Use of Novel High-Protein Functional Food Products as Part of a Calorie-Restricted Diet to Reduce Insulin Resistance and Increase Lean Body Mass in Adults: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Foods
2.2. Subjects
2.3. Study Design
2.4. Blood Analyses
2.5. Statistical Analysis
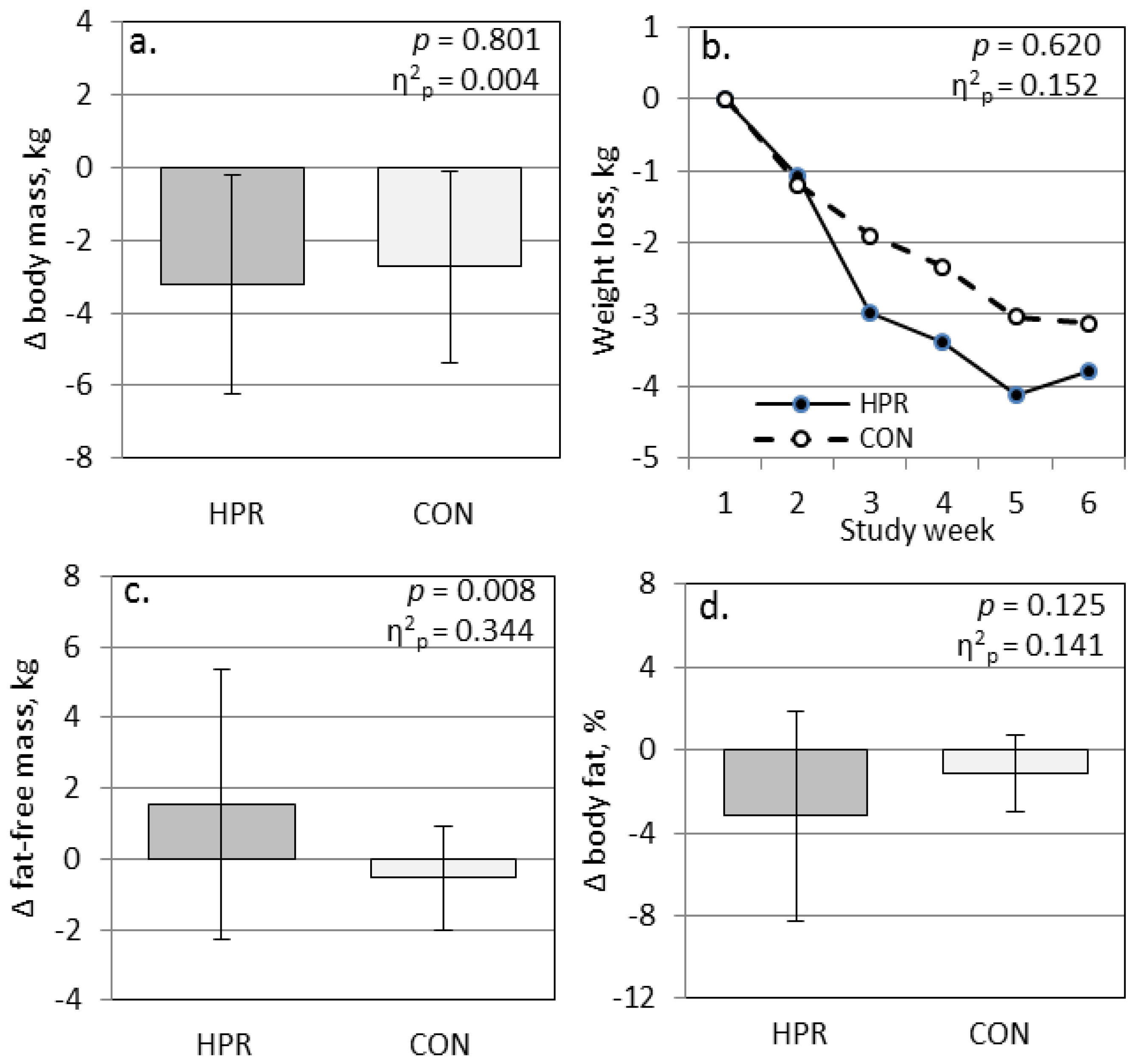
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Di Meo, S.; Iossa, S.; Venditti, P. Skeletal muscle insulin resistance: Role of mitochondria and other ROS sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Olefsky, J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2016, 12, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.P.; Racette, S.B.; Villareal, D.T.; Fontana, L.; Steger-May, K.; Schechtman, K.B.; Klein, S.; Holloszy, J.O.; Washington University School of Medicine CALERIE Group. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: A randomized controlled trial. Am. J. Clin. Nutr. 2006, 84, 1033–1042. [Google Scholar] [PubMed]
- Ross, R.; Dagnone, D.; Jones, P.J.; Smith, H.; Paddags, A.; Hudson, R.; Janssen, I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann. Intern. Med. 2000, 133, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Markovic, T.P.; Jenkins, A.B.; Campbell, L.V.; Furler, S.M.; Kraegen, E.W.; Chisholm, D.J. The determinants of glycemic responses to diet restriction and weight loss in obesity and NIDDM. Diabetes Care 1998, 21, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.; Dyer, K.; Buckley, J.; Brinkworth, G.; Coates, A.; Parfitt, G.; Howe, P.; Noakes, M.; Murphy, K. Effects of low-fat diets differing in protein and carbohydrate content on cardiometabolic risk factors during weight loss and weight maintenance in obese adults with type 2 diabetes. Nutrients 2016, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.J.; Choi, J.H.; Yoo, S.H.; Bae, J.C.; Kim, W.J.; Choi, E.S.; Park, S.E.; Park, C.Y.; Park, S.W.; Oh, K.W.; et al. The association of unintentional changes in weight, body composition, and homeostasis model assessment index with glycemic progression in non-diabetic healthy subjects. Diabetes Metab. J. 2011, 35, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.W.; Sitren, H.S.; Daniels, M.J.; Langkamp-Henken, B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: A meta-regression. Am. J. Clin. Nutr. 2006, 83, 260–274. [Google Scholar] [PubMed]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Margolis, L.M.; Orr, J.S. Optimized dietary strategies to protect skeletal muscle mass during periods of unavoidable energy deficit. FASEB J. 2015, 29, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Reaven, G.; Abbasi, F.; Lamendola, C.; Saad, M.; Waters, D.; Simon, J.; Krauss, R.M. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am. J. Cardiol. 2005, 96, 399–404. [Google Scholar] [CrossRef] [PubMed]
- König, D.; Kookhan, S.; Schaffner, D.; Deibert, P.; Berg, A. A meal replacement regimen improves blood glucose levels in prediabetic healthy individuals with impaired fasting glucose. Nutrition 2014, 30, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Armstrong, C.L.; Leidy, H.J.; Campbell, W.W. Normal vs. high-protein weight loss diets in men: Effects on body composition and indices of metabolic syndrome. Obesity 2013, 21, E204–E210. [Google Scholar] [CrossRef] [PubMed]
- Mamerow, M.M.; Mettler, J.A.; English, K.L.; Casperson, S.L.; Arentson-Lantz, E.; Sheffield-Moore, M.; Layman, D.K.; Paddon-Jones, D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J. Nutr. 2014, 144, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Areta, J.L.; Burke, L.M.; Ross, M.L.; Camera, D.M.; West, D.W.; Broad, E.M.; Jeacocke, N.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 2013, 591, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Curis, E.; Hamon-Vilcot, B.; Nicolis, I.; Chrétien, P.; Schauer, N.; Vincent, J.P.; Cynober, L.; Aussel, C. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: A randomized controlled trial. Clin. Nutr. 2013, 32, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Flechtner-Mors, M.; Boehm, B.O.; Wittmann, R.; Thoma, U.; Ditschuneit, H.H. Enhanced weight loss with protein-enriched meal replacements in subjects with the metabolic syndrome. Diabetes Metab. Res. Rev. 2010, 26, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.; Bae, W.K.; Choi, J.K.; Kim, H.J.; Cho, B. Efficacy of low-calorie, partial meal replacement diet plans on weight and abdominal fat in obese subjects with metabolic syndrome: A double-blind, randomised controlled trial of two diet plans—one high in protein and one nutritionally balanced. Int. J. Clin. Pract. 2009, 63, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Laleg, K.; Barron, C.; Santé-Lhoutellier, V.; Walrand, S.; Micard, V. Protein enriched pasta: Structure and digestibility of its protein network. Food Funct. 2016, 7, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, S.R.; With, E.; Rehfeld, J.F.; Kulseng, B.; Truby, H.; Martins, C. The impact of rate of weight loss on body composition and compensatory mechanisms during weight reduction: A randomized control trial. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur. J. Clin. Nutr. 2013, 67, 759–764. [Google Scholar] [CrossRef] [PubMed]
- De Luis, D.A.; Sagrado, M.G.; Conde, R.; Aller, R.; Izaola, O. The effects of two different hypocaloric diets on glucagon-like peptide 1 in obese adults, relation with insulin response after weight loss. J. Diabetes Complicat. 2009, 23, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L. Physiological adaptations to weight loss and factors favouring weight regain. Int. J. Obes. 2015, 39, 1188–1896. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, L.; Lu, J.; Mu, Y. High-protein breakfast promotes weight loss by suppressing subsequent food intake and regulating appetite hormones in obese Chinese adolescents. Horm. Res. Paediatr. 2015, 83, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, M.P.; Westerterp, K.R.; Adam, T.C.; Luscombe-Marsh, N.D.; Westerterp-Plantenga, M.S. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am. J. Clin. Nutr. 2006, 83, 89–94. [Google Scholar] [PubMed]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Bolanowski, M.; Nilsson, B.E. Assessment of human body composition using dual-energy x-ray absorptiometry and bioelectrical impedance analysis. Med. Sci. Monit. 2001, 7, 1029–1033. [Google Scholar] [PubMed]
- Fulgoni, V.L. Current protein intake in America: Analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr. 2008, 87, 1554S–1557S. [Google Scholar] [PubMed]
| Cereal | Orzo Pasta Dish | Fusilli Pasta Dish | Total | |||||
|---|---|---|---|---|---|---|---|---|
| CON | HPR | CON | HPR | CON | HPR | CON (% en) | HPR (% en) | |
| Energy, kcal | 260 | 270 | 324 | 364 | 324 | 358 | 935 | 954 |
| Protein, g | 4 | 21 | 11 | 25 | 7 | 24 | 22 (9) | 70 (29) |
| Fat, g | 5 | 11 | 9 | 12 | 9 | 11 | 23 (22) | 34 (32) |
| Carbohydrate, g | 52 | 24 | 53 | 39 | 55 | 39 | 160 (68) | 92 (39) |
| Fiber, g | 2 | 5 | 5 | 6 | 4 | 5 | 11 | 16 |
| Charateristic | CON | HPR | P |
|---|---|---|---|
| Gender, M/F | 1/10 | 5/5 | |
| Age, years | 45.6 ± 12.0 | 41.9 ± 12.6 | 0.875 |
| Body weight, kg | 86.1 ± 23.1 | 103.7 ± 13.9 | 0.310 |
| Body mass index, kg/m2 | 30.8 ± 7.6 | 33.7 ± 4.7 | 0.561 |
| Fat-free mass, kg | 50.8 ± 12.3 | 64.8 ± 11.3 | 0.464 |
| Body fat, % | 40.0 ± 7.9 | 38.1 ± 11.1 | 0.382 |
| TG/HDL ratio | 3.5 ± 3.0 | 3.3 ± 1.8 | 0.176 |
| Physical activity, MET h/week | 41.2 ± 25.5 | 54.6 ± 33.8 | 0.649 |
| POMS score | 13.5 ± 27.4 | 13.3 ± 20.9 | 0.956 |
| Characteristic | CON | HPR | P |
|---|---|---|---|
| Energy, kcal | 1184 ± 745 | 1206.0 ± 665 | 0.917 |
| Protein, g | 59 ± 43 | 54 ± 23 | 0.767 |
| Carbohydrate, g | 113 ± 70 | 134 ± 68 | 0.651 |
| Total fat, g | 55 ± 46 | 52 ± 41 | 0.765 |
| Saturated fat, g | 16 ± 10 | 16 ± 13 | 0.864 |
| Fiber, g | 14 ± 10 | 17 ± 10 | 0.628 |
| Total energy, kcal | 2119 ± 745 | 2160 ± 665 | 0.961 |
| Energy deficit, kcal | 310 ± 520 | 493 ± 571 | 0.522 |
| Group | Baseline | Week 6 | 6-Week Change | P | η2p |
|---|---|---|---|---|---|
| Plasma glucose, mg/dL CON | 91.0 ± 8.4 | 92.0 ± 9.3 | 1.1 ± 7.3 | 0.242 | 0.071 |
| HPR | 90.0 ± 11.4 | 87.6 ± 9.1 | −2.4 ± 5.8 | ||
| Plasma insulin, mU/mL CON | 16.7 ± 5.4 | 13.3 ± 4.0 | −3.4 ± 2.8 | 0.017 | 0.292 |
| HPR | 22.1 ± 12.1 | 15.4 ± 7.9 | −6.7 ± 5.0 | ||
| HOMA-IR CON | 3.8 ± 1.4 | 3.1 ± 1.1 | −0.7 ± 0.7 | 0.020 | 0.280 |
| HPR | 5.1 ± 3.4 | 3.5 ± 2.1 | −1.7 ± 1.4 | ||
| GLP-1, pmol/L ** CON | 7.3 ± 5.4 | 7.7 ± 6.2 | 0.4 ± 1.1 | 0.021 | 0.263 |
| HPR | 5.5 ± 3.0 | 4.8 ± 3.0 | −0.6 ± 0.8 | ||
| PYY, pmol/L † CON | 29.5 ± 25.1 | 20.6 ± 21.7 | −9.0 ± 13.6 | 0.241 | 0.075 |
| HPR | 34.1 ± 30.3 | 14.1 ± 19.5 | −20.0 ± 27.5 | ||
| HMW Adiponectin, μg/mL CON | 3.0 ± 1.3 | 3.0 ± 1.3 | +0.21 ± 2.26 | 0.828 | 0.003 |
| HPR | 1.6 ± 1.3 | 1.3 ± 0.9 | +0.03 ± 1.16 | ||
| Total cholesterol, mg/dL CON | 193.0 ± 25.7 | 175.5 ± 25.1 | −17.5 ± 18.7 | 0.276 | 0.065 |
| HPR | 175.0 ± 19.8 | 149.4 ± 21.6 | −25.6 ± 23.4 | ||
| LDL cholesterol, mg/dL † CON | 117.4 ± 38.6 | 107.5 ± 29.6 | −9.9 ± 19.0 | 0.506 | 0.024 |
| HPR | 108.5 ± 19.0 | 93.0 ± 24.3 | −15.6 ± 19.3 | ||
| HDL cholesterol, mg/dL CON | 49.8 ± 17.4 | 50.7 ± 14.2 | 0.9 ± 11.1 | 0.644 | 0.012 |
| HPR | 43.2 ± 12.1 | 43.4 ± 8.8 | 0.3 ± 7.3 | ||
| Triglycerides, mg/dL † CON | 138.3 ± 81.0 | 104.3 ± 44.3 | −34.1 ± 58.3 | 0.711 | 0.007 |
| HPR | 125.6 ± 49.3 | 83.4 ± 27.6 | −42.1 ± 36.1 | ||
| Chol/HDL ratio † CON | 4.3 ± 1.4 | 3.8 ± 1.4 | −0.5 ± 0.8 | 0.404 | 0.037 |
| HPR | 4.4 ± 1.3 | 3.6 ± 1.0 | −0.8 ± 0.7 | ||
| TG/HDL ratio † CON | 3.5 ± 3.0 | 2.4 ± 1.6 | −1.1 ± 2.1 | 0.882 | 0.001 |
| HPR | 3.3 ± 1.8 | 2.1 ± 1.0 | −1.2 ± 1.1 |
| Group | Baseline | Week 6 | 6-Week Change | P | η2p |
|---|---|---|---|---|---|
| Total antioxidant capacity CON | 2.0 ± 0.7 | 2.0 ± 0.6 | 0.1 ± 0.5 | 0.410 | 0.036 |
| HPR | 1.9 ± 0.5 | 1.8 ± 0.6 | −0.1 ± 0.5 | ||
| hsCRP, mg/L ** CON | 3.5 ± 3.9 | 3.4 ± 3.5 | −0.2 ± 1.2 | 0.533 | 0.022 |
| HPR | 3.0 ± 2.7 | 2.4 ± 1.7 | −0.6 ± 1.7 | ||
| 8-Isoprostane, pg/mL ** CON | 18.8 ± 4.5 | 18.5 ± 4.8 | −0.3 ± 3.8 | 0.909 | 0.001 |
| HPR | 19.0 ± 10.1 | 19.1 ± 3.6 | 0.1 ± 10.0 | ||
| TBARS, nmol/mL CON | 1.7 ± 0.7 | 1.7 ± 0.8 | −0.03 ± 1.0 | 0.467 | 0.028 |
| HPR | 2.1 ± 0.6 | 1.8 ± 0.4 | −0.3 ± 0.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, C.S.; Sears, B.; Perry, M.; Knurick, J.R. Use of Novel High-Protein Functional Food Products as Part of a Calorie-Restricted Diet to Reduce Insulin Resistance and Increase Lean Body Mass in Adults: A Randomized Controlled Trial. Nutrients 2017, 9, 1182. https://doi.org/10.3390/nu9111182
Johnston CS, Sears B, Perry M, Knurick JR. Use of Novel High-Protein Functional Food Products as Part of a Calorie-Restricted Diet to Reduce Insulin Resistance and Increase Lean Body Mass in Adults: A Randomized Controlled Trial. Nutrients. 2017; 9(11):1182. https://doi.org/10.3390/nu9111182
Chicago/Turabian StyleJohnston, Carol S., Barry Sears, Mary Perry, and Jessica R. Knurick. 2017. "Use of Novel High-Protein Functional Food Products as Part of a Calorie-Restricted Diet to Reduce Insulin Resistance and Increase Lean Body Mass in Adults: A Randomized Controlled Trial" Nutrients 9, no. 11: 1182. https://doi.org/10.3390/nu9111182





