Lipid-Lowering Effects of Pediococcus acidilactici M76 Isolated from Korean Traditional Makgeolli in High Fat Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of LAB Test Samples
2.2. Animals and Diets
2.3. Animal Treatment and Biochemical Assays
2.4. Analysis of Insulin and Leptin
2.5. Hepatic mRNA Expression Analysis of Lipid-Regulating Genes
| Primer | Sequence | |
|---|---|---|
| ACC | Forward | 5′-CCA ACA TGA GGA CTA TAA CTT CCT-3′ |
| Reverse | 5′-TAC ATA CGT GCC GTC AGG CTT CAC-3′ | |
| FAS | Forward | 5′-AGG GGT CGA CCT GGT CCT CA-3′ |
| Reverse | 5′-GCC ATG CCC AGA GGG TGG TT-3′ | |
| PPARγ | Forward | 5′-GCT GTT ATG GGT GAA ACT CTG-3′ |
| Reverse | 5′-ATA AGG TGG AGA TGC AGG TTC-3′ | |
| CPT-1 | Forward | 5′-AAA GAT CAA TCG GAC CCT AGA CA-3′ |
| Reverse | 5′-CAG CGA GTA GCG CAT AGT CA-3′ | |
| β-actin | Forward | 5′-GTG GGG CGC CCC AGG CAC CAG-3′ |
| Reverse | 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′ |
2.6. Statistical Analyses
3. Results
3.1. Body Weight, Epididymal Fat Pad Weight, Food Intake, and Energy Intake
| Groups | ND | HD | HD-PR | HD-PA |
|---|---|---|---|---|
| Initial body weight (g) | 18.86 ± 0.77 NS | 19.00 ± 0.43 | 19.05 ± 1.43 | 19.13 ± 0.68 |
| Final body weight (g) | 23.72 ± 1.07 c | 40.69 ± 3.58 a | 39.37 ± 2.60 ab | 37.63 ± 4.38 b |
| Epididymal fat (g) | 0.43 ± 0.19 c | 3.02 ± 0.82 a | 2.53 ± 0.48 ab | 2.26 ± 0.58 b |
| Feed intake (g/day) | 2.06 ± 0.05 c | 2.43 ± 0.18 a | 2.19 ± 0.07 bc | 2.32 ± 0.11 ab |
| Energy intake (kcal/day) | 7.92 ± 0.21 c | 12.71 ± 0.93 a | 11.45 ± 0.36 b | 12.14 ± 0.55 ab |
3.2. Serum and Hepatic Lipid Profiles
| Groups | ||||
|---|---|---|---|---|
| ND | HD | HD-PR | HD-PA | |
| Serum (mg/dl) | ||||
| Total cholesterol | 109.80 ± 8.51 c | 212.40 ± 22.60 a | 207.00 ± 22.51 a | 187.40 ± 16.60 b |
| Triglyceride | 110.80 ± 46.60 NS | 130.20 ± 53.66 | 121.40 ± 49.08 | 140.00 ± 58.80 |
| Liver (mg/g) | ||||
| Total cholesterol | 3.82 ± 2.15 c | 12.35 ± 4.83 a | 10.51 ± 5.18 ab | 8.59 ± 4.93 b |
| Triglyceride | 48.31 ± 12.45 c | 99.49 ± 36.16 a | 88.73 ± 29.49 ab | 79.94 ± 24.66 b |
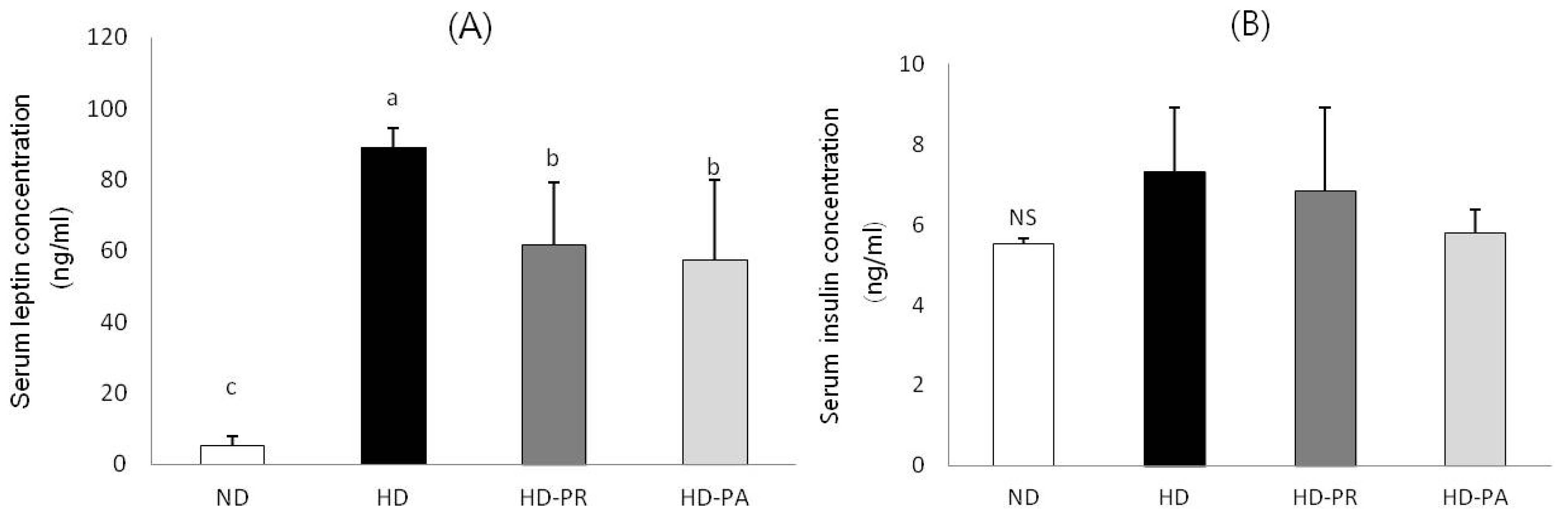
3.3. Serum Leptin and Insulin Concentrations
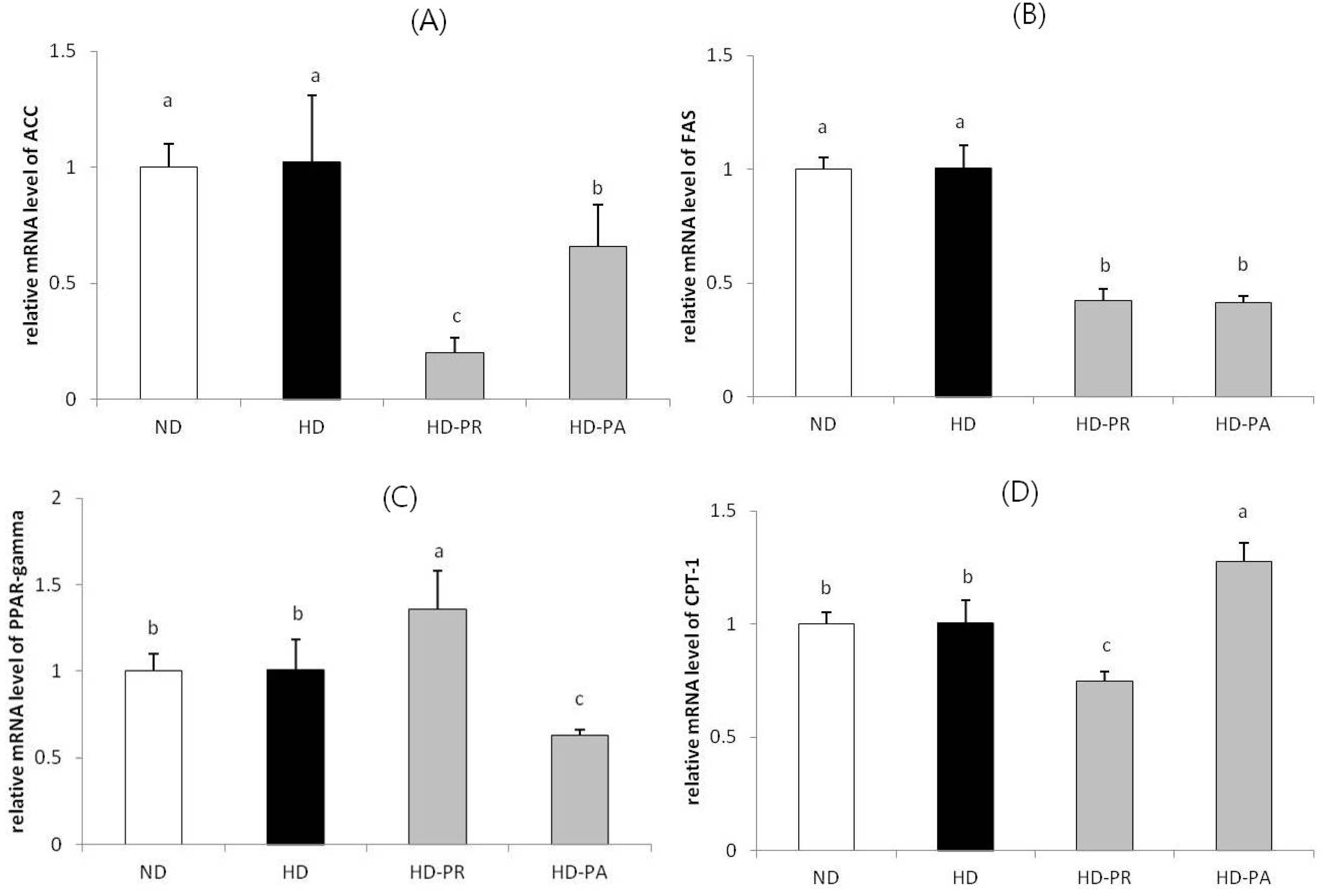
3.4. Hepatic mRNA Level
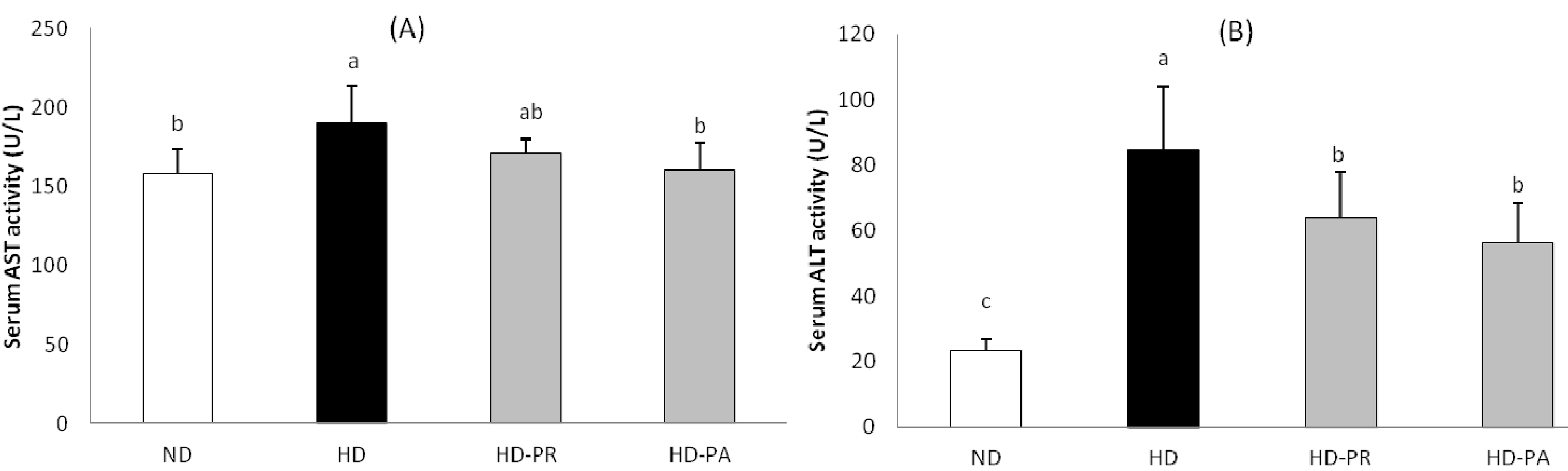
3.5. Hepatic Toxicity Assay
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ramachandran, A.; Chamukuttan, S.; Shetty, S.A.; Arun, N.; Susairaj, P. Obesity in Asia-is it different from rest of the world. Diabetes Metab. Res. Rev. 2012, 28, 47–51. [Google Scholar] [CrossRef]
- Grundy, S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007, 56, 2655–2667. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, J.H.; Bai, D.H.; Ahn, B. Feasibility of brewing makgeolli using Pichia anomala Y 197-13, a non-Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2012, 22, 1749–1757. [Google Scholar] [CrossRef]
- Lee, J.W.; Shim, J.Y. Quality characteristics of Makgeolli during freezing storage. Food Eng. Progr. 2010, 14, 328–334. [Google Scholar]
- Jin, J.; Kim, S.-Y.; Jin, Q.; Eom, H.-J.; Han, N.-S. Diversity analysis of lactic acid bacteria in Takju, Korean rice wine. J. Microbiol. Biotechnol. 2008, 18, 1678–1682. [Google Scholar]
- Seo, D.H.; Jung, J.H.; Kim, H.Y.; Kim, Y.R.; Ha, S.J.; Kim, Y.C.; Park, C.S. Identification of lactic acid bacteria involved in traditional Korean rice wine fermentation. Food Sci. Biotechnol. 2007, 16, 994–998. [Google Scholar]
- Kim, H.R.; Lee, A.R.; Kim, J.H.; Ahn, B.H. Microbial dynamics of commercial makgeolli depending on the storage temperature. J. Microbiol. Biotechnol. 2012, 22, 1101–1106. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.; Park, P.; Kang, H.I.; Ryu, E.K.; Kim, S.M. Effects of storage temperature and time on the biogenic amine content and microflora in Korean turbid rice wine, Makgeolli. Food Chem. 2011, 128, 87–92. [Google Scholar] [CrossRef]
- Dicks, L.M.; Botes, M. Probiotic lactic acid bacteria in the gastro-intestinal tract: Health benefits, safety and mode of action. Benef. Microbes 2010, 1, 11–29. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Cheng, P.C.; Pan, T.M. Anti-obesity effects of gut microbiota are associated with lactic acid bacteria. Appl. Microbiol. Biotechnol. 2014, 98, 1–10. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 2007, 23, 62–68. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar]
- Mi-Ok, S.; Dae-Yeon, K.; Mi-Hyang, K.; Song-Ja, B. Effect of growth inhibition and quinone reductase activity stimulation of makgeoly fractions in various cancer cells. J. Korean Soc. Food Sci. Nutr. 2008, 37, 288–293. [Google Scholar] [CrossRef]
- Bae, S.H.; Choi, J.W.; Ra, K.S.; Yu, K.W.; Shin, K.S.; Park, S.S.; Suh, H.J. Anti-complementary activity of enzyme-treated traditional Korean rice wine (Makgeolli) hydrolysates. J. Sci. Food Agric. 2012, 92, 1765–1770. [Google Scholar] [CrossRef]
- Bae, S.H.; Jung, E.Y.; Kim, S.Y.; Shin, K.S.; Suh, H.J. Antioxidant and immuno-modulating activities. J. Food Biochem. 2010, 34, 233–248. [Google Scholar] [CrossRef]
- Jeong, J.W.; Nam, P.W.; Lee, S.J.; Lee, K.G. Antioxidant activities of Korean rice wine concentrates. J. Agric. Food Chem. 2011, 59, 7039–7044. [Google Scholar] [CrossRef]
- Kim, J.E.; Jung, S.K.; Lee, S.J.; Lee, K.W.; Kim, G.W.; Lee, H.J. Nuruk extract inhibits lipopolysaccharide-induced production of nitrite and interleukin-6 in RAW 264.7 cells through blocking activation of p38 mitogen-activated protein kinase. J. Microbiol. Biotechnol. 2008, 18, 1423–1426. [Google Scholar]
- Song, R.; Jeong, D.Y.; Cha, Y.S.; Baik, S.H. Exopolysaccharide produced by Pediococcus acidilactici M76 isolated from the Korean traditional rice wine, Makgeolli. J. Microbiol. Biotechnol. 2013, 23, 681–688. [Google Scholar] [CrossRef]
- Beena Divya, J.; Kulangara Varsha, K.; Madhavan Nampoothiri, K.; Ismail, B.; Pandey, A. Probiotic fermented foods for health benefits. Eng. Life Sci. 2012, 12, 377–390. [Google Scholar] [CrossRef]
- Zhao, X.R.; Higashikawa, F.; Noda, M.; Kawamura, Y.; Matoba, Y.; Kumagai, T.; Sugiyama, M. The obesity and fatty liver are reduced by plant-derived pediococcus pentosaceus LP28 in high fat diet-induced obese mice. PLos One 2012, 7, 1–8. [Google Scholar]
- Lee, H.Y.; Park, J.H.; Seok, S.H.; Baek, M.W.; Kim, D.J.; Lee, K.E.; Paek, K.S.; Lee, Y.; Park, J.H. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim. Biophys. ActaMol. Cell Biol. Lipids 2006, 1761, 736–744. [Google Scholar]
- Takemura, N.; Okubo, T.; Sonoyama, K. Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Exp. Biol. Med. 2010, 235, 849–856. [Google Scholar] [CrossRef]
- An, H.M.; Park, S.Y.; Lee, D.K.; Kim, J.R.; Cha, M.K.; Lee, S.W.; Lim, H.T.; Kim, K.J.; Ha, N.J. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011, 10, 1–8. [Google Scholar] [CrossRef]
- Yin, Y.-N.; Yu, Q.-F.; Fu, N.; Liu, X.-W.; Lu, F.-G. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J. Gastroenterol. 2010, 16, 3394. [Google Scholar] [CrossRef]
- Banks, W.A.; Coon, A.B.; Robinson, S.M.; Moinuddin, A.; Shultz, J.M.; Nakaoke, R.; Morley, J.E. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 2004, 53, 1253–1260. [Google Scholar]
- West, D.B.; Boozer, C.N.; Moody, D.L.; Atkinson, R.L. Dietary obesity in nine inbred mouse strains. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992, 262, R1025–R1032. [Google Scholar]
- Park, J.A.; Pichiah, P.B.T.; Yu, J.J.; Oh, S.H.; Daily, J.W.; Cha, Y.S. Anti-obesity effect of kimchi fermented with Weissella koreensis OK1-6 as starter in high-fat diet-induced obese C57BL/6J mice. J. Appl. Microbiol. 2012, 113, 1507–1516. [Google Scholar] [CrossRef]
- Lee, K.; Paek, K.; Lee, H.; Park, J.H.; Lee, Y. Antiobesity effect of trans-10, cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J. Appl. Microbiol. 2007, 103, 1140–1146. [Google Scholar] [CrossRef]
- Mandal, V.; Sen, S.; Mandal, N. Effect of prebiotics on bacteriocin production and cholesterol lowering activity of Pediococcus acidilactici lab 5. World J. Microbiol. Biotechnol. 2009, 25, 1837–1847. [Google Scholar] [CrossRef]
- Bukowska, H.; Pieczul-Mroz, J.; Jastrzebska, M.; Chelstowski, K.; Naruszewicz, M. Decrease in fibrinogen and ldl-cholesterol levels upon supplementation of diet with lactobacillus plantarum in subjects with moderately elevated cholesterol. Atherosclerosis 1998, 137, 437–438. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, J.G.; Shin, Y.W.; Kim, S.H.; Whang, K.Y. Effect of dietary inclusion of Lactobacillus acidophilus atcc 43121 on cholesterol metabolism in rats. J. Microbiol. Biotechnol. 2007, 17, 655–662. [Google Scholar]
- Wang, Y.; Xu, N.; Xi, A.; Ahmed, Z.; Zhang, B.; Bai, X. Effects of Lactobacillus Plantarum MA2 isolated from tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 2009, 84, 341–347. [Google Scholar] [CrossRef]
- Xie, N.; Cui, Y.; Yin, Y.N.; Zhao, X.; Yang, J.W.; Wang, Z.G.; Fu, N.; Tang, Y.; Wang, X.H.; Liu, X.W.; et al. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement. Altern. Med. 2011, 11, 53. [Google Scholar] [CrossRef]
- Tok, E.; Aslim, B. Cholesterol removal by some lactic acid bacteria that can be used as probiotic. Microbiol. Immunol. 2010, 54, 257–264. [Google Scholar]
- Maeda, H.; Zhu, X.; Omura, K.; Suzuki, S.; Kitamura, S. Effects of an exopolysaccharide (kefiran) on lipids, blood pressure, blood glucose, and constipation. Biofactors 2004, 22, 197–200. [Google Scholar] [CrossRef]
- Ryu, M.H.; Cha, Y.S. The effects of a high-fat or high-sucrose diet on serum lipid profiles, hepatic acyl-coa synthetase, carnitine palmitoyltransferase-I, and the acetyl-coa carboxylase mRNA levels in rats. J. Biochem. Mol. Biol. 2003, 36, 312–318. [Google Scholar] [CrossRef]
- O’onnor, B.J.; Kathamna, B.; Tavill, A.S. Nonalcoholic fatty liver (NASH syndrome). Gastroenterologist 1997, 5, 316–329. [Google Scholar]
- Naidu, A.; Bidlack, W.; Clemens, R. Probiotic spectra of lactic acid bacteria (LAB). Crit. Rev. Food Sci. Nutr. 1999, 39, 13–126. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Wu, T.; Qi, X.M.; Liu, Y.; Guo, J.; Zhu, R.Y.; Chen, W.; Zheng, X.D.; Yu, T. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem. 2013, 141, 482–487. [Google Scholar] [CrossRef]
- Park, J.E.; Oh, S.H.; Cha, Y.S. Lactobacillus plantarum LG42 isolated from gajami sik-hae decreases body and fat pad weights in diet-induced obese mice. J. Appl. Microbiol. 2013, 116, 145–156. [Google Scholar] [CrossRef]
- Szanto, S.; Yudkin, J. The effect of dietary sucrose on blood lipids, serum insulin, platelet adhesiveness and body weight in human volunteers. Postgrad. Med. J. 1969, 45, 602–607. [Google Scholar] [CrossRef]
- Holl, M.G.; Allen, L.H. Sucrose ingestion, insulin response and mineral metabolism in humans. J. Nutr. 1987, 117, 1229–1233. [Google Scholar]
- Harwood, H.J.; Petras, S.F.; Shelly, L.D.; Zaccaro, L.M.; Perry, D.A.; Makowski, M.R.; Hargrove, D.M.; Martin, K.A.; Tracey, W.R.; Chapman, J.G.; et al. Isozyme-nonselective N-substituted bipiperidylcarboxamide acetyl-CoA carboxylase inhibitors reduce tissue malonyl-CoA concentrations, inhibit fatty acid synthesis, and increase fatty acid oxidation in cultured cells and in experimental animals. J. Biol. Chem. 2003, 278, 37099–37111. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Progr. Lipid Res. 2009, 48, 1–26. [Google Scholar] [CrossRef]
- Yoo, S.R.; Kim, Y.J.; Park, D.Y.; Jung, U.J.; Jeon, S.M.; Ahn, Y.T.; Huh, C.S.; McGregor, R.; Choi, M.S. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obes. Silv. Spring 2013, 21, 2571–2578. [Google Scholar] [CrossRef]
- Kersten, S.; Desvergne, B.; Wahli, W. Roles of PPARs in health and disease. Nature 2000, 405, 421–424. [Google Scholar] [CrossRef]
- Unger, R.H.; Zhou, Y.T.; Orci, L. Regulation of fatty acid homeostasis in cells: Novel role of leptin. Proc. Natl. Acad. Sci. USA 1999, 96, 2327–2332. [Google Scholar] [CrossRef]
- O’Neill, H.M.; Holloway, G.P.; Steinberg, G.R. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: Implications for obesity. Mol. Cell. Endocrinol. 2012, 366, 135–151. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moon, Y.-J.; Baik, S.-H.; Cha, Y.-S. Lipid-Lowering Effects of Pediococcus acidilactici M76 Isolated from Korean Traditional Makgeolli in High Fat Diet-Induced Obese Mice. Nutrients 2014, 6, 1016-1028. https://doi.org/10.3390/nu6031016
Moon Y-J, Baik S-H, Cha Y-S. Lipid-Lowering Effects of Pediococcus acidilactici M76 Isolated from Korean Traditional Makgeolli in High Fat Diet-Induced Obese Mice. Nutrients. 2014; 6(3):1016-1028. https://doi.org/10.3390/nu6031016
Chicago/Turabian StyleMoon, Yeon-Jeong, Sang-Ho Baik, and Youn-Soo Cha. 2014. "Lipid-Lowering Effects of Pediococcus acidilactici M76 Isolated from Korean Traditional Makgeolli in High Fat Diet-Induced Obese Mice" Nutrients 6, no. 3: 1016-1028. https://doi.org/10.3390/nu6031016




