Effects of Fusarium Head Blight on Wheat Grain and Malt Infected by Fusarium culmorum
Abstract
:1. Introduction
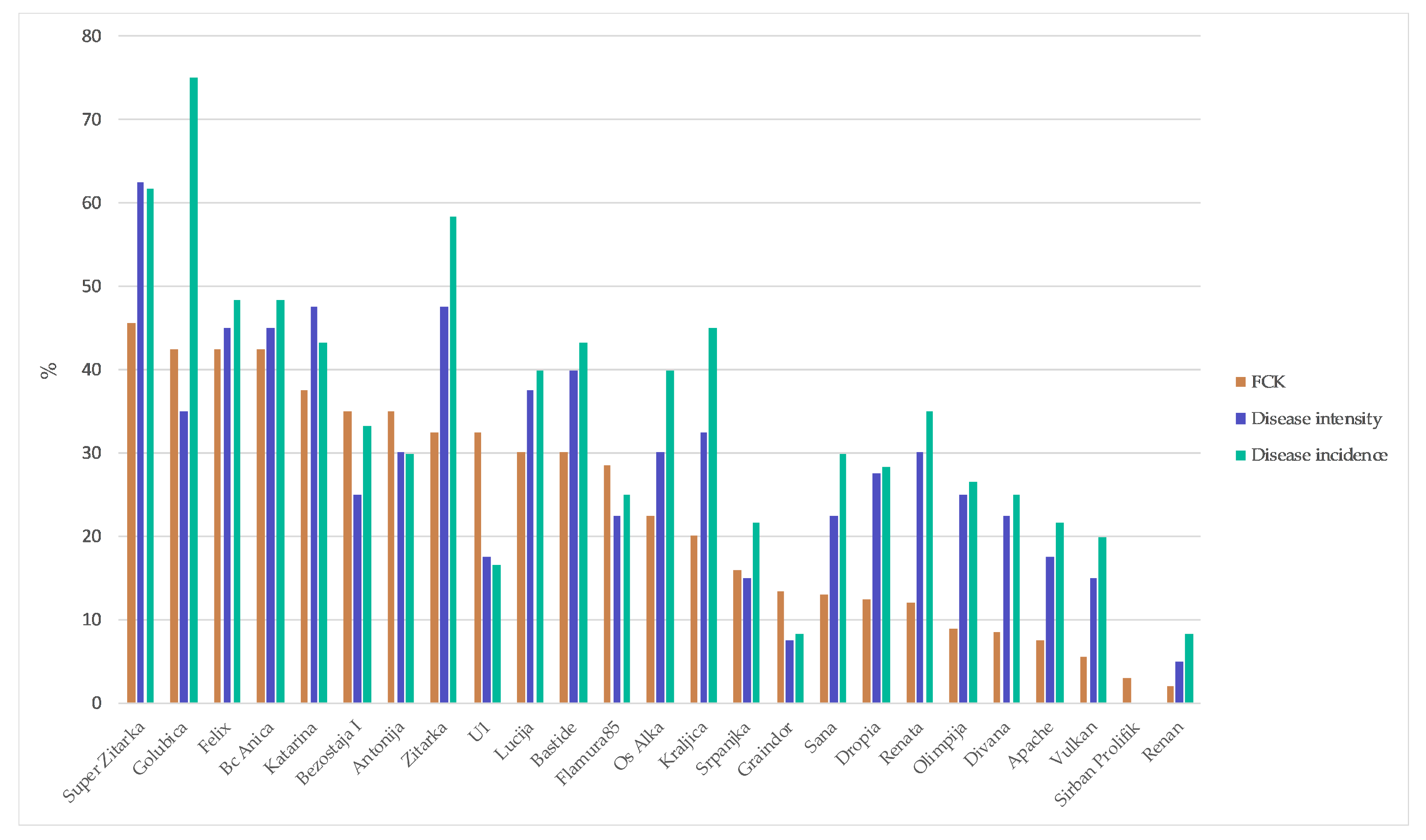
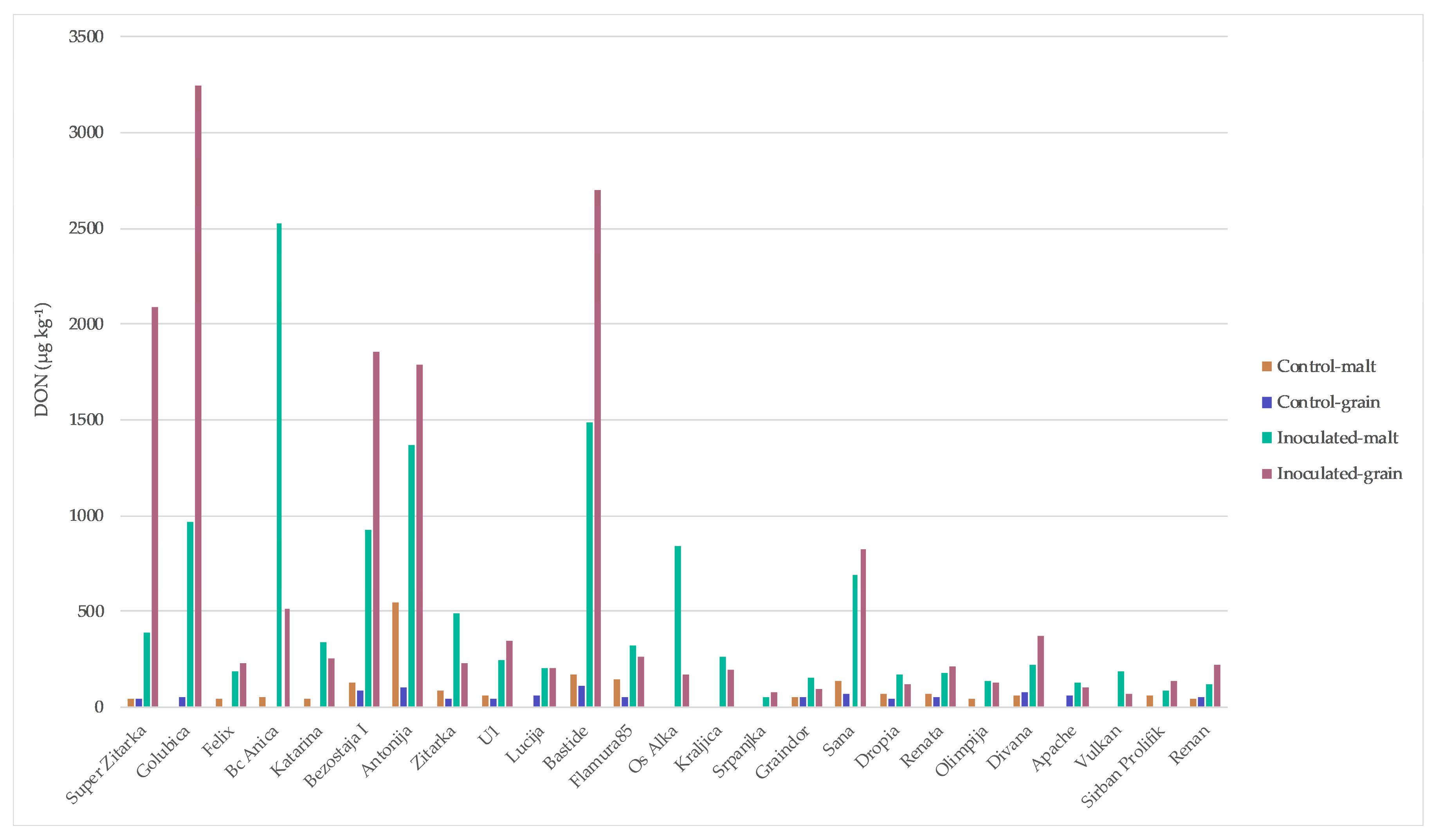
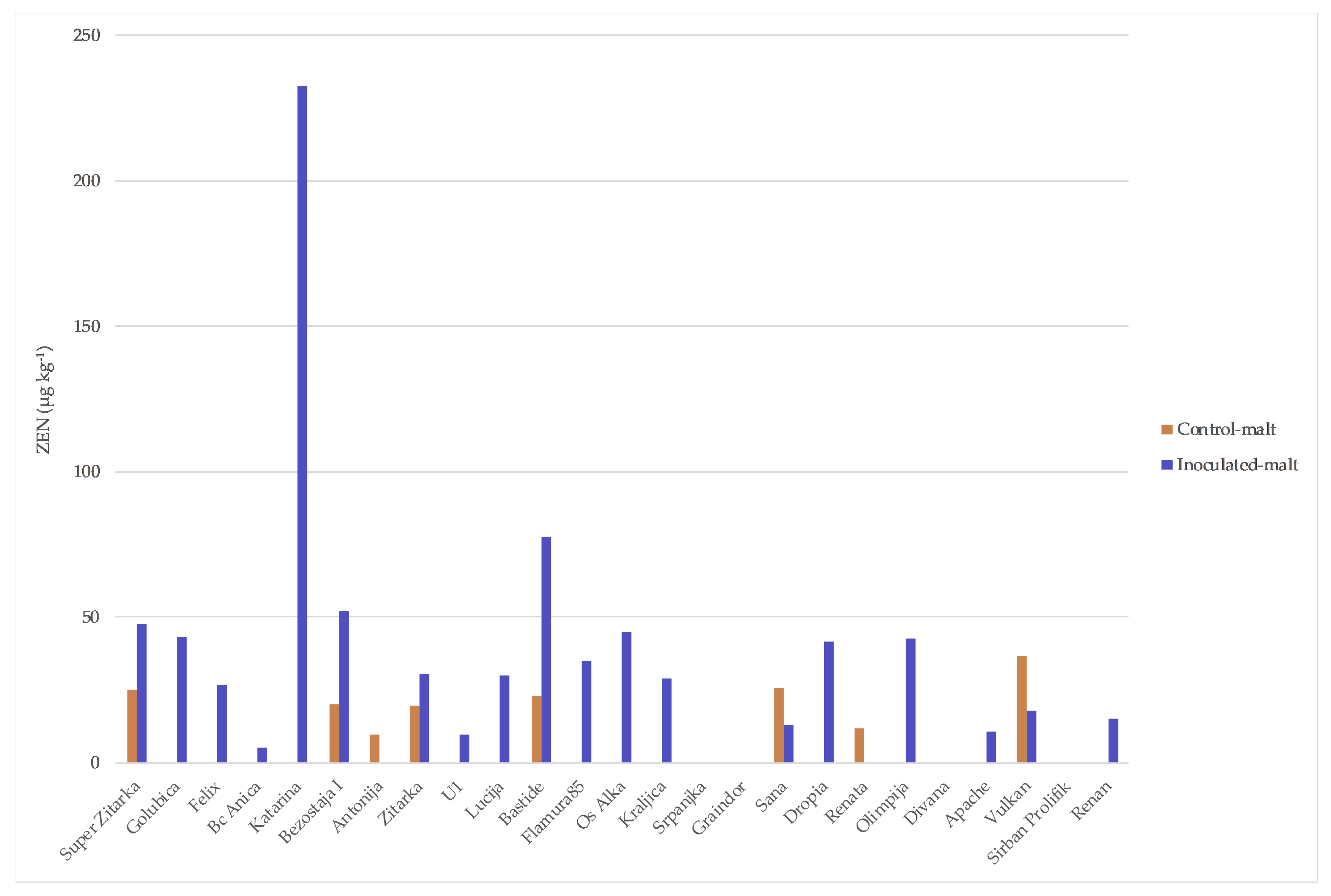
2. Results
3. Discussion
4. Materials and Methods
4.1. Inoculum Production
4.2. Field Trials
4.3. Malting
4.4. Mycotoxin Analyses
4.5. Method Validation
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lemmens, M.; Bűrstmayr, H.; Krska, R.; Schuhmacher, R.; Grausgruber, H.; Ruckenbauer, P. The effect of inoculation treatment and long-term application of moisture on Fusarium head blight symptoms and deoxynivalenol contamination in wheat grains. Eur. J. Plant Pathol. 2004, 110, 229–308. [Google Scholar] [CrossRef]
- Perkowski, J.; Kiecana, I.; Kaczmarek, Z. Natural occurrence and distribution of Fusarium toxins in contaminated barley cultivars. Eur. J. Plant Pathol. 2003, 109, 331–339. [Google Scholar] [CrossRef]
- Lindblad, M.; Gidlund, A.; Sulyok, M.; Borjesson, T.; Krska, R.; Olsen, M.; Fredlund, E. Deoxynivalenol and other selected Fusarium toxins in Swedish wheat—Occurrence and correlation to specific Fusarium species. Int. J. Food Sci. Technol. 2015, 167, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Spanic, V.; Lemmens, M.; Drezner, G.; Dvojkovic, K. Interellations between heiht of winter wheat genotypes and resistance to fusarium head blight (FHB). Rom. Agric. Res. 2011, 28, 43–48. [Google Scholar]
- Miedaner, T.; Wilde, F.; Korzun, V.; Ebmeyer, E.; Schmolke, M.; Hartl, L.; Schon, C.C. Marker selection for Fusarium head blight resistance based on quantitative trait loci (QTL) from two European sources compared to phenotypic selection in winter wheat. Euphytica 2009, 166, 219–227. [Google Scholar] [CrossRef]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, H.; Lemmens, M.; Fedak, G.; Ruckenbauer, P. Back-cross reciprocal monosomic analysis of Fusarium head blight resistance in wheat (Triticum aestivum L.). Theor. Appl. Genet. 1999, 98, 76–85. [Google Scholar] [CrossRef]
- Draeger, R.; Gosman, N.; Steed, A.; Chandler, E.; Thomsett, M.; Srinivasachary, J.; Schondelmaier, H.; Buerstmayer, M.; Lemmens, M.; Schmolke, A.; et al. Identification of QTLs for resistance to Fusarium head blight, DON accumulation and associated traits in the winter wheat variety Arina. Theor. Appl. Genet. 2007, 115, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W.; Christensen, J.J. Factors affecting resistance of wheat to scab by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Miller, J.D.; Arnison, P.G. Degradation of deoxynivalenol by suspension cultures of the Fusarium head blight resistant wheat cultivar Frontana. Can. J. Plant Pathol. 1986, 8, 147–150. [Google Scholar] [CrossRef]
- Mesterhazy, A. Types and components of resistance to Fusarium head blight of wheat. Plant Breed. 1995, 114, 337–386. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Bartok, T.; Mirocha, G.; Komoroczy, R. Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breed. 1999, 118, 97–110. [Google Scholar] [CrossRef]
- Havlová, P.; Lancová, K.; Vánová, M.; Havel, J.; Hajslová, J. The effect of fungicidal treatment on selected quality parameters of barley and malt. J. Agric. Food Chem. 2006, 54, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Skrbic, B.; Malachová, A.; Zivancev, J.; Vepríková, Z.; Hajslová, J. Fusarium mycotoxins in wheat samples harvested in Serbia: A preliminary survey. Food Control 2011, 22, 1261–1267. [Google Scholar] [CrossRef]
- Da Rocha, M.E.B.; da Chagas Oliveira Freire, F.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Spanic, V.; Lemmens, M.; Drezner, G. Morphological and molecular identification of Fusarium species associated with head blight on wheat in East Croatia. Eur. J. Plant Pathol. 2010, 128, 511–516. [Google Scholar] [CrossRef]
- Siegel, D.; Babuscio, T. Mycotoxin management in the European cereal trading sector. Food Control 2011, 22, 1145–1153. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Foglia, P.; Gubbiotti, R.; Roccia, C.; Samperi, R.; Laganà, A. Development and validation of a liquid chromatography/atmospheric pressure photoionization-tandem mass spectrometric method for the analysis of mycotoxins subjected to commission regulation (EC) No. 1881/2006 in cereals. J. Chromatogr. A 2010, 1217, 6044–6051. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Hall, C.E. Mold and mycotoxin problem encountered during malting and brewing. Int. J. Food Microbiol. 2007, 119, 89–94. [Google Scholar] [CrossRef] [PubMed]
- EC. European Commission Recommendation on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products; EC 165/2013; Official Journal of the European Union: Brussels, Belgium, 2006; Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013H0165&from=en (accessed on 26 December 2017).
- Wolf-Hall, C.E.; Schwarz, P.B. Mycotoxins and fermentation—Beer production. Adv. Exp. Med. 2002, 504, 217–226. [Google Scholar]
- Justé, A.; Malfliet, S.; Lenaerts, M.; de Cooman, L.; Aerts, G.; Willems, K.A.; Lievens, B. Microflora during Malting of Barley: Overview and Impact on Malt Quality. Brew. Sci. 2011, 64, 22–31. [Google Scholar]
- Milani, J.; Maleki, G. Effects of processing on mycotoxin stability in cereals. J. Sci. Food Agric. 2014, 94, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.B.; Beattie, S.; Casper, H.H. Relationship between Fusarium infestation of barley and the gushing potential of malt. J. Inst. Brew. 1996, 102, 93–96. [Google Scholar] [CrossRef]
- Lowe, D.P.; Arendt, E.K. The Use and Effects of Lactic Acid Bacteria in Malting and Brewingwith Their Relationships to Antifungal Activity, Mycotoxins and Gushing: A Review. J. Inst. Brew. 2004, 110, 163–180. [Google Scholar] [CrossRef]
- Schwarz, P.B.; Horsley, R.D.; Steffenson, B.J.; Salas, B.; Barr, J.M. Quality Risks Associated with the Utilization of Fusarium Head Blight Infected Malting Barley. J. Am. Soc. Brew. Chem. 2006, 64, 1–7. [Google Scholar] [CrossRef]
- Váňová, M.; Hajšlová, J.; Havlová, P.; Matušinsky, P.; Lancová, K.; Spitzerová, D. Effect of spring barley protection on the production of Fusarium spp. mycotoxins in grain and malt using fungicides in field trials. Plant Soil Environ. 2004, 50, 447–455. [Google Scholar]
- Schapira, S.F.D.; Whitehead, M.P.; Flannigan, B. Effects of the mycotoxins diacetoxyscirpenol and deoxynivalenol on malting characteristics of barley. J. Inst. Brew. 1989, 95, 415–417. [Google Scholar] [CrossRef]
- Strub, C.; Pocaznoi, D.; Lebrihi, A.; Fournier, R.; Mathieu, F. Influence of barley malting operating parameters on T-2 and HT-2 toxinogenesis of Fusarium langsethiae, a worrying contaminant of malting barley in Europe. Food Addit. Contam. Part A 2010, 27, 1247–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancova, K.; Hajslova, J.; Poustka, J.; Krplova, A.; Zachariasova, M.; Dostalek, P.; Sachambula, L. Transfer of Fusarium mycotoxins and ‘masked’ deoxynivalenol (deoxynivalenol-3-glucoside) from field barley through malt to beer. Food Addit. Contam. Part A 2008, 25, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.B.; Casper, H.H.; Barr, J.M. Survey of the natural occurrence of deoxynivalenol (vomitoxin) in barley grown in Minnesota, North Dakota and South Dakota during 1993. Tech. Q. Master Brew. Assoc. Am. 1995, 3, 190–194. [Google Scholar]
- Schwarz, P.B.; Casper, H.H.; Barr, J.M.; Musial, M. Impact of Fusarium head blight on the malting and brewing quality of barley. Cereal Res. Commun. 1997, 25, 813–814. [Google Scholar]
- Sarlin, T.; Nakari-Setala, T.; Linder, M.; Penttila, M.; Haikara, A. Fungal hydrophobins as predictors of the gushing activity of malt. J. Inst. Brew. 2005, 111, 105–111. [Google Scholar] [CrossRef]
- Habler, K.; Hofer, K.; Geißinger, C.; Schüler, J.; Hückelhoven, R.; Hess, M.; Gastl, M.; Rychlik, M. Fate of Fusarium Toxins during the Malting Process. Agric. Food Chem. 2016, 64, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.M.; Mauch, A.; Jacob, F.; Waters, D.M.; Arendt, E.K. Fundamental study on the influence of Fusarium infection on quality and ultrastructure of barley malt. Int. J. Food Microbiol. 2012, 156, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Badeck, F.W.; Lattanzio, V.M.T.; Pascale, M.; Terzi, V. Occurrence of Fusarium langsethiae and T-2 and HT-2 Toxins in Italian Malting Barley. Toxins 2016, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Snijders, C.H.A.; Van Eeuwijk, F.A. Genotype X strain interactions for resistance to Fusarium head blight caused by Fusarium culmorum in winter wheat. Theor. Appl. Genet. 1991, 81, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, Y.; Shao, S.; Cai, Z.; Feng, L.; Pan, H.; Wang, Z. Simultaneous determination of multi-component mycotoxin contaminants in foods and feeds by ultra-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2007, 1143, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Malachová, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chrom. A 2014, 1362, 145–156. [Google Scholar] [CrossRef] [PubMed]
- EC. European Commission Decision on Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results; EC 657/2002; Official Journal of the European Communities: Grand Duchy, Luxembourg, 2002; Available online: http://eurl.craw.eu/img/page/Legislation/657_2002_EN.pdf (accessed on 26 December 2017).

| Trait | Control | Inoculum | ||
|---|---|---|---|---|
| Malt | Grain | Malt | Grain | |
| FCK ** | 0.21 | 0.10 | 0.53 * | 0.54 * |
| Intensity | 0.05 | 0.00 | 0.39 | 0.38 |
| Incidence | −0.06 | 0.01 | 0.42 | 0.55 * |
| Varieties | Origin * | Year of Release | Susceptibility ** to Fusarium |
|---|---|---|---|
| GOLUBICA | HR, AIO | 1997 | S |
| SUPER ZITARKA | HR, AIO | 1997 | S |
| BC ANICA | HR, BC | 2010 | S |
| SANA | HR, BC | 1983 | MS |
| ŽITARKA | HR, AIO | 1985 | MS |
| OS ALKA | HR, AIO | 2003 | MS |
| KATARINA | HR, AIO | 2006 | MS |
| BASTIDE | FRA | 2003 | MS |
| FELIX | HR, AIO | 2007 | MS |
| U1 | HR, AIO | 1936 | MR |
| BEZOSTAYA-1 | Former USSR | 1955 | MR |
| SRPANJKA | HR, AIO | 1989 | MR |
| FLAMURA 85 | ROM | 1989 | MR |
| DIVANA | HR, JS | 1995 | MR |
| APACHE | FRA | 1998 | MR |
| LUCIJA | HR, AIO | 2001 | MR |
| DROPIA | ROM | 2006 | MR |
| RENATA | HR, AIO | 2006 | MR |
| OLIMPIJA | HR, AIO | 2009 | MR |
| VULKAN | HR, AIO | 2009 | MR |
| KRALJICA | HR, AIO | 2010 | MR |
| ANTONIJA | HR, AIO | 2011 | MR |
| SIRBAN PROLIFIC | HU | 1905 | R |
| RENAN | FRA | 1991 | R |
| GRAINDOR | FRA | 2006 | R |
| Analyte | Polarity | Retention Time (min) | RE (%) | RA (%) | SSE (%) | RSD Interday | RSD Intraday | LOD Matrix (ng g−1) | LOQ Matrix (ng g−1) |
|---|---|---|---|---|---|---|---|---|---|
| NIV | − | 2.4 | 81 | 70 | 70 | 5.3% | 9.6% | 6.1 | 21.0 |
| DON | − | 2.5 | 102 | 101 | 99 | 6.0% | 10.4% | 4.8 | 15.1 |
| FUS-X | + | 2.6 | 72 | 64 | 89 | 2.8% | 4.8% | 2.7 | 9.8 |
| 3-ADON | + | 3.1 | 92 | 88 | 96 | 5.1% | 7.1% | 5.15 | 16.2 |
| DAS | + | 14.9 | 84 | 97 | 117 | 7.9% | 21.6% | 2.1 | 7.1 |
| HT-2 | + | 18.4 | 70 | 60 | 86 | 7.1% | 14.0% | 1.8 | 6.2 |
| T-2 | + | 19.6 | 72 | 75 | 107 | 7.4% | 12.8% | 2.3 | 6.6 |
| ZEN | − | 19.8 | 73 | 82 | 109 | 11.2% | 13.6% | 1.2 | 3.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanic, V.; Marcek, T.; Abicic, I.; Sarkanj, B. Effects of Fusarium Head Blight on Wheat Grain and Malt Infected by Fusarium culmorum. Toxins 2018, 10, 17. https://doi.org/10.3390/toxins10010017
Spanic V, Marcek T, Abicic I, Sarkanj B. Effects of Fusarium Head Blight on Wheat Grain and Malt Infected by Fusarium culmorum. Toxins. 2018; 10(1):17. https://doi.org/10.3390/toxins10010017
Chicago/Turabian StyleSpanic, Valentina, Tihana Marcek, Ivan Abicic, and Bojan Sarkanj. 2018. "Effects of Fusarium Head Blight on Wheat Grain and Malt Infected by Fusarium culmorum" Toxins 10, no. 1: 17. https://doi.org/10.3390/toxins10010017





