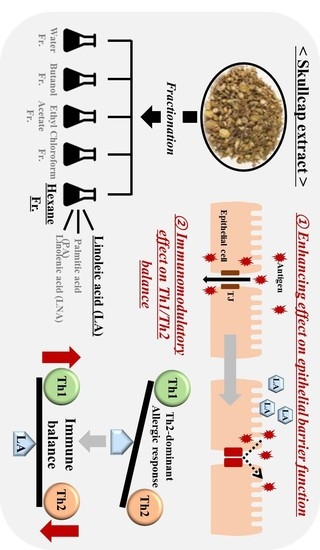
Skullcap (Scutellaria Baicalensis) Hexane Fraction Inhibits the Permeation of Ovalbumin and Regulates Th1/2 Immune Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
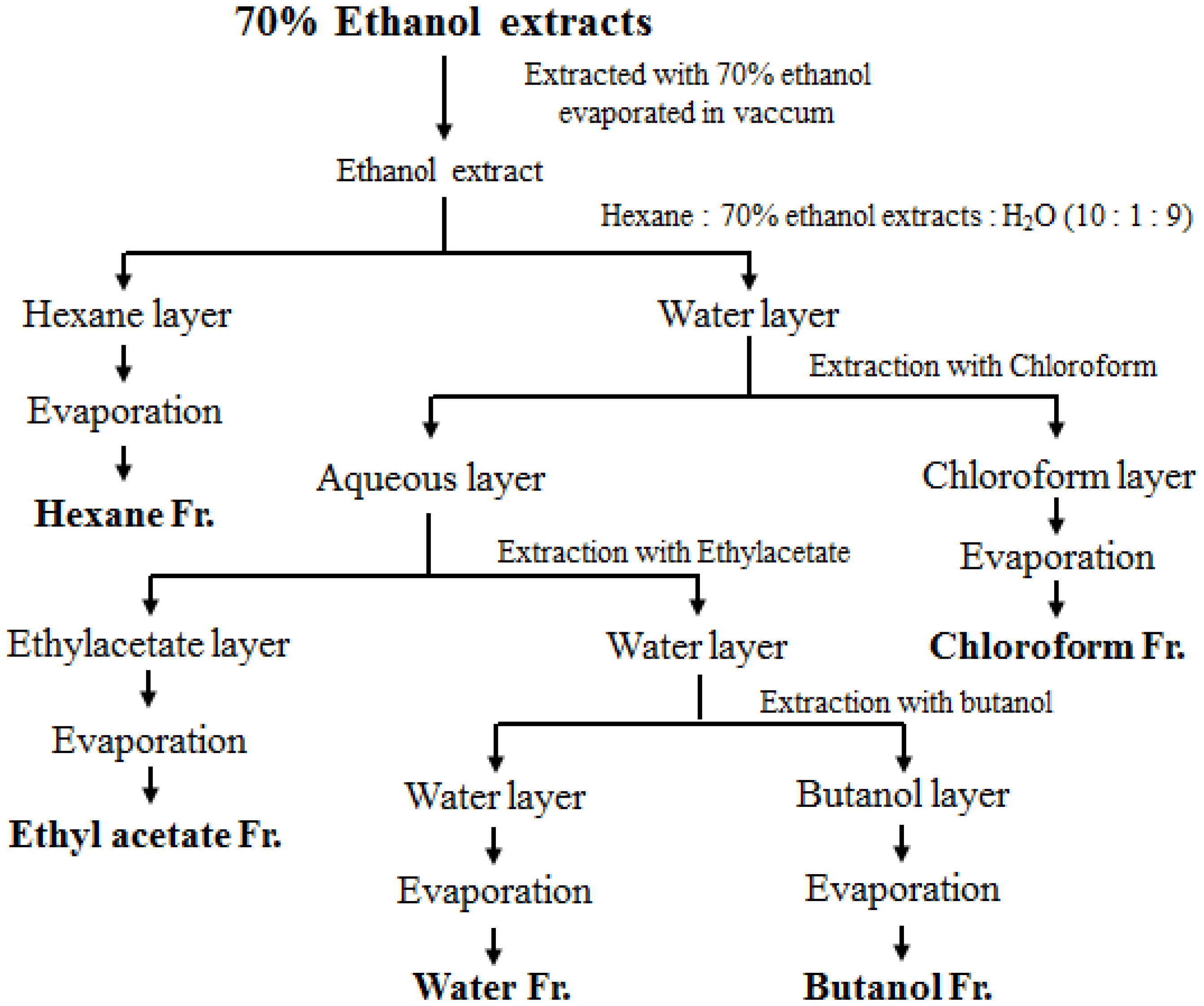
2.2. Sample Preparation
2.3. Sensitization with OVA and Preparation of Splenocyte Cultures
2.4. Measurement of Cytokine Levels Using ELISA
2.5. Measurement of Transepithelial Electrical Resistance
2.6. Analysis of Active Components Using High-Performance Liquid Chromatography (HPLC)
2.7. Fatty Acid Analysis
2.8. Statistical Analysis
3. Results
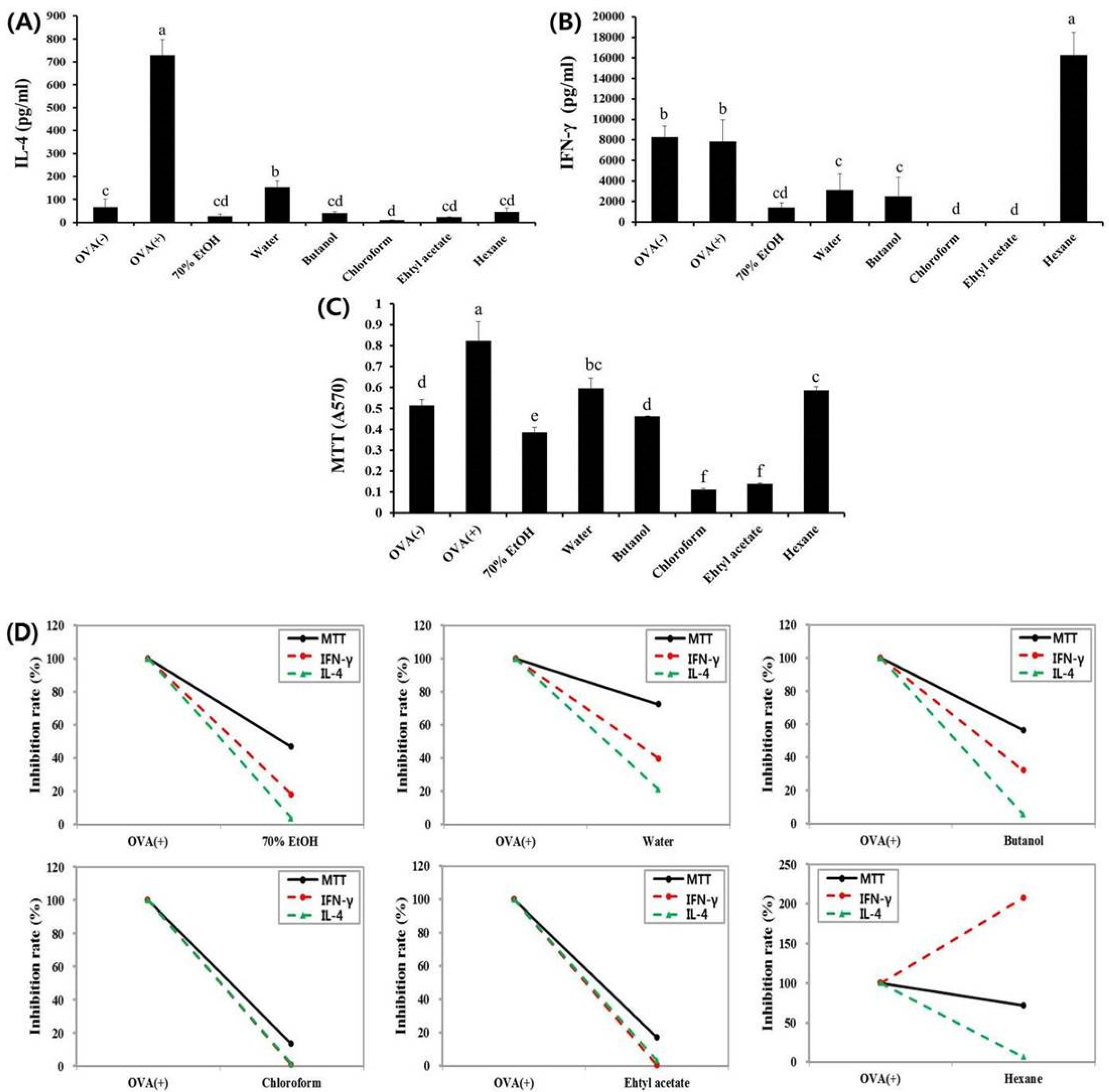
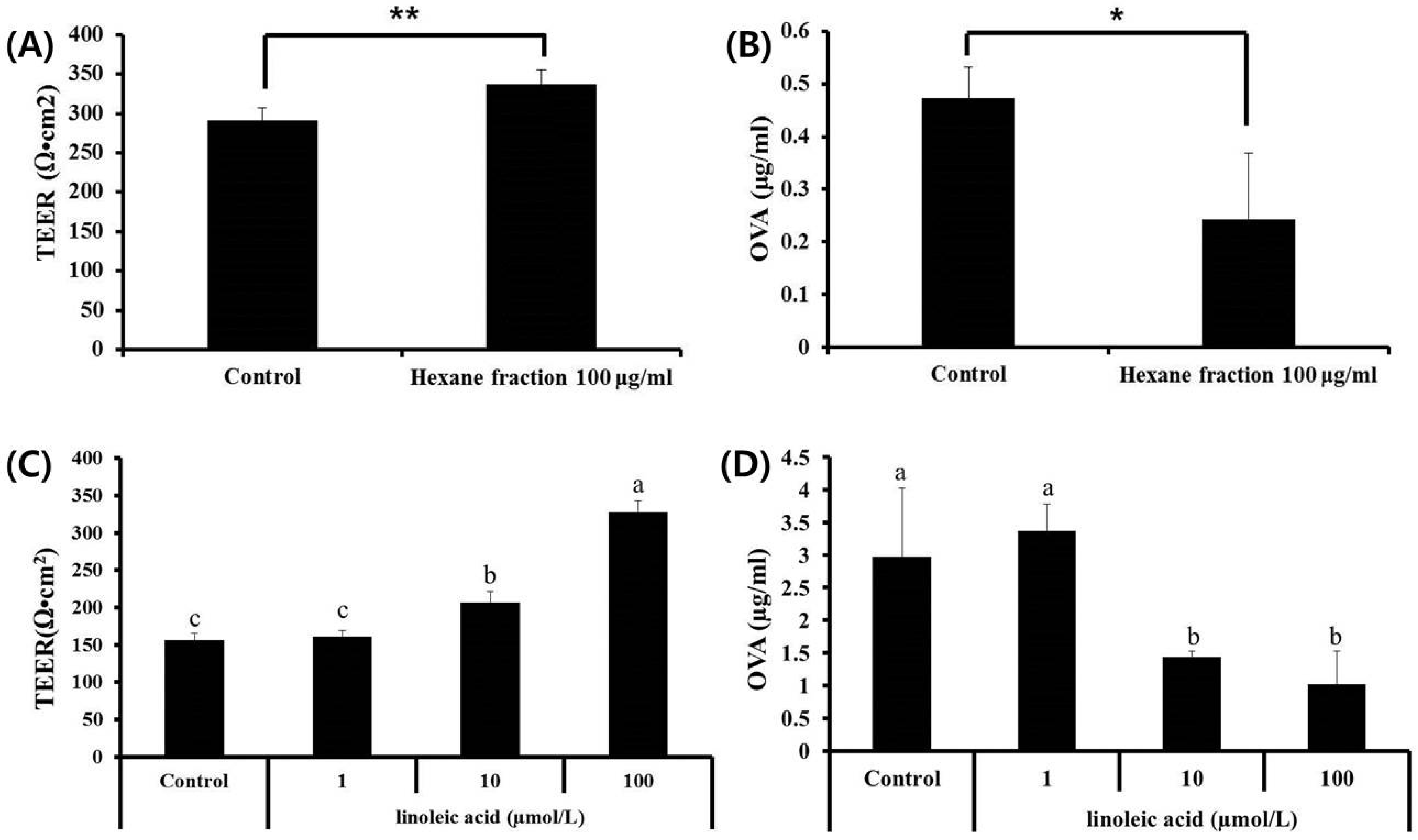
3.1. Effects of Fractions Obtained from Skullcap Extracts
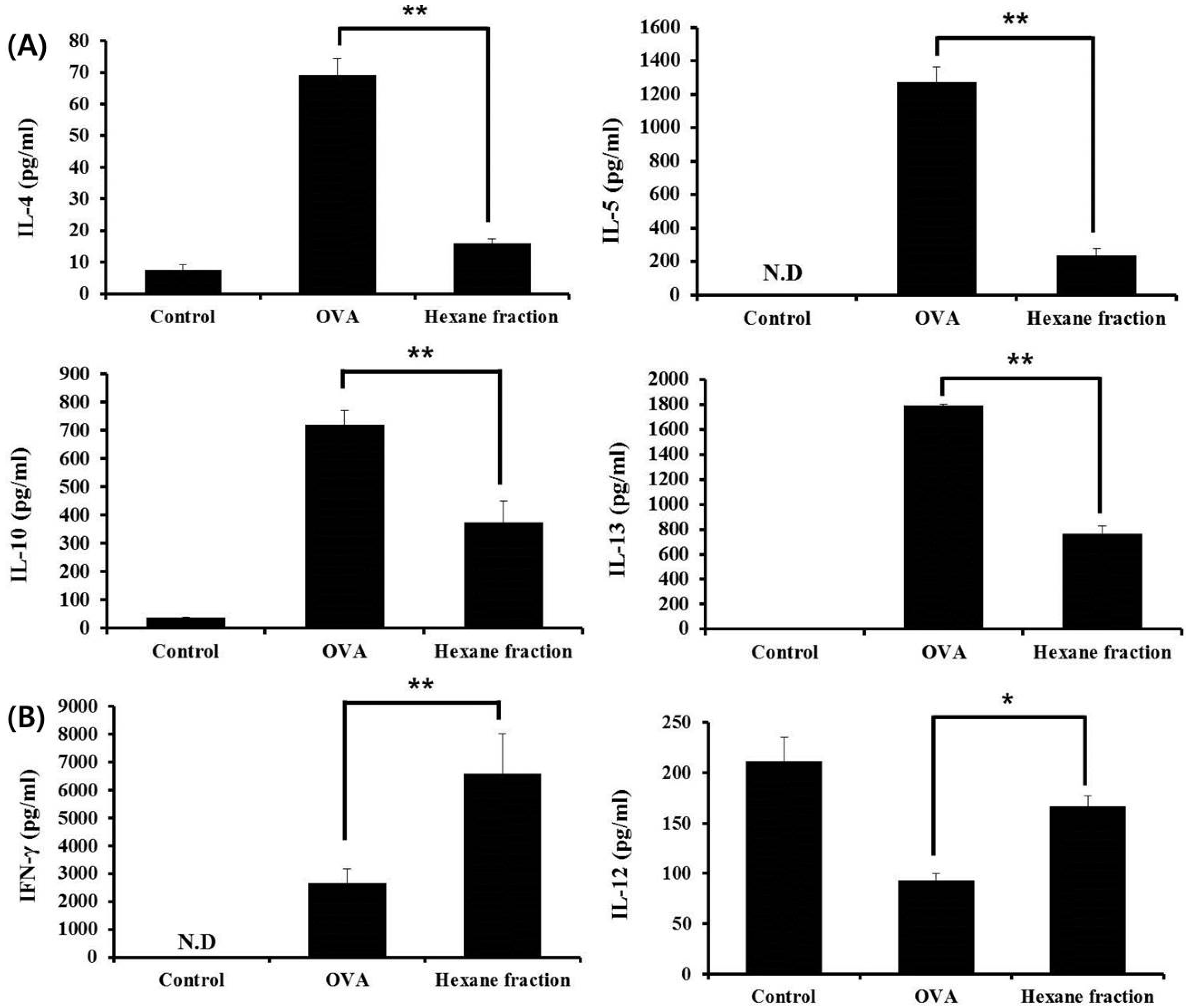
3.2. Hexane Fraction of Skullcap Regulates Th1- and Th2-Mediated Cytokines in Splenocytes from OVA-Sensitized Mice
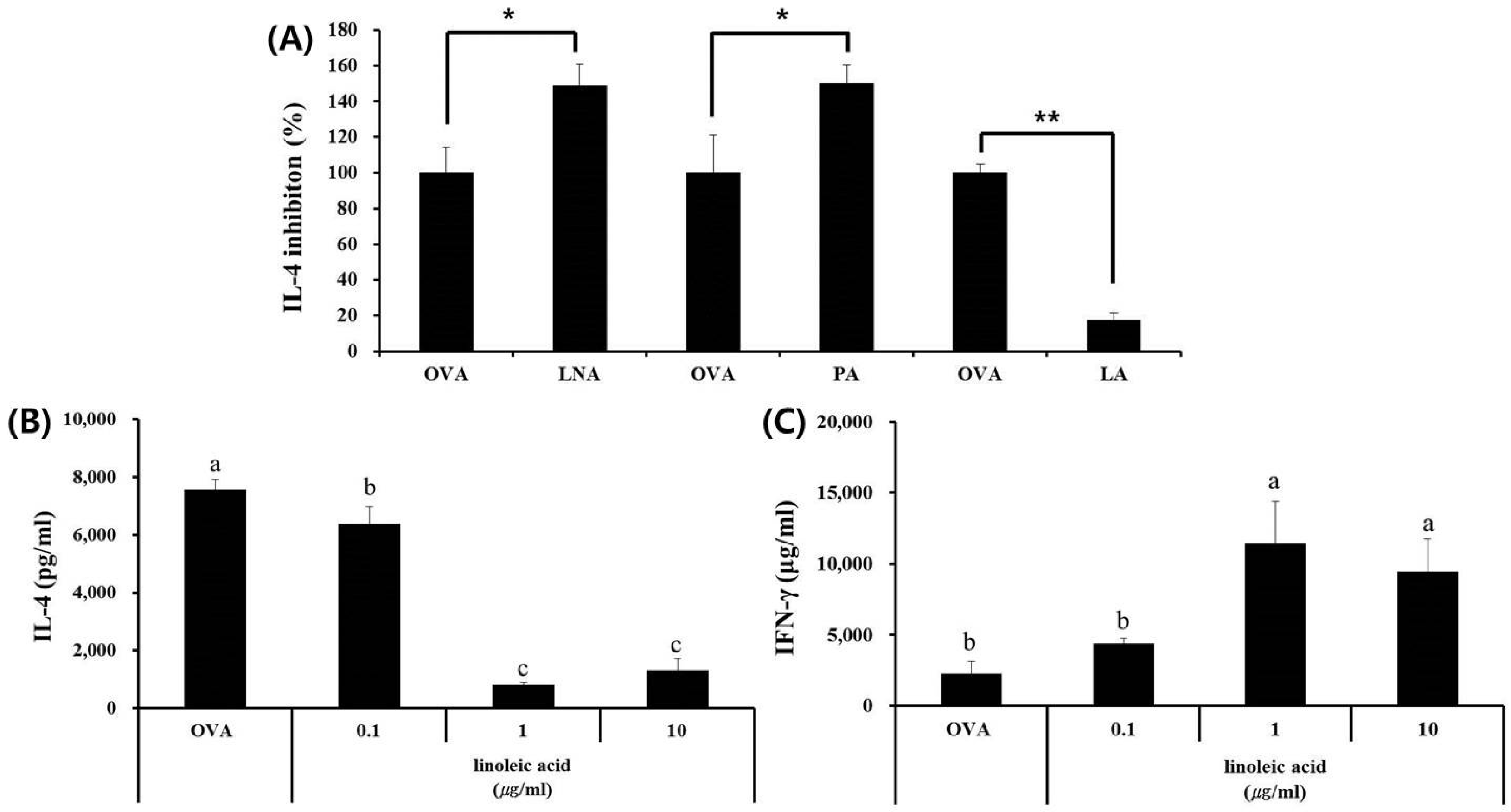
3.3. Fatty Acid Analysis of Skullcap Hexane Fraction
3.4. LA, but Not PA or LNA, Regulates Th1/Th2 Immune Balance
3.5. Effects of Hexane Fraction and LA on Transepithelial Electrical Resistance in Caco-2 Cell Monolayers
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of cutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lian, X.Y.; Li, S.; Stringer, J.L. Characterization of chemical ingredients and anticonvulsant activity of American skullcap (Scutellaria lateriflora). Phytomedicine 2009, 16, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Sanchez-Medina, A.; Pendry, B.A.; Hughes, M.J.; Webb, G.P.; Corcoran, O. Validation of a HPLC method for flavonoid biomarkers in sckullcap (Scutellaria) and its use to illustrate wide variability in the quality of commercial tinctures. J. Pharm. Sci. 2008, 11, 77–87. [Google Scholar]
- Shin, H.S.; Bae, M.J.; Jung, S.Y.; Shon, D.H. Inhibitory effect of skullcap (Scutellaria baicalensis) extract on ovalbumin permeation in vitro and in vivo. Food Chem. 2013, 140, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Bae, M.J.; Choi, D.W.; Shon, D.H. Skullcap (Scutellaria baicalensis) extract and its active compound, wogonin, inhibit ovalbumin-induced Th2-mediated response. Molecules 2014, 19, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Dai, S.X.; Chi, H.G.; Li, T.; He, Z.W.; Wang, J.; Ye, C.G.; Huang, G.L.; Zhao, B.; Li, W.Y.; et al. Baicalin attenuates TNBS-induced colitis in rats by modulating the Th17/Treg paradigm. Arch. Pharm. Res. 2015, 38, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.J.; Shin, H.S.; See, H.J.; Jung, S.Y.; Kwon, D.A.; Shon, D.H. Baicalein induces CD4 + Foxp3 + T cells and enhances intestinal barrier function in a mouse model of food allergy. Sci. Rep. 2016, 6, 32225. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Immunologic influences on allergy and the Th1/Th2 balance. J. Allergy Clin. Immunol. 2004, 113, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Bae, M.J.; Jung, S.Y.; Shon, D.H. Preventive effects of skullcap (Scutellaria baicalensis) extract in a mouse model of food allergy. J. Ethnopharmacol. 2014, 153, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Kaminogawa, S.; Hachimura, S.; Nakajjima-adachi, H.; Totsuka, M. Food allergens and mucosal immune systems with special reference to recognition of food allergens by gut-associated lymphoid tissue. Allergol. Int. 1999, 48, 15–23. [Google Scholar] [CrossRef]
- Kobayashi, S.; Watanabe, J.; Fukushi, E.; Kawabata, J.; Nakajima, M.; Watanabe, M. Polyphenols from some foodstuffs as inhibitors of ovalbumin permeation through Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2003, 67, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Zhang, J.W. Surfactants enhance the tight-junction permeability of food allergens in human intestinal epithelial Caco-2 cells. Int. Arch. Allergy Immunol. 2003, 30, 135–142. [Google Scholar] [CrossRef]
- AOAC International. Methyl esters of fatty acids in oils and fats. AOAC official method 963.22. In Official Methods of Analysis; AOAC International: Arlington, VA, USA, 2000. [Google Scholar]
- Yang, J.; Yang, X.; Li, M. Baicalin, a natural compound, promotes regulatory T cell differentiation. BMC Complement. Altern. Med. 2012, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, Z.; Xing, Y.; Gao, Y.; Ma, T.; Lou, L.; Lou, J.; Gao, Y.; Wang, S.; Wang, Y. Baicalin reduces the permeability of blood-brain barrier during hypoxia in vitro by increasing the expression of tight junction proteins in brain microvascular endothelial cells. J. Ethnopharmacol. 2012, 141, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Nusrat, A. Dynamic regulation of epithelial cell fate and barrier function by intercellular junctions. Ann. N. Y. Acad. Sci. 2009, 1165, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, T.; Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Finotto, S.; Glimcher, L.H. The role of Th1/Th2 polarization in mucosal immunity. Nat. Med. 2002, 8, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Gassler, N.; Rohr, C.; Schneider, A.; Kartenbeck, J.; Bach, A.; Obermuller, N.; Fotto, H.; Autschbach, F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am. J. Physiol. Cell Physiol. 2001, 281, G216–G228. [Google Scholar]
- Li, N.; Gu, L.; Qu, L.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine attenuates pro-inflammatory cytokine-induced tight junction disruption in an in vitro model of intestinal epithelial cells. Eur. J. Pharm. Sci. 2010, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Bae, M.J.; Jung, S.Y.; See, H.J.; Kim, Y.T.; Do, J.R.; Back, S.Y.; Choi, S.W.; Shon, D.H. Enhancing effect of trachelogenin from Trachelospermi caulis extract on intestinal barrier function. Biol. Pharm. Bull. 2015, 38, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Molina, E.; López-Fandiño, R. Hypoallergenic hydrolysates of egg white proteins modulate allergen responses induced ex vivo on spleen cells from sensitized mice. Food Res. Intern. 2016, 89, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, S.; Zuercher, A.W.; Julien-Javaux, F.; Perrot, M.; Mercenier, A. Characterization of candidate anti-allergic probiotic strains in a model of Th2-skewed human peripheral blood mononuclear cells. Int. Arch. Allergy Immunol. 2013, 161, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, B.; Eo, H.; Park, K.; Kim, Y.; Lee, H.J.; Son, M.; Chang, Y.S.; Cho, S.H.; Kim, S.; et al. Control of IgE and selective T(H)1 and T(H)2 cytokines by PG102 isolated from Actinidia arguta. J. Allergy Clin. Immunol. 2005, 116, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Umeda-Sawada, R.; Fujiwara, Y.; Ushiyama, I.; Sagawa, S.; Morimitsu, Y.; Kawashima, H.; Ono, Y.; Kiso, Y.; Matsumoto, A.; Seyama, Y. Distribution and metabolism of dihomo-γ-linolenic acid (DGLA, 20:3n-6) by oral supplementation in rats. Biosci. Biotechnol. Biochem. 2006, 70, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.J.; Kim, H.K.; Lim, S.; Lee, S.Y.; Shin, H.S.; Kim, J.E.; Im, S.H.; Kim, S.Y. Lactobacillus pentosus KF340 alleviates house dust mite-induced murine atopic dermatitis via the secretion of IL-10-producing splenic B10 cells. J. Funct. Foods 2016, 26, 258–267. [Google Scholar] [CrossRef]
- Smith, D.L.; Willis, A.L.; Nguyen, N.; Conner, D.; Zahedi, S.; Fulks, J. Eskimo plasma constituents, dihomo-γ-linolenic acid, eicosapentaenoic acid and docosahexaenoic acid inhibit the release of atherogenic mitogens. Lipids 1989, 24, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Hamazaki, T.; Taki, H.; Yamazaki, K.; Kobayashi, M. Intravenous infusion of tridihomo-γ-linoleoyl-glycerol reduces leukotriene B4 production in the rat and rabbit. Clin. Sci. 1993, 84, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Tateishi, N.; Shiraishi, A.; Teraoka, N.; Tanaka, T.; Tanaka, A.; Matsuda, H.; Kiso, Y. Oral administration of dihomo-γ-linolenic acid prevents development of atopic dermatitis in NC/Nga Mice. Lipids 2008, 43, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, C.J.; Thijs, C.; Henquet, C.J.; van Houwelingen, A.C.; Dagnelie, P.C.; Schrander, J.; Menheere, P.P.; van den Brandt, P.A. γ-Linolenic acid supplementation for prophylaxis of atopic dermatitis-a randomized controlled trial in infants at high familial risk. Am. J. Clin. Nutr. 2003, 77, 943–951. [Google Scholar] [PubMed]
- Kawamura, A.; Ooyama, K.; Kojima, K.; Kachi, H.; Abe, T.; Amano, K.; Aoyama, T. Dietary supplementation of Gamma-Linolenic acid improves skin parameters in subjects with dry skin and mild atopic dermatitis. J. Oleo Sci. 2011, 60, 597–607. [Google Scholar] [CrossRef] [PubMed]
| Chemical Formula | Fatty Acid | Composition Ratio (%) per 100 g Fat |
|---|---|---|
| C16:0 | Palmitic acid | 17.7 |
| C18:0 | Stearic acid | 1.6 |
| C18:1 | Oleic acid | 3.6 |
| C18:2 | Linoleic acid | 59.7 |
| C18:3 | Linolenic acid | 12.0 |
| C20:0 | Arachidic acid | 1.0 |
| C20:2 | Eicosadienoic acid | 0.8 |
| C22:0 | Behenic acid | 2.0 |
| C24:0 | Lignoceric acid | 1.2 |
| Unknown | 0.4 | |
| Total | 100.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.Y.; Lee, S.-Y.; Choi, D.W.; See, H.-J.; Kwon, D.-A.; Do, J.-R.; Shon, D.-H.; Shin, H.S. Skullcap (Scutellaria Baicalensis) Hexane Fraction Inhibits the Permeation of Ovalbumin and Regulates Th1/2 Immune Responses. Nutrients 2017, 9, 1184. https://doi.org/10.3390/nu9111184
Jung SY, Lee S-Y, Choi DW, See H-J, Kwon D-A, Do J-R, Shon D-H, Shin HS. Skullcap (Scutellaria Baicalensis) Hexane Fraction Inhibits the Permeation of Ovalbumin and Regulates Th1/2 Immune Responses. Nutrients. 2017; 9(11):1184. https://doi.org/10.3390/nu9111184
Chicago/Turabian StyleJung, Sun Young, So-Young Lee, Dae Woon Choi, Hye-Jeong See, Da-Ae Kwon, Jeong-Ryong Do, Dong-Hwa Shon, and Hee Soon Shin. 2017. "Skullcap (Scutellaria Baicalensis) Hexane Fraction Inhibits the Permeation of Ovalbumin and Regulates Th1/2 Immune Responses" Nutrients 9, no. 11: 1184. https://doi.org/10.3390/nu9111184