virus, a naturally attenuated wild Orthopoxvirus. Virus Genes 2001, 23, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Rebanho Bovino Brasileiro Cresce e Chega a 212.3 Milhões de Cabeças de Gado. 2015. Available online: http://www.brasil.gov.br/ (accessed on 16 October 2017).
- Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Dados de Rebanho Bovino e Bubalino No Brasil—2015. Available online: http://www.agricultura.gov.br/ (accessed on 10 September 2017).
- Quiner, C.A.; Nakazawa, Y. Ecological niche modeling to determine potential niche of Vaccinia virus: A case only study. Int. J. Health Geogr. 2017, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.L.; Almeida, G.M.; Oliveira, D.B.; Franco-Luiz, A.P.; Campos, R.K.; Guedes, M.I.; Fonseca, F.G.; Trindade, G.S.; Drumond, B.P.; Kroon, E.G.; et al. Characterization of a new Vaccinia virus isolate reveals the C23L gene as a putative genetic marker for autochthonous Group 1 Brazilian Vaccinia virus. PLoS ONE 2012, 7, e50413. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.G.; Barros, C.B.; Appolinário, C.M.; Antunes, J.M.; Mioni, M.S.; Bacchiega, T.S.; Allendorf, S.D.; Vicente, A.F.; Fonseca, C.R.; Megid, J. Dogs and opossums positive for Vaccinia virus during outbreak affecting cattle and humans, São Paulo State, Brazil. Emerg. Infect. Dis. 2016, 22, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.B.; Miranda, J.B.; Almeida, G.G.; de Oliveira, J.S.; Pinheiro, M.S.; Gonçalves, S.A.; dos Reis, J.K.P.; Gonçalves, R.; Ferreira, P.C.P.; Bonjardim, C.A.; et al. Detection of Vaccinia virus in urban domestic cats, Brazil. Emerg. Infect. Dis. 2017, 23, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Souza Trindade, G.; Pereira-Oliveira, G.; Oliveira Figueiredo, P.; Costa, G.; Moreira Franco-Luiz, A.P.; Lopes Assis, F.; Bretas de Oliveira, D.; Mattos Paim, L.R.; Araújo Oliveira, C.E.; et al. Detection of Vaccinia virus during an outbreak of exanthemous oral lesions in Brazilian equids. Equine Vet. J. 2017, 49, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Silva-Fernandes, A.T.; Lima, L.S.; Campos, R.K.; Guedes, M.I.; Cota, M.M.; Assis, F.L.; Borges, I.A.; Souza-Júnior, M.F.; Lobato, Z.I.; et al. Vaccinia virus infection in monkeys, Brazilian Amazon. Emerg. Infect. Dis. 2010, 16, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.V.; Medaglia, M.L.G.; Soares, H.S.; Quixabeira-Santos, J.C.; Gennari, S.M.; Damaso, C.R. Presence of neutralizing antibodies to Orthopoxvirus in capybaras (Hydrochoerus hydrochaeris) in Brazil. J. Infect. Dev. Ctries. 2014, 8, 1646–1649. [Google Scholar] [CrossRef] [PubMed]
- Dutra, L.A.; de Freitas Almeida, G.M.; Oliveira, G.P.; Abrahão, J.S.; Kroon, E.G.; de Souza Trindade, G. Molecular evidence of Orthopoxvirus DNA in capybara (Hydrochoerus hydrochaeris) stool samples. Arch. Virol. 2017, 162, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Drumond, B.P.; Leite, J.A.; da Fonseca, F.G.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Brazilian Vaccinia virus strains are genetically divergent and differ from the Lister vaccine strain. Microbes Infect. 2008, 10, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Oliveira, T.M.; Campos, R.K.; Madureira, M.C.; Kroon, E.G.; Lobato, Z.I. Bovine vaccinia outbreaks: Detection and isolation of vaccinia virus in milk samples. Foodborne Pathog. Dis. 2009, 6, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.G.; Bacchiega, T.S.; Appolinário, C.M.; Vicente, A.F.; Allendorf, S.D.; Antunes, J.M.A.P.; Moreira, S.A.; Legatti, E.; Fonseca, C.R.; Pituco, E.M.; et al. Serological study of vaccinia virus reservoirs in areas with and without official reports of outbreaks in cattle and humans in São Paulo, Brazil. Arch. Virol. 2013, 158, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Trindade, G.S.; Emerson, G.L.; Sammons, S.; Frace, M.; Govil, D.; Fernandes Mota, B.E.; Abrahão, J.S.; de Assis, F.L.; Olsen-Rasmussen, M.; Goldsmith, C.S.; et al. Serro 2 virus highlights the fundamental genomic and biological features of a natural Vaccinia virus infecting humans. Viruses 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.K.; Brum, M.C.; Nogueira, C.E.; Drumond, B.P.; Alves, P.A.; Siqueira-Lima, L.; Assis, F.L.; Trindade, G.S.; Bonjardim, C.A.; Ferreira, P.C.; et al. Assessing the variability of Brazilian Vaccinia virus isolates from a horse exanthematic lesion: Coinfection with distinct viruses. Arch. Virol. 2011, 156, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Drumond, B.P.; de Souza Trindade, G.; da Silva-Fernandes, A.T.; Ferreira, J.M.S.; Alves, P.A.; Campos, R.K.; Siqueira, L.; Bonjardim, C.A.; Ferreira, P.C.P.; et al. Rapid detection of Orthopoxvirus by semi-nested PCR directly from clinical specimens: A useful alternative for routine laboratories. J. Med. Virol. 2010, 82, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. Improved sensitivity of profile searches through the use of sequence weights and gap excision. Comput. Appl. Biosci. 1994, 10, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Multiple alignment using hidden Markov models. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1995, 3, 114–120. [Google Scholar] [PubMed]
- Leite, J.A.; Drumond, B.P.; de Souza Trindade, G.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Brazilian Vaccinia virus strains show genetic polymorphism at the ati gene. Virus Genes 2007, 35, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Viana, F.; Ribeiro, S. Surto de varíola bovina no município de Prata—MG. Bras. Med. Vet. Zootec. 1985, 38, 323–330. [Google Scholar]
- Rodrigues-Da-Silva, G.; Rabello, S.I.; Angulo, J.J. Epidemic of variola minor in a suburb of São Paulo. Public Health Rep. 1963, 78, 165–174. [Google Scholar] [CrossRef] [PubMed]
- De Quadros, C.C.; Morris, L.; da Costa, E.A.; Arnt, N.; Tigre, C.H. Epidemiology of variola minor in Brazil based on a study of 33 outbreaks. Bull. World Health Organ. 1972, 46, 165–171. [Google Scholar] [PubMed]
- Behbehani, A.M. The smallpox story: Life and death of an old disease. Microbiol. Rev. 1983, 47, 455–509. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro de Geografia e Estatística (IBGE). 2016. Available online: https://www.ibge.gov.br/ (accessed on 16 October 2016).
- Empresa Brasileira de Pesquisa Agrocpecuária (EMBRAPA). 2016. Available online: https://www.embrapa.br/ (accessed on 22 October 2016).
- Trindade, G.S.; Lobato, Z.I.; Drumond, B.P.; Leite, J.A.; Trigueiro, R.C.; Guedes, M.I.; Da Fonseca, F.G.; Dos Santos, J.R.; Bonjardim, C.A.; Ferreira, P.C.; et al. Short report: Isolation of two vaccinia virus strains from a single bovine vaccinia outbreak in rural area from Brazil: Implications on the emergence of zoonotic orthopoxviruses. Am. J. Trop. Med. Hyg. 2006, 75, 486–490. [Google Scholar] [PubMed]
- Da Fonseca, F.G.; Kroon, E.G.; Nogueira, M.L.; de Souza Trindade, G. Zoonotic Vaccinia virus outbreaks in Brazil. Future Virol. 2011, 6, 697–707. [Google Scholar] [CrossRef]
- Costa, G.B.; Augusto, L.T.S.; Leite, J.A.; Ferreira, P.C.P.; Bonjardim, C.A.; Abrahão, J.S.; Kroon, E.G.; Moreno, E.C.; de Souza Trindade, G. Seroprevalence of Orthopoxvirus in rural Brazil: Insights into anti-OPV immunity status and its implications for emergent zoonotic OPV. Virol. J. 2016, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.B.; Borges, I.A.; Alves, P.A.; Miranda, J.B.; Luiz, A.P.M.; Ferreira, P.C.; Abrahão, J.S.; Moreno, E.C.; Kroon, E.G.; de Souza Trindade, G. Alternative routes of zoonotic Vaccinia virus transmission, Brazil. Emerg. Infect. Dis. 2015, 21, 2244–2246. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.S.; Costa, G.B.; Luiz, A.P.M.F.; Leite, J.A.; Bonjardim, C.A.; Abrahão, J.S.; Drumond, B.P.; Kroon, E.G.; de Souza Trindade, G. Cross-sectional study involving healthcare professionals in a Vaccinia virus endemic area. Vaccine 2017, 35, 3281–3285. [Google Scholar] [CrossRef] [PubMed]
- Moojen, V.; Riet-Correa, F.; Roehe, P.M.; Weiblen, R. Viroses Confundíveis Com Febre Aftosa; Ciencia Rural: Santa Maria, Brazil, 1996. [Google Scholar]
- Quixabeira-Santos, J.C.; Medaglia, M.L.G.; Pescador, C.A.; Damaso, C.R. Animal movement and establishment of vaccinia virus Cantagalo strain in Amazon biome, Brazil. Emerg. Infect. Dis. 2011, 17, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Laguardia-Nascimento, M.; Sales, É.B.; Gasparini, M.R.; de Souza, N.M.; da Silva, J.A.G.; Souza, G.G.; Carani, F.R.; dos Santos, A.F.; Rivetti Júnior, A.V.; Camargos, M.F.; et al. Detection of multiple viral infections in cattle and buffalo with suspected vesicular disease in Brazil. J. Vet. Diagn. Investig. 2016, 28, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Gurav, Y.K.; Raut, C.G.; Yadav, P.D.; Tandale, B.V.; Sivaram, A.; Pore, M.D.; Basu, A.; Mourya, D.T.; Mishra, A.C. Buffalopox outbreak in humans and animals in Western Maharashtra, India. Prev. Vet. Med. 2011, 100, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Silva Gomes, J.A.; de Araújo, F.F.; Trindade, G.D.S.; Quinan, B.R.; Drumond, B.P.; Ferreira, J.M.S.; Mota, B.E.F.; Nogueira, M.L.; Kroon, E.G.; Abrahão, J.S.; et al. Immune modulation in primary Vaccinia virus zoonotic human infections. Clin. Dev. Immunol. 2012, 2012. [Google Scholar] [CrossRef]
- Alves, P.A.; Figueiredo, P.O.; de Oliveira, C.H.; Barbosa, J.D.; Lima, D.H.; Bomjardim, H.A.; Silva, N.S.; Campos, K.F.; Oliveira, C.M.C.; Barbosa-Stancioli, E.F.; et al. Occurrence of Pseudocowpox virus associated to Bovine viral diarrhea virus-1, Brazilian Amazon. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Laguardia-Nascimento, M.; de Oliveira, A.P.F.; Azevedo, I.C.; Júnior, A.V.R.; Camargos, M.F.; Júnior, A.A.F. Spread of poxviruses in livestock in Brazil associated with cases of double and triple infection. Arch. Virol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.; Damon, I.; Fulginiti, V.; Weber, S.G.; Kahana, M.; Stein, S.L.; Gerber, S.I.; Garcia-Houchins, S.; Lederman, E.; Hruby, D.; et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin. Infect. Dis. 2008, 46, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Lederman, E.; Miramontes, R.; Openshaw, J.; Olson, V.A.; Karem, K.L.; Marcinak, J.; Panares, R.; Staggs, W.; Allen, D.; Weber, S.G.; et al. Eczema vaccinatum resulting from the transmission of Vaccinia virus from a smallpox vaccinee: An investigation of potential fomites in the home environment. Vaccine 2009, 27, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Batista, V.H.; Scremin, J.; Aguiar, L.M.; Schatzmayr, H.G. Vulvar infection and possible human-to-human transmission of bovine poxvirus disease. Virus Rev. Res. 2009. [Google Scholar] [CrossRef]
- Hughes, C.M.; Blythe, D.; Li, Y.; Reddy, R.; Jordan, C.; Edwards, C.; Adams, C.; Conners, H.; Rasa, C.; Wilby, S.; et al. Vaccinia virus infections in martial arts gym, Maryland, USA, 2008. Emerg. Infect. Dis. 2011, 17, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Young, G.E.; Hidalgo, C.M.; Sullivan-Frohm, A.; Schulte, C.; Davis, S.; Kelly-Cirino, C.; Egan, C.; Wilkins, K.; Emerson, G.L.; Noyes, K.; et al. Secondary and tertiary transmission of vaccinia virus from US military service member. Emerg. Infect. Dis. 2011, 17, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, E.R.; Olive, D.S.; Brundage, J.F.; Clark, L.L. Contact transmission of vaccinia virus from smallpox vaccinees in the United States, 2003–2011. Vaccine 2012, 30, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Pereira Oliveira, G.; Fernandes, A.T.S.; de Assis, F.L.; Alves, P.A.; Luiz, A.P.M.F.; Figueiredo, L.B.; de Almeida, C.M.C.; Travassos, C.E.P.F.; de Souza Trindade, G.; Abrahão, J.S.; et al. Intrafamilial transmission of Vaccinia virus during a bovine Vaccinia outbreak in Brazil: A new insight in viral transmission chain. Am. J. Trop. Med. Hyg. 2014, 90, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Swanepoel, R.; Hewson, R.; Nizam, M.; Ahmed, A.; Husain, A.; Grobbelaar, A.; Bewley, K.; Mioulet, V.; Dowsett, B.; et al. Nosocomial buffalopoxvirus infection, Karachi, Pakistan. Emerg. Infect. Dis. 2007, 13, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, P.; Zange, S.; Ibrahim, S.; Zoeller, G.; Herbstreit, F.; Meyer, H. Generalized cowpox virus infection in a patient with HIV, Germany, 2012. Emerg. Infect. Dis. 2016, 22, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Mota, B.E.; Trindade, G.S.; Diniz, T.C.; da Silva-Nunes, M.; Braga, E.M.; Urbano-Ferreira, M.; Rodrigues, G.O.L.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Seroprevalence of orthopoxvirus in an Amazonian rural village, Acre, Brazil. Arch. Virol. 2010, 155, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Essbauer, S.; Meyer, H.; Porsch-Özcürümez, M.; Pfeffer, M. Long-lasting stability of vaccinia virus (orthopoxvirus) in food and environmental samples. Zoonoses Public Health 2007, 54, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; de Souza Trindade, G.; Ferreira, J.M.S.; Campos, R.K.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Long-lasting stability of Vaccinia virus strains in murine feces: Implications for virus circulation and environmental maintenance. Arch. Virol. 2009, 154, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.M.; Rehfeld, I.S.; Siqueira, J.M.F.; Abrahao, J.S.; Campos, R.K.; dos Santos, A.K.R.; Cerqueira, M.M.O.; Kroon, E.G.; Lobato, Z.I. Vaccinia virus is not inactivated after thermal treatment and cheese production using experimentally contaminated milk. Foodborne Pathog. Dis. 2010, 7, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.M.; Guedes, M.I.M.C.; Rehfeld, I.S.; Matos, A.C.D.; Rivetti, A.V., Jr.; Alves, P.A.; Galinari, G.C.F.; Cerqueira, M.M.O.P.; Abrahão, J.S.; Lobato, Z.I.P. Detection of Vaccinia virus in milk: Evidence of a systemic and persistent infection in experimentally infected cows. Foodborne Pathog. Dis. 2015, 12, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Geessien Kroon, E.; Santos Abrahão, J.; de Souza Trindade, G.; Pereira Oliveira, G.; Luiz, M.F.; Paula, A.; Barbosa Costa, G.; Teixeira Lima, M.; Silva Calixto, R.; de Oliveira, D.B.; et al. Natural Vaccinia virus infection: diagnosis, isolation, and characterization. Curr. Protoc. Microbiol. 2016, 42. [Google Scholar] [CrossRef]
- Rehfeld, I.S.; Guedes, M.I.M.C.; Fraiha, A.L.S.; Costa, A.G.; Matos, A.C.D.; Fiúza, A.T.L.; Lobato, Z.I.P. Vaccinia virus transmission through experimentally contaminated milk using a murine model. PLoS ONE 2015, 10, e0127350. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, K.M.P.M.B.; Manly, B.; Verdade, L.M. The influence of environmental variables on capybara (Hydrochoerus hydrochaeris: Rodentia, Hydrochoeridae) detectability in anthropogenic environments of southeastern Brazil. Popul. Ecol. 2010, 52, 263–270. [Google Scholar] [CrossRef]
- Kugelman, J.R.; Johnston, S.C.; Mulembakani, P.M.; Kisalu, N.; Lee, M.S.; Koroleva, G.; McCarthy, S.E.; Gestole, M.C.; Wolfe, N.D.; Fair, J.N.; et al. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014, 20, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Nalca, A.; Zumbrun, E.E. ACAM2000: The new smallpox vaccine for United States Strategic National Stockpile. Drug Des. Dev. Ther. 2010, 4, 71–79. [Google Scholar] [CrossRef]
- Olson, V.A. Are we prepared in case of a possible smallpox-like disease emergence? Viruses 2017, 9, 242. [Google Scholar] [CrossRef]
- Quenelle, D.C.; Prichard, M.N.; Keith, K.A.; Hruby, D.E.; Jordan, R.; Painter, G.R.; Robertson, A.; Kern, E.R. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob. Agents Chemother. 2007, 51, 4118–4124. [Google Scholar] [CrossRef] [PubMed]
- Lederman, E.R.; Davidson, W.; Groff, H.L.; Smith, S.K.; Warkentien, T.; Li, Y.; Wilkins, K.A.; Karem, K.L.; Akondy, R.S.; Ahmed, R.; et al. Progressive vaccinia: Case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J. Infect. Dis. 2012, 206, 1372–1385. [Google Scholar] [CrossRef] [PubMed]

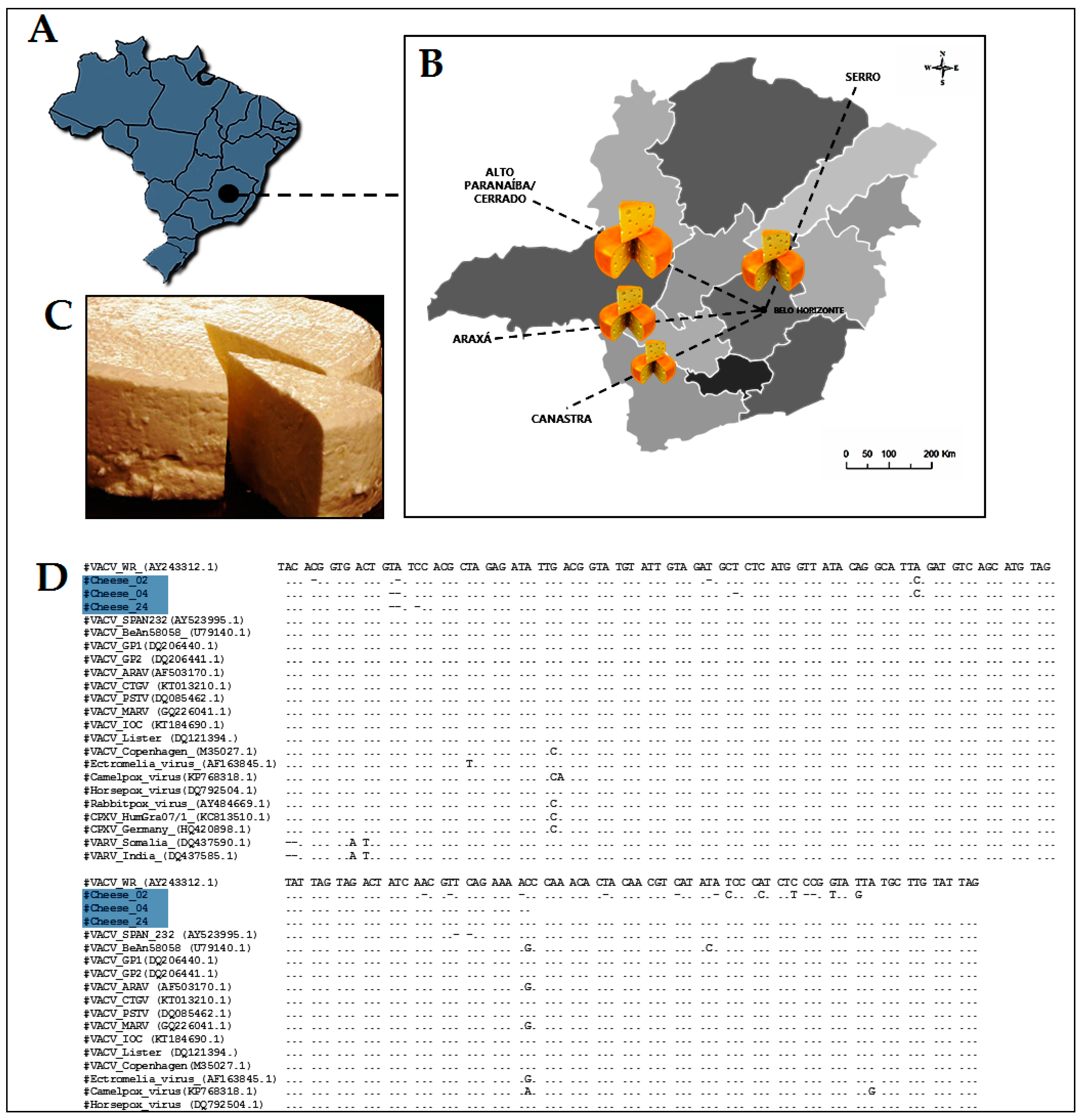
| Sample | Date (Month/Year) | Dairy Basin | Nested-PCR C11R | Real-Time PCR A56R |
|---|---|---|---|---|
| 01 | June/2015 | Serro | + | − |
| 02 | June/2015 | Serro | + | − |
| 03 | June/2015 | Serro | − | − |
| 04 | June/2015 | Serro | + | − |
| 05 | June/2015 | Serro | − | − |
| 06 | June/2015 | Serro | + | − |
| 07 | February/2016 | Serro | + | − |
| 08 | March/2016 | Serro | − | − |
| 09 | April/2016 | Serro | − | − |
| 10 | April/2016 | Serro | − | − |
| 11 | April/2016 | Alto do Paranaíba/Cerrado | + | + |
| 12 | May/2016 | Araxá | + | − |
| 13 | August/2016 | Araxá | − | + |
| 14 | September/2016 | Araxá | − | − |
| 15 | September/2016 | Araxá | − | + |
| 16 | September/2016 | Alto do Paranaíba/Cerrado | − | − |
| 17 | October/2016 | Araxá | − | − |
| 18 | March/2017 | Serro | − | − |
| 19 | March/2017 | Alto do Paranaíba/Cerrado | − | − |
| 20 | March/2017 | Araxá | − | − |
| 21 | April/2017 | Araxá | − | − |
| 22 | April/2017 | Serro | − | − |
| 23 | April/2017 | Serro | − | − |
| 24 | April/2017 | Serro | + | − |
| 25 | May/2017 | Araxá | − | − |
| 26 | May/2017 | Serro | − | − |
| 27 | May/2017 | Alto do Paranaíba/Cerrado | − | − |
| 28 | May/2017 | Serro | − | − |
| 29 | May/2017 | Canastra | − | − |
| 30 | June/2017 | Araxá | − | − |
| 31 | June/2017 | Serro | − | − |
| 32 | June/2017 | Serro | − | − |
| 33 | June/2017 | Araxá | − | − |
| 34 | June/2017 | Serro | − | − |
| 35 | June/2017 | Serro | − | − |
| 36 | June/2017 | Araxá | − | − |
| 37 | June/2017 | Serro | − | − |
| 38 | June/2017 | Araxá | − | − |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.S.d.; Figueiredo, P.D.O.; Costa, G.B.; Assis, F.L.d.; Drumond, B.P.; Da Fonseca, F.G.; Nogueira, M.L.; Kroon, E.G.; Trindade, G.D.S. Vaccinia Virus Natural Infections in Brazil: The Good, the Bad, and the Ugly. Viruses 2017, 9, 340. https://doi.org/10.3390/v9110340
Oliveira JSd, Figueiredo PDO, Costa GB, Assis FLd, Drumond BP, Da Fonseca FG, Nogueira ML, Kroon EG, Trindade GDS. Vaccinia Virus Natural Infections in Brazil: The Good, the Bad, and the Ugly. Viruses. 2017; 9(11):340. https://doi.org/10.3390/v9110340
Chicago/Turabian StyleOliveira, Jaqueline Silva de, Poliana De Oliveira Figueiredo, Galileu Barbosa Costa, Felipe Lopes de Assis, Betânia Paiva Drumond, Flávio Guimarães Da Fonseca, Maurício Lacerda Nogueira, Erna Geessien Kroon, and Giliane De Souza Trindade. 2017. "Vaccinia Virus Natural Infections in Brazil: The Good, the Bad, and the Ugly" Viruses 9, no. 11: 340. https://doi.org/10.3390/v9110340






