Hyperspectral Time Series Analysis of Native and Invasive Species in Hawaiian Rainforests
Abstract
:1. Introduction
2. Materials and Methods
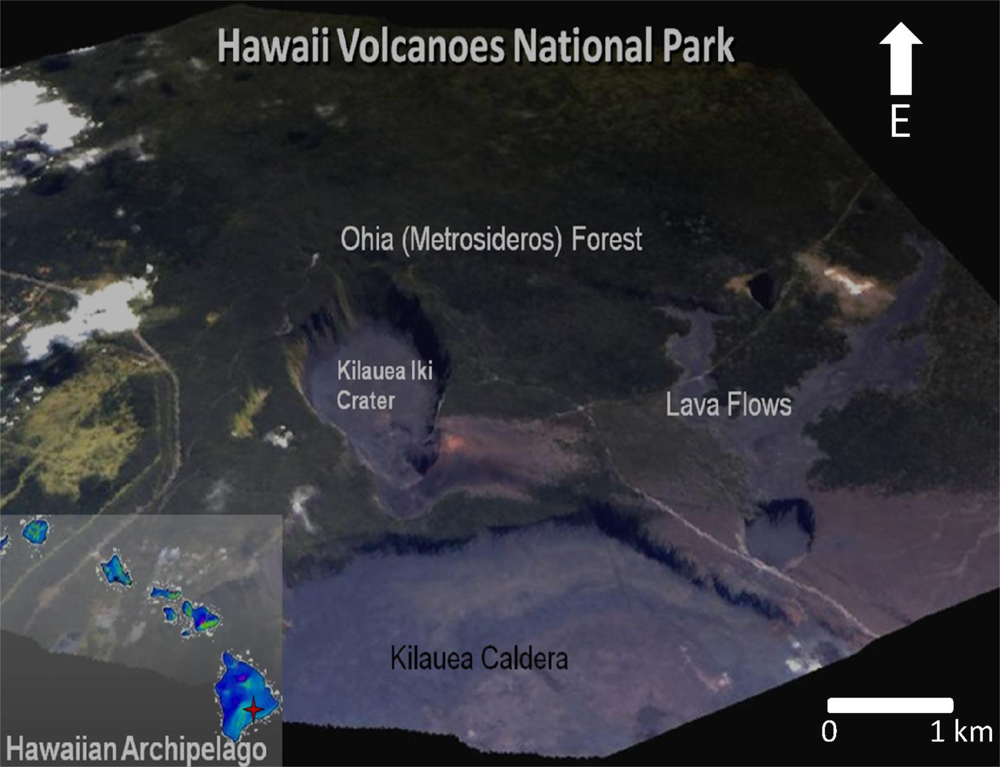
2.1. Study Area and Remote Sensing
2.2. Spectral Separability
3. Results and Discussion
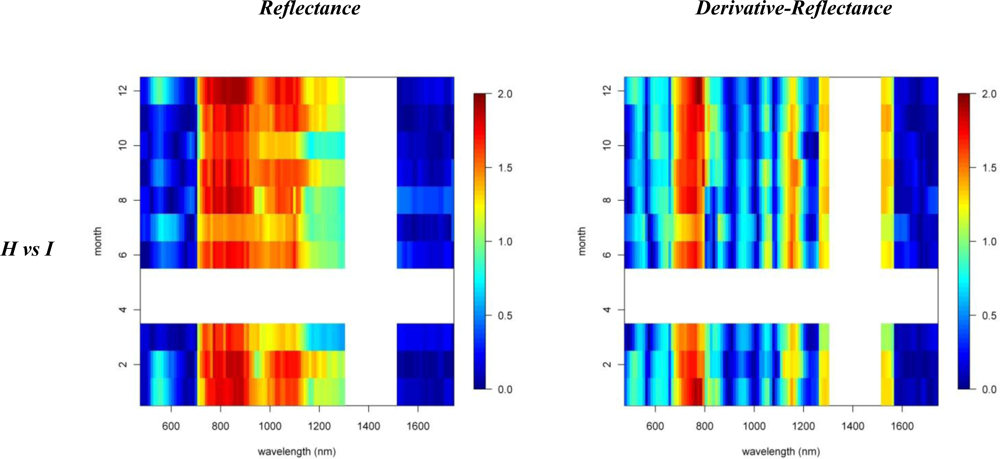
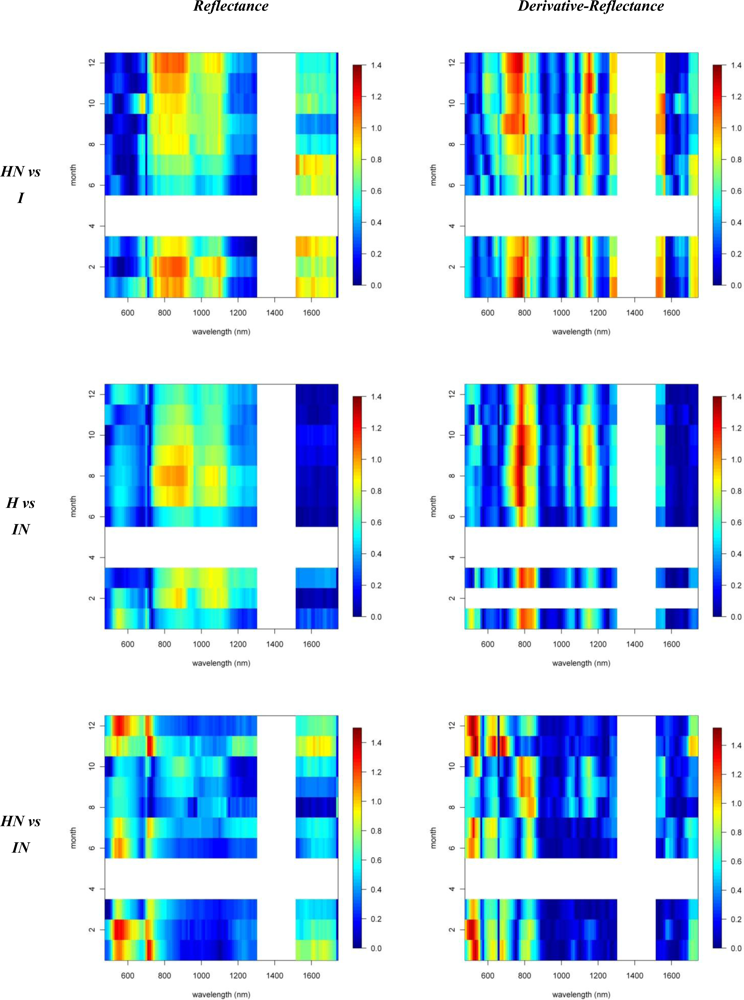
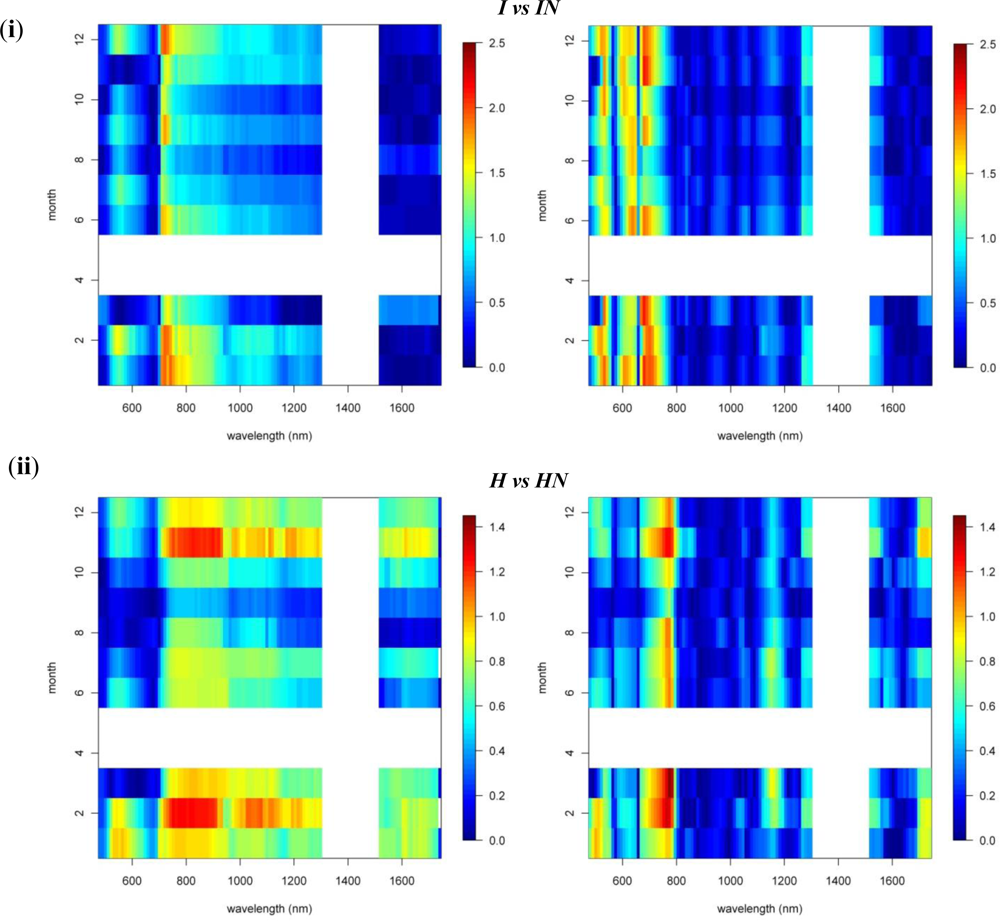
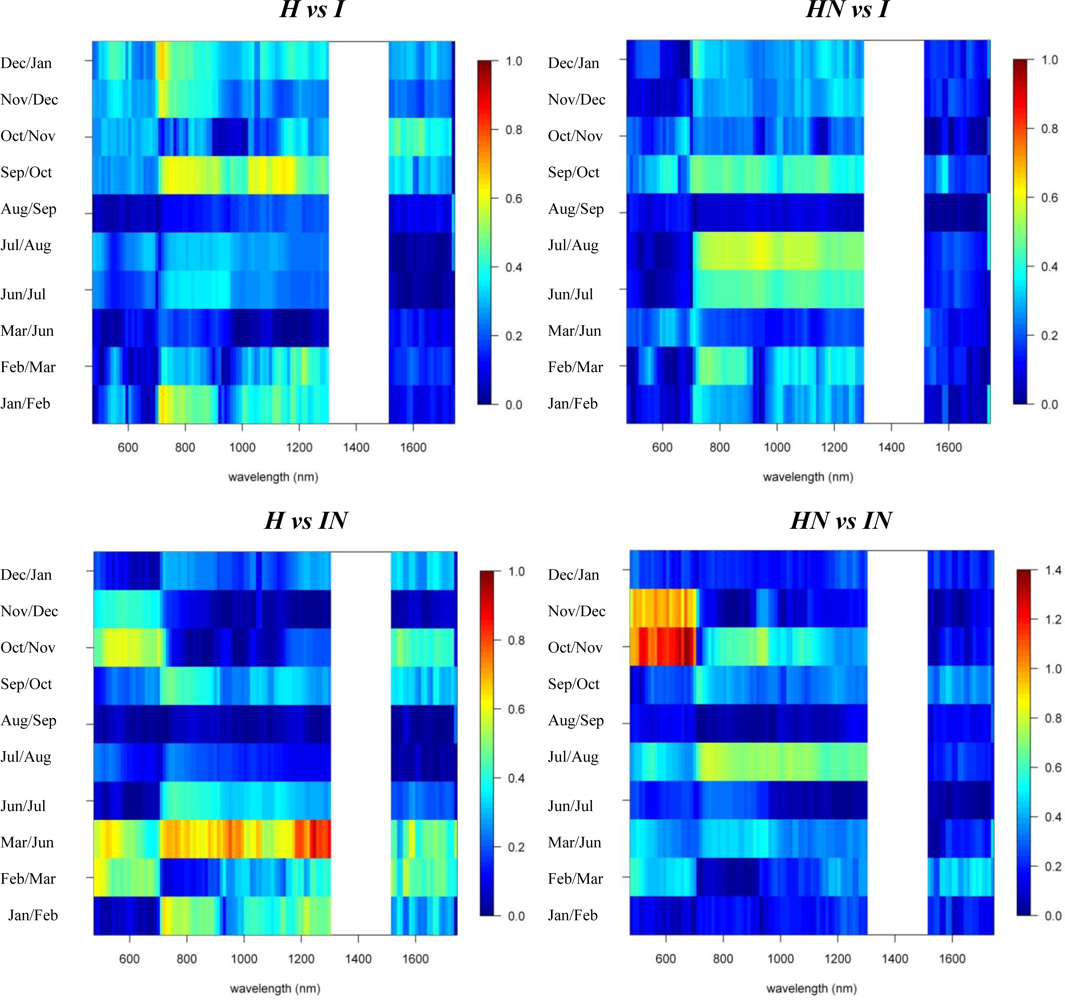
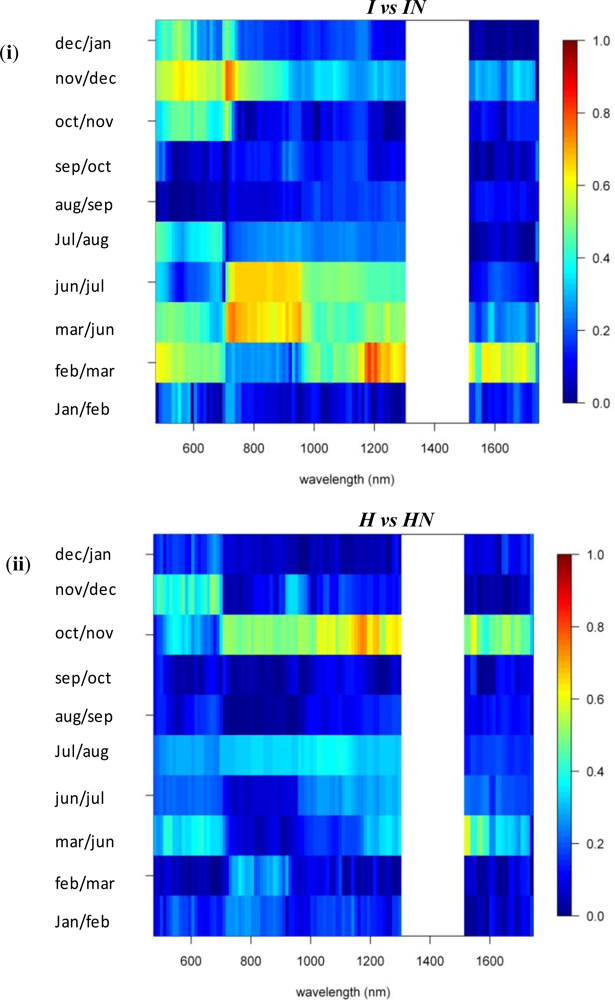
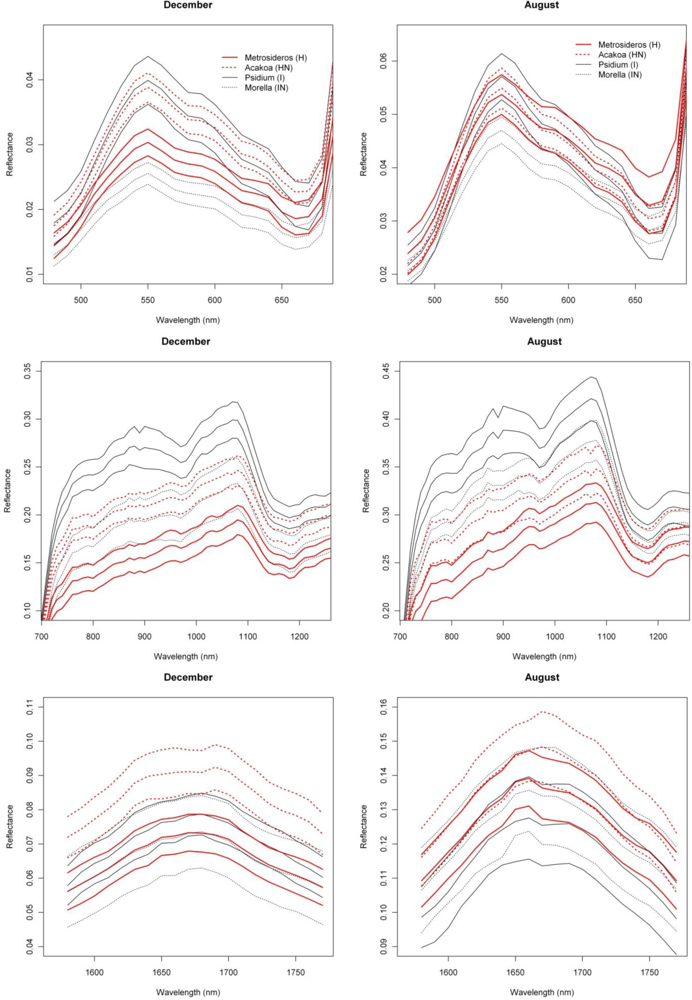
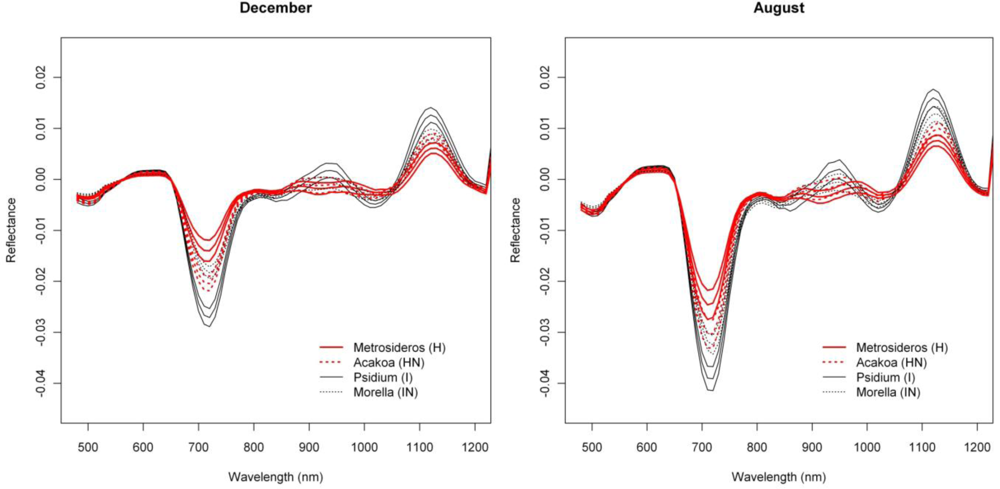
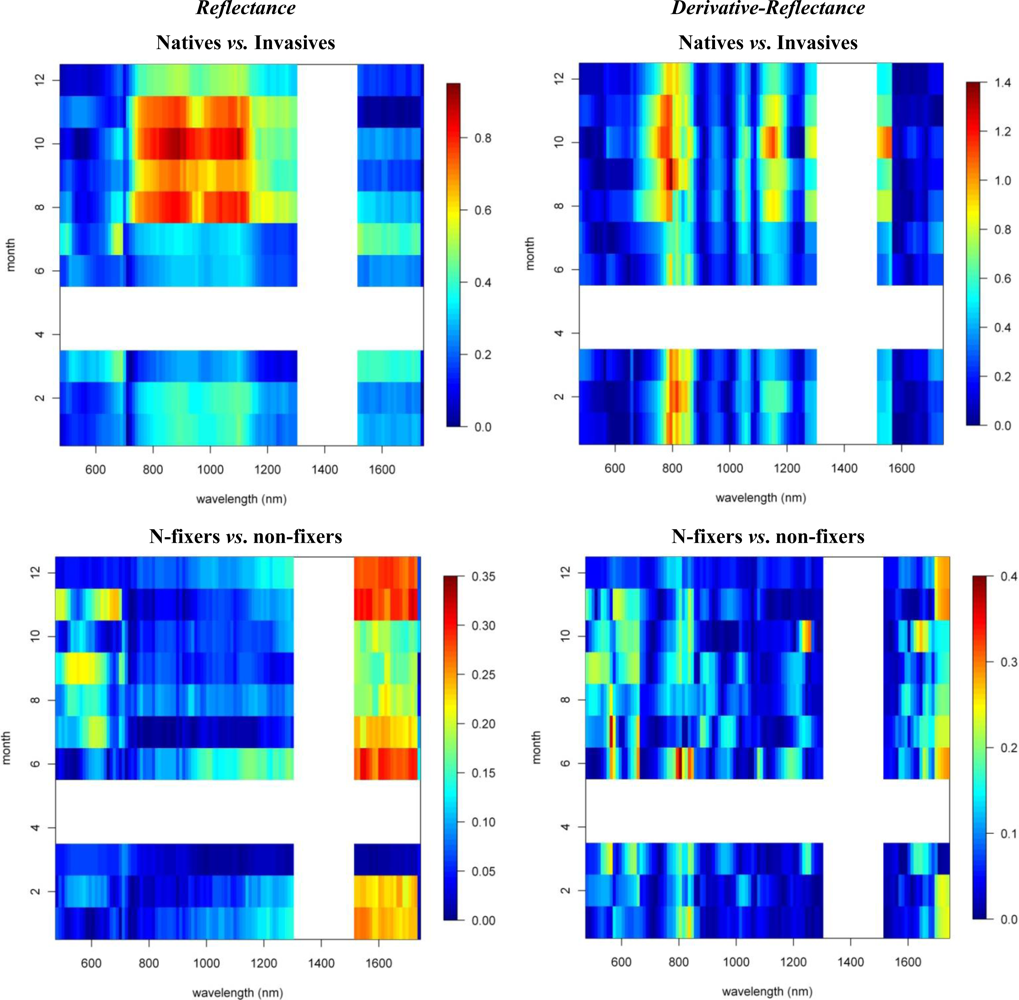
3.1. Species Comparisons
3.2. Invasive vs. Native
3.3. N-Fixing vs. Non-Fixing
4. Conclusions
Acknowledgments
References
- Vitousek, P.M.; Walker, L.R. Biological invasion by Myrica faya in Hawai’i: Plant demography, nitrogen fixation, ecosystem effects. Ecol. Monogr 1989, 59, 247–265. [Google Scholar]
- Asner, G.P.; Jones, M.O.; Martin, R.E.; Knapp, D.E.; Hughes, R.F. Remote sensing of native and invasive species in Hawaiian rainforests. Remote Sens. Environ 2008, 112, 1912–1926. [Google Scholar]
- Mack, R.N.; Simberloff, D.; Londsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl 2000, 10, 689–710. [Google Scholar]
- He, K.S.; Rocchini, D.; Neteler, M.; Nagendra, H. Benefits of hyperspectral remote sensing for tracking plant invasions. Divers. Distrib 2011, 17, 381–392. [Google Scholar]
- Asner, G.P.; Knapp, D.E.; Kennedy-Bowdoin, T.; Jones, M.O.; Martin, R.E.; Boardman, J.; Hughes, R.F. Invasive species detection in Hawaiian rainforests using airborne imaging spectroscopy and LiDAR. Remote Sens. Environ 2008, 112, 1942–1955. [Google Scholar]
- Dennison, P.E.; Roberts, D.A. The effects of vegetation phenology on endmember selection and species mapping in Southern California Chaparral. Remote Sens. Environ 2003, 87, 123–135. [Google Scholar]
- Hesketh, M.; Sanchez-Azofeifa, G.A. The effect of seasonal spectral variation on species classification in the Panamanian Tropical Forest. Remote Sens. Environ 2012, 118, 73–82. [Google Scholar]
- Somers, B.; Asner, G.P. Invasive species mapping in Hawaiian rainforests using multi-temporal Hyperion spaceborne imaging spectroscopy. IEEE J. Sel. Top. Appl. Earth Obs 2012. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Walker, L.R.; Whiteacre, L.D.; Mueller-Dombois, D.; Matson, P.A. Biological invasion by Myrica faya alters ecosystem development in Hawaii. Science 1987, 238, 802–804. [Google Scholar]
- D’Antonio, C.M.; Vitousek, P.M. Biological invasions by exotic grasses, the grass/fire cycle, and global change. Ann. Rev. Ecol. Systemat 1992, 23, 63–87. [Google Scholar]
- Giambelluca, T.W.; Martin, R.E.; Asner, G.P.; Huang, M.; Mudd, R.G.; Nullet, M.A.; DeLay, J.K.; Foote, D. Evapotranspiration and energy balance of native wet montane cloud forest in Hawaii. Agr. For. Meteorol 2009, 149, 230–243. [Google Scholar]
- Asner, G.P.; Martin, R.E.; Carlson, K.M.; Rascher, U.; Vitousek, P.M. Vegetation-climate interactions among native and invasive species in Hawaiian rainforest. Ecosystems 2006, 9, 1041–1054. [Google Scholar]
- Giambelluca, T.M.; Nullet, M.A.; Schroeder, T.A. Rainfall Atlas of Hawaii; Department of Land and Natural Resources: Honolulu, HI, USA, 1986. [Google Scholar]
- Armstrong, R.W. Atlas of Hawaii, 2nd ed; University of Hawaii Press: Honolulu, HI, USA, 1983. [Google Scholar]
- Harrington, R.A.; Fownes, J.H.; Meinzer, F.C.; Scowcroft, P.G. Forest growth along a rainfall gradient in Hawaii: Acacia koa stand structure, productivity, foliar nutrients, and water- and nutrient-use efficiencies. Oecologia 1995, 102, 277–284. [Google Scholar]
- Smith, C.W. Impact of Alien Plants on Hawai’i’s Native Biota. In Hawai’i’s Terrestrial Ecosystems: Preservation and Management; Stone, C.P., Scott, J.M., Eds.; University of Hawaii Cooperative National Park Resources Studies Unit: Honolulu, HI, USA, 1985; pp. 180–250. [Google Scholar]
- Asner, G.P.; Knapp, D.E.; Kennedy-Bowdoin, T.; Jones, M.O.; Martin, R.E.; Boardman, J.; Field, C.B. Carnegie Airborne Observatory: In-flight fusion of hyperspectral imaging and waveform light detection and ranging (wLiDAR) for three-dimensional studies of ecosystems. J. Appl. Remote Sens 2007, 1. n°013536. [Google Scholar]
- Asner, G.P.; Heidebrecht, K.B. Imaging spectroscopy for desertification studies: Comparing AVIRIS and EO-1 Hyperion in Argentina drylands. IEEE Trans. Geosci. Remote Sens 2003, 41, 1283–1296. [Google Scholar]
- Asner, G.P.; Vitousek, P.M. Remote Analysis of biological invasion and biogeochemical change. Proc. Natl. Acad. Sci. USA 2005, 102, 4383–4386. [Google Scholar]
- Somers, B.; Delalieux, S.; Verstraeten, W.; van Aardt, J.; Albrigo, G.; Coppin, P. An automated waveband selection technique for optimized hyperspectral mixture analysis. Int. J. Remote Sens 2010, 31, 5549–5568. [Google Scholar]
- Somers, B.; Delalieux, S.; Stuckens, J.; Verstraeten, W.; Coppin, P. A weighted linear spectral mixture analysis approach to address endmember variability in agricultural production systems. Int. J. Remote Sens 2009, 30, 139–147. [Google Scholar]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem 1964, 36, 1627–1639. [Google Scholar]
- Somers, B.; Delalieux, S.; Verstraeten, W.; Verbesselt, J.; Lhermitte, S.; Coppin, P. Magnitude and shape related feature integration in hyperspectral mixture analysis to monitor weeds in citrus orchards. IEEE Trans. Geosci. Remote Sens 2009, 47, 3630–3642. [Google Scholar]
- Thenkabail, P.S.; Enclona, E.A.; Ashton, M.S.; van der Meer, B. Accuracy assessments of hyperspectral waveband performance for vegetation analysis applications. Remote Sens. Environ 2004, 91, 354–376. [Google Scholar]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ 1989, 30, 271–278. [Google Scholar]
- Yoder, B.J.; Pettigrew-Crosby, R.E. Predicting nitrogen and chlorophyll content and concentrations from reflectance spectra (300–2500 nm) at leaf and canopy scales. Remote Sens. Environ 1995, 53, 199–211. [Google Scholar]
- Martin, M.E.; Aber, J.D. High spectral resolution remote sensing of forest canopy lignin, nitrogen, and ecosystem processes. Ecol. Appl 1997, 7, 431–444. [Google Scholar]
- Smith, M.L.; Martin, M.E.; Plourde, L.; Ollinger, S.V. Analysis of hyperspectral data for estimation of temperate forest canopy nitrogen concentrations: Comparison between an airborne (AVIRIS) and spaceborne (Hyperion) sensor. IEEE Trans. Geosci. Remote Sens 2003, 41, 1332–1337. [Google Scholar]
- Hughes, R.F.; Denslow, J.S. Invasion by a N2-fixing tree alters function and structure in wet lowland forests of Hawaii. Ecol. Appl 2005, 15, 1615–1628. [Google Scholar]
- Durand, L.Z.; Goldstein, G. Photosynthesis, photoinhibition, and nitrogen use efficiency in native and invasive tree ferns in Hawaii. Oecologia 2001, 126, 345–354. [Google Scholar]
- Baruch, Z.; Goldstein, G. Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 1999, 121, 183–192. [Google Scholar]
- Clark, M.; Roberts, D.A. Species-level differences in hyperspectral metrics among tropical rainforest trees as determined by a tree-based classifier. Remote Sens 2012, 4, 1820–1855. [Google Scholar]
- Mirik, M.; Ansley, R.J. Utility of satellite and aerial images for quantification of canopy cover and infilling rates of the invasive woody species honey mesquite on rangeland. Remote Sens 2012, 4, 1947–1962. [Google Scholar]
- Evangelista, P.H.; Stohlgren, T.J.; Morisette, J.T.; Kumar, S. Mapping invasive Tamarisk: A comparison of single scene and time-series analyses of remotely sensed data. Remote Sens 2009, 1, 519–533. [Google Scholar]
| 2004 | 2005 | 2006 | 2007 | |
|---|---|---|---|---|
| January | X | X | ||
| February | X | |||
| March | X | |||
| April | ||||
| May | ||||
| June | X | X | ||
| July | X | X | ||
| August | XX | X | X | X |
| September | XX | |||
| October | XX | |||
| November | X | X | ||
| December | X | X | X | |
| HN | IN | I | |
|---|---|---|---|
| Winter | Summer | Winter | |
| H | R690–920 nm (SI > 1) | D720–770 nm, Tfeb–jun, 700–1250 nm (0.7–1) | R770–870 nm (>1.9) |
| Winter | Winter | ||
| HN | D520 nm (>1.5) | D760–770 nm (1–1.2) | |
| All seasons | |||
| IN | D530 nm, D630 nm, D690 nm (1–2) |
Share and Cite
Somers, B.; Asner, G.P. Hyperspectral Time Series Analysis of Native and Invasive Species in Hawaiian Rainforests. Remote Sens. 2012, 4, 2510-2529. https://doi.org/10.3390/rs4092510
Somers B, Asner GP. Hyperspectral Time Series Analysis of Native and Invasive Species in Hawaiian Rainforests. Remote Sensing. 2012; 4(9):2510-2529. https://doi.org/10.3390/rs4092510
Chicago/Turabian StyleSomers, Ben, and Gregory P. Asner. 2012. "Hyperspectral Time Series Analysis of Native and Invasive Species in Hawaiian Rainforests" Remote Sensing 4, no. 9: 2510-2529. https://doi.org/10.3390/rs4092510




