Synchrotron-Based Pencil Beam Scanning Nozzle with an Integrated Mini-Ridge Filter: A Dosimetric Study to Optimize Treatment Delivery
Abstract
:1. Introduction
2. Results
2.1. Effect of IMRF on Integral Depth Dose and Spot Size
2.2. Phantom Planning Study
2.3. Clinical Case Planning Study
3. Discussion
4. Materials and Methods
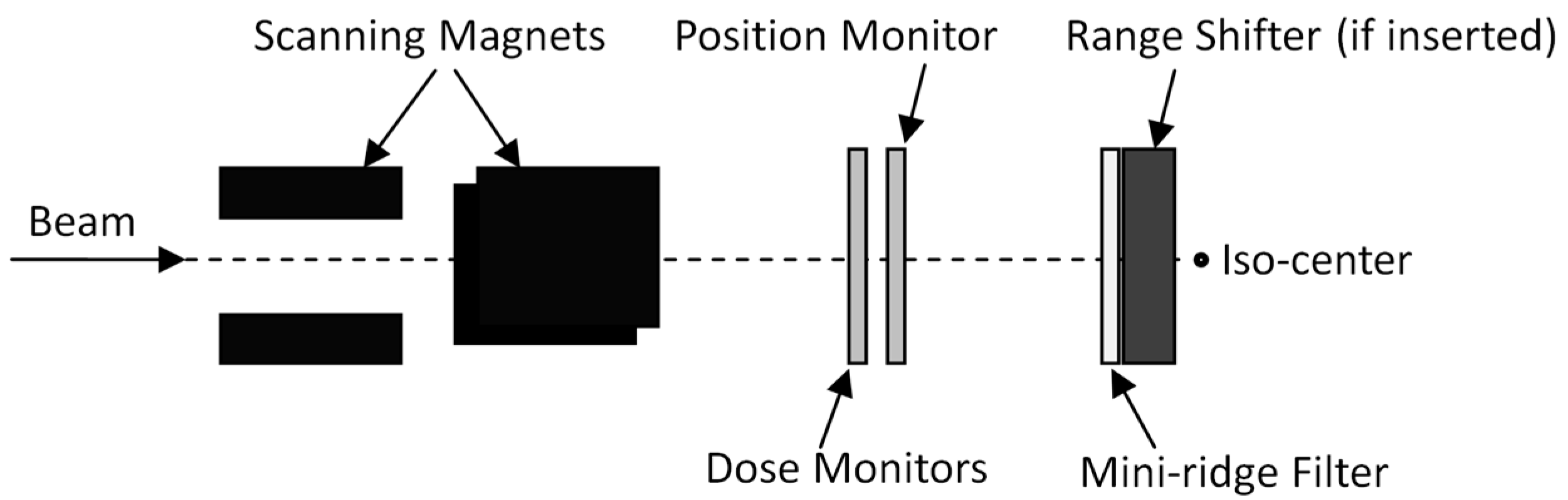
4.1. Treatment Delivery System
4.2. Treatment Planning System
4.3. Phantom Planning Study
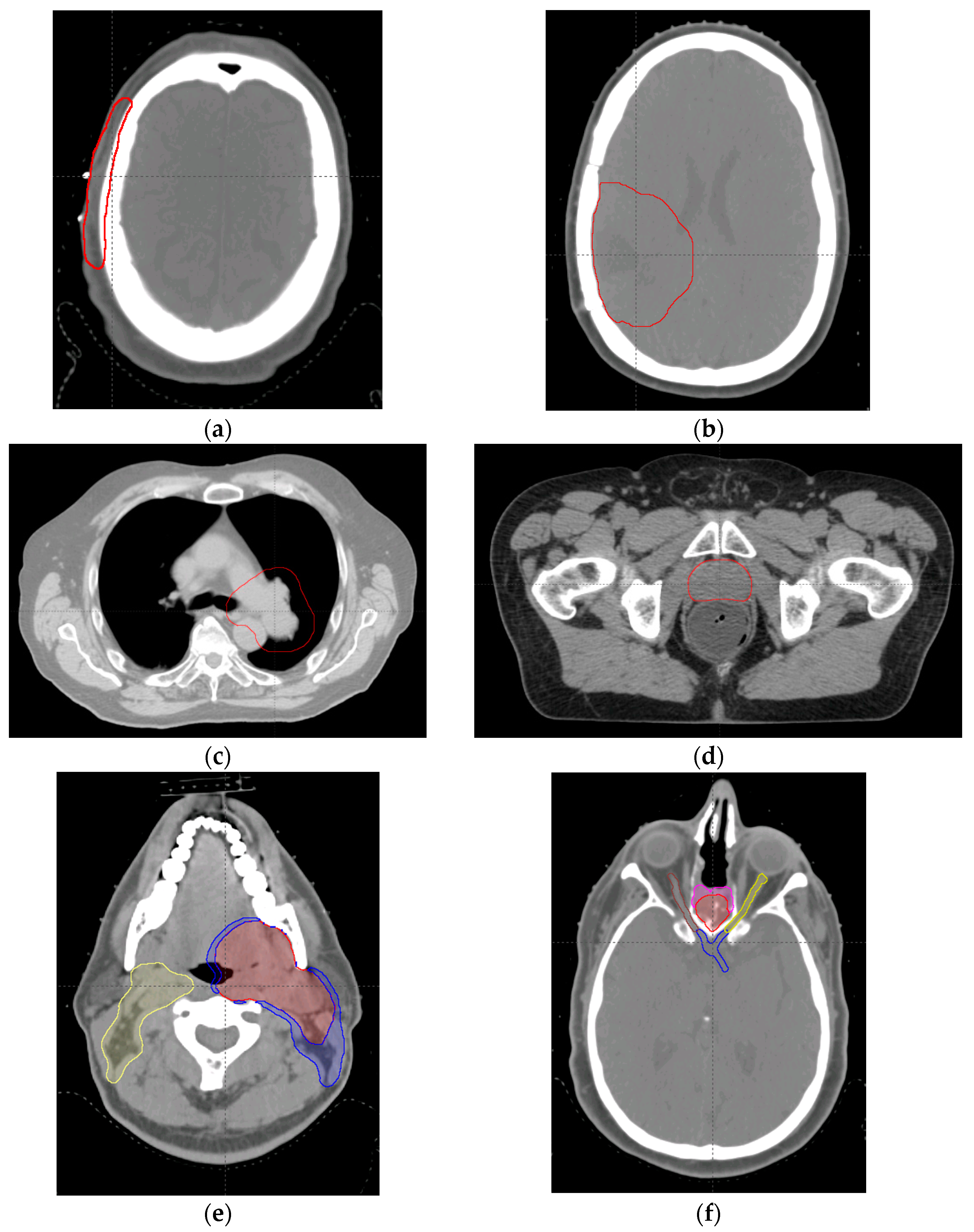
4.4. Clinical Case Planning Study
4.5. Beam Delivery Efficiency
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schippers, M. Proton beam production and dose delivery techniques. In Principles and Practice of Proton Beam Therapy; Das, I.J., Paganetti, H., Eds.; AAPM Monograph, 2015 Summer School; Medical Physics Publishing: Madison, WI, USA, 2015; Chapter 6. [Google Scholar]
- Li, Y.; Kardar, L.; Li, X.; Li, H.; Cao, W.; Chang, J.Y.; Liao, L.; Zhu, R.X.; Sahoo, N.; Gillin, M.; et al. On the interplay effects with proton scanning beams in stage III lung cancer. Med. Phys. 2014, 41, 021721. [Google Scholar] [CrossRef] [PubMed]
- Courneyea, L.; Beltran, C.; Tseung, H.S.; Yu, J.; Herman, M.G. Optimizing mini-ridge filter thickness to reduce proton treatment times in a spot-scanning synchrotron system. Med. Phys. 2014, 41, 061713. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Fujii, Y.; Takao, S.; Yamada, T.; Matsuzaki, Y.; Miyamoto, N.; Takayanagi, T.; Fujitaka, S.; Shimizu, S.; Shirato, H.; et al. Development and evaluation of a short-range applicator for treating superficial moving tumors with respiratory-gated spot-scanning proton therapy using real-time image guidance. Phys. Med. Biol. 2016, 61, 1515–1531. [Google Scholar] [CrossRef] [PubMed]
- Fujitaka, S.; Takayanagi, T.; Fujimoto, R.; Fujii, Y.; Nishiuchi, H.; Ebina, F.; Okazaki, T.; Hiramoto, K.; Sakae, T.; Terunuma, T. Reduction of the number of stacking layers in proton uniform scanning. Phys. Med. Biol. 2009, 54, 3101–3111. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, B.; Kanai, T.; Futami, Y.; Shimbo, M.; Urakabe, E. Ridge filter design and optimization for the broad-beam three-dimensional irradiation system for heavy-ion radiotherapy. Med. Phys. 2000, 27, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Weber, U.; Kraft, G. Design and construction of a ripple filter for a smoothed depth dose distribution in conformal particle therapy. Phys. Med. Biol. 1999, 44, 2765–2775. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Gillin, M.; Bues, M.; Zhu, X.R.; Suzuki, K.; Mohan, R.; Woo, S.; Lee, A.; Komaki, R.; Cox, J.; et al. The M.D. Anderson proton therapy system. Med. Phys. 2009, 36, 4068–4083. [Google Scholar] [CrossRef] [PubMed]
- Lomax, A.; Bolsi, A.; Albertini, F.; Weber, D. Treatment planning for pencil beam scanning. In Principles and Practice of Proton Beam Therapy; Das, I.J., Paganetti, H., Eds.; AAPM Monograph, 2015 Summer School; Medical Physics Publishing: Madison, WI, USA, 2015; Chapter 24. [Google Scholar]
- Zhu, X.; Poenisch, F.; Li, H.; Zhang, X.; Sahoo, N.; Gillin, M. Field shaping: Scanning beam. In Principles and Practice of Proton Beam Therapy; Das, I.J., Paganetti, H., Eds.; AAPM Monograph, 2015 Summer School; Medical Physics Publishing: Madison, WI, USA, 2015; Chapter 9. [Google Scholar]
- Van de Water, T.A.; Lomax, A.J.; Bijl, H.P.; Schilstra, C.; Hug, E.B.; Langendijk, J.A. Using a reduced spot size for intensity-modulated proton therapy potentially improves salivary gland-sparing in oropharyngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e313–e319. [Google Scholar] [CrossRef] [PubMed]
- Langner, U.W.; Eley, J.G.; Dong, L.; Langen, K. Comparison of multi-institutional varian probeam pencil beam scanning proton beam commissioning data. J. Appl. Clin. Med. Phys. 2017, 18, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sahoo, N.; Zhang, X.; Robertson, D.; Li, H.; Choi, S.; Lee, A.; Gillin, M. Intensity modulated proton therapy treatment planning using single-field optimization: The impact of monitor unit constraints on plan quality. Med. Phys. 2010, 37, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Grassberger, C.; Dowdell, S.; Lomax, A.; Sharp, G.; Shackleford, J.; Choi, N.; Willers, H.; Paganetti, H. Motion interplay as a function of patient parameters and spot size in spot scanning proton therapy for lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Lim, G.; Liao, L.; Li, Y.; Jiang, S.; Li, X.; Li, H.; Suzuki, K.; Zhu, X.R.; Gomez, D.; et al. Proton energy optimization and reduction for intensity-modulated proton therapy. Phys. Med. Biol. 2014, 59, 6341–6354. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Kadowaki, T.; Uchiyama, H.; Fujimoto, T.; Takada, E.; Shirai, T.; Furukawa, T.; Mizushima, K.; Takeshita, E.; Katagiri, K.; et al. Multiple-energy operation with extended flattops at HIMAC. Nuclear Inst. Methods Phys. Res. A 2010, 624, 33–38. [Google Scholar] [CrossRef]
- Widesott, L.; Lomax, A.J.; Schwarz, M. Is there a single spot size and grid for intensity modulated proton therapy? Simulation of head and neck, prostate and mesothelioma cases. Med. Phys. 2012, 39, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Tryggestad, E.; Younkin, J.E.; Keole, S.R.; Furutani, K.M.; Kang, Y.; Herman, M.G.; Bues, M. Using experimentally determined proton spot scanning timing parameters to accurately model beam delivery time. Med. Phys. 2017, 44, 5081–5088. [Google Scholar] [CrossRef] [PubMed]
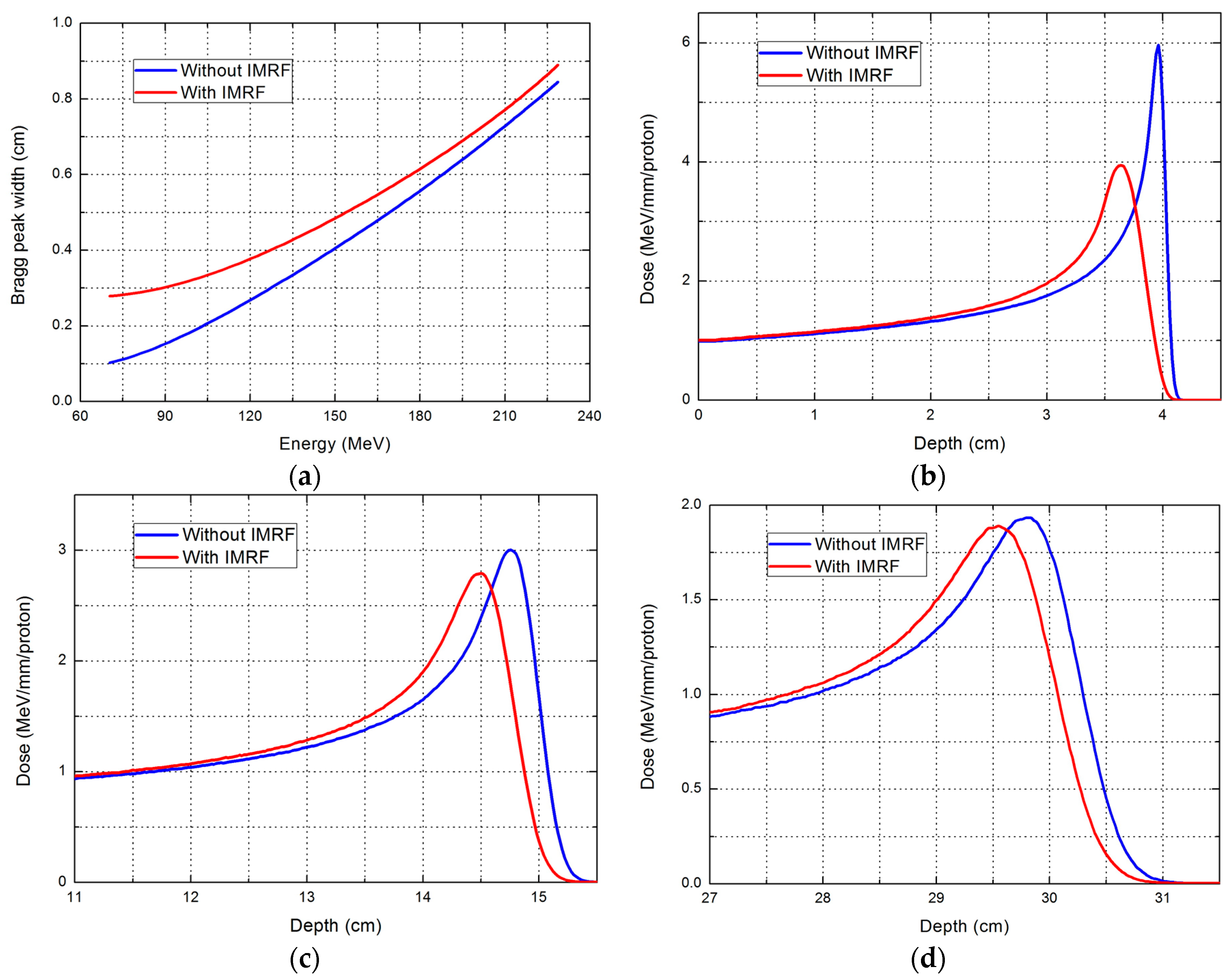
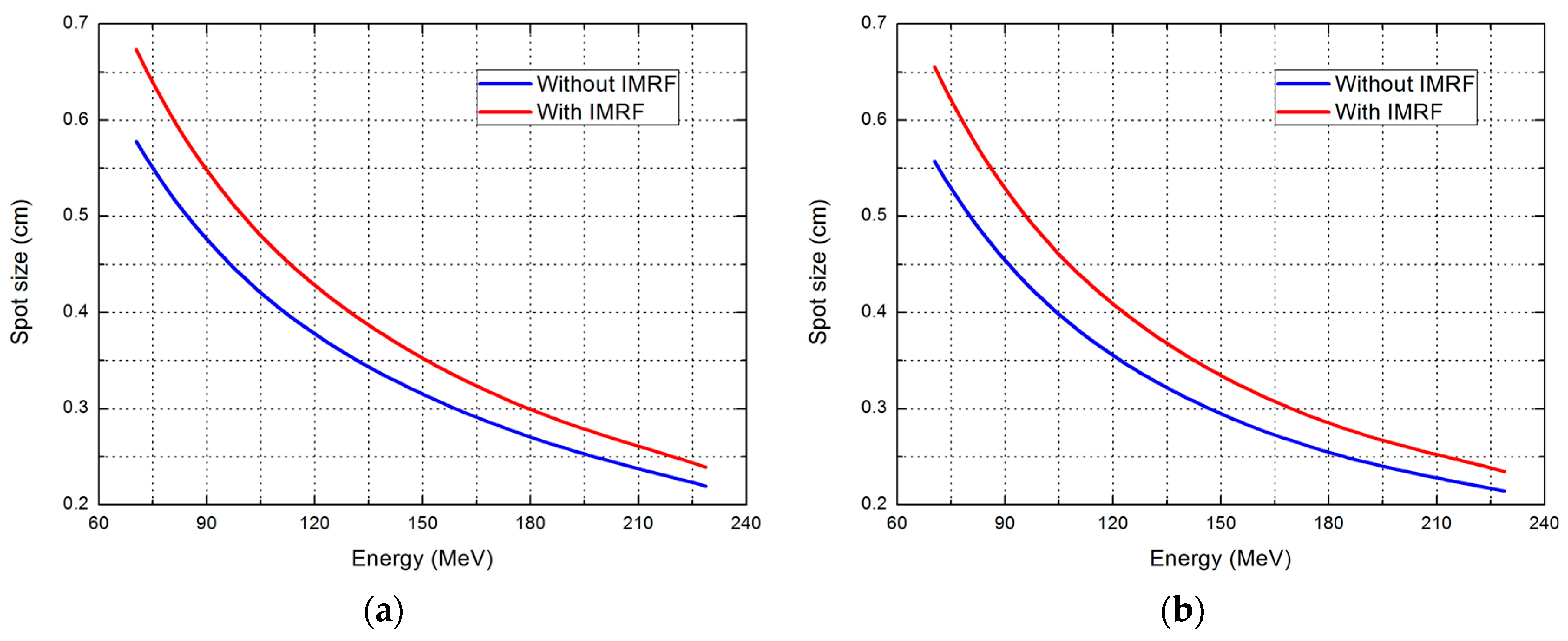
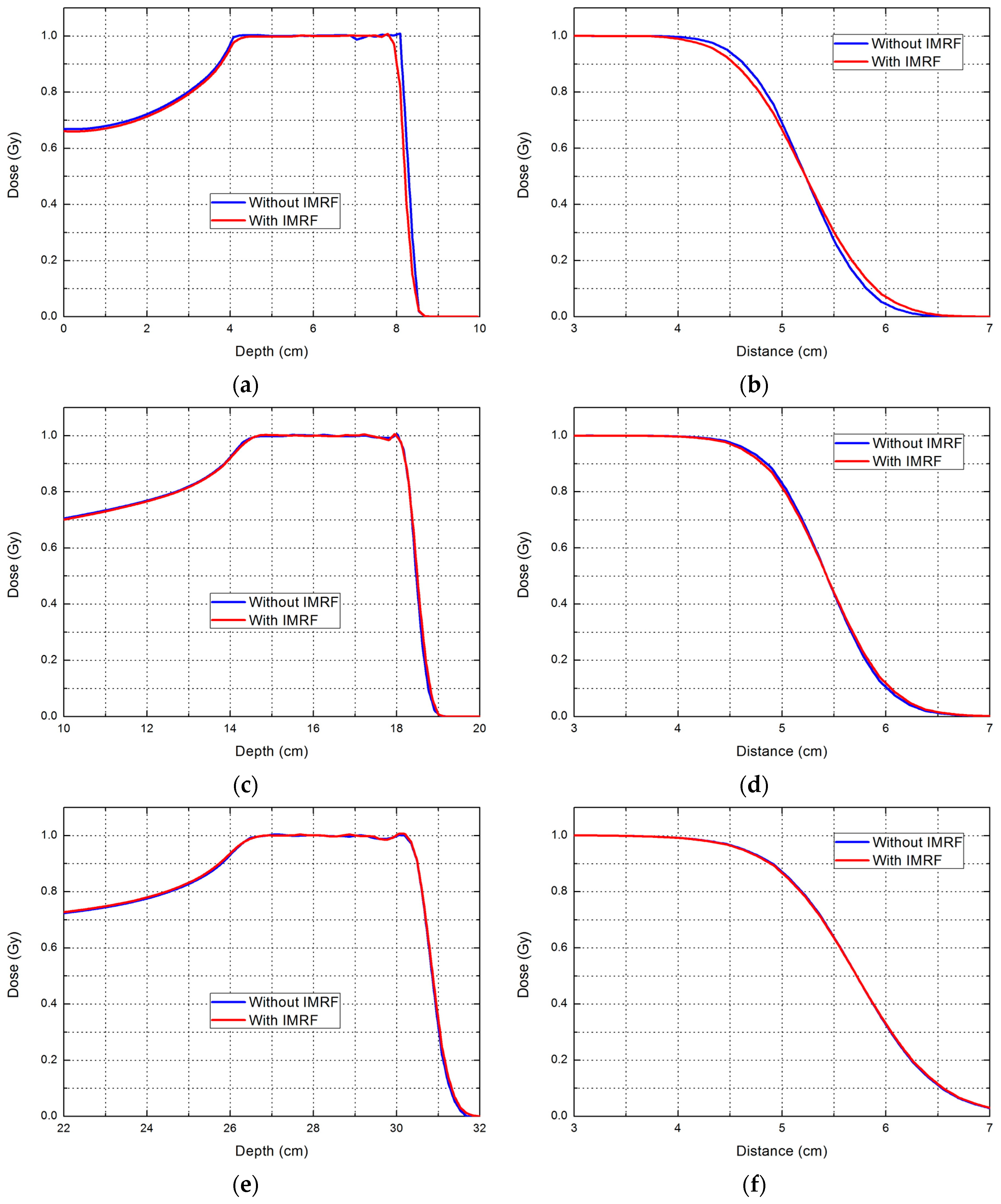
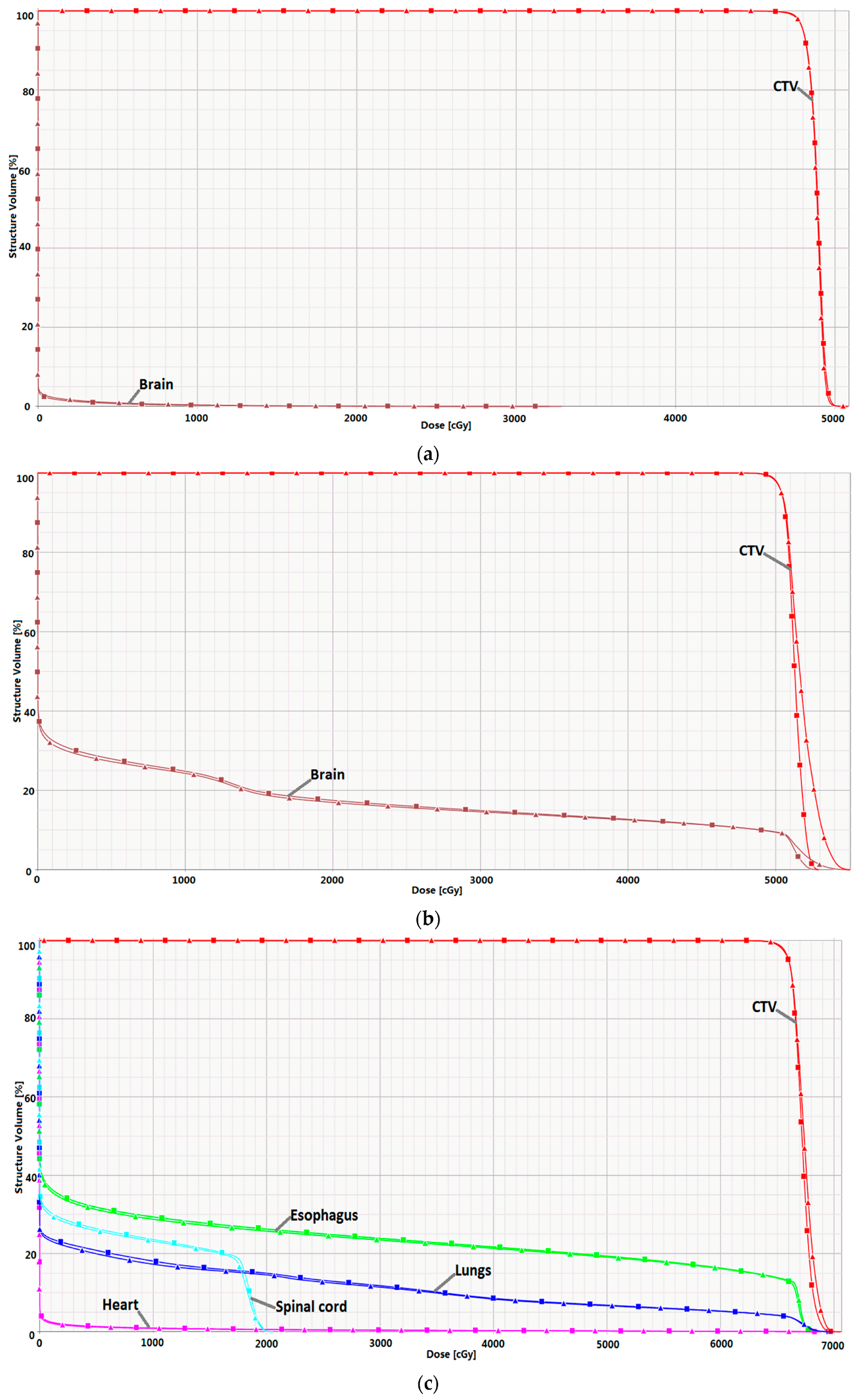

| Range (cm) | Parameter | Without IMRF | With IMRF | Reduction (%) |
|---|---|---|---|---|
| 8 | Number of layers | 36 | 14 | 61.1% |
| Post-processed spots | 10,693 | 4624 | 56.8% | |
| Beam delivery time (s) | 100.3 | 46.8 | 53.3% | |
| 10 | Number of layers | 24 | 14 | 41.7% |
| Post-processed spots | 7225 | 4624 | 36.0% | |
| Beam delivery time (s) | 70.1 | 46.1 | 34.2% | |
| 14 | Number of layers | 16 | 15 | 6.3% |
| Post-processed spots | 5202 | 4913 | 5.6% | |
| Beam delivery time (s) | 50.2 | 47.9 | 4.6% | |
| 18 | Number of layers | 11 | 11 | 0.0% |
| Post-processed spots | 3757 | 3757 | 0.0% | |
| Beam delivery time (s) | 37.4 | 37.5 | −0.3% | |
| 22 | Number of layers | 9 | 9 | 0.0% |
| Post-processed spots | 3179 | 3179 | 0.0% | |
| Beam delivery time (s) | 32.5 | 32.6 | −0.3% | |
| 26 | Number of layers | 8 | 8 | 0.0% |
| Post-processed spots | 2890 | 2890 | 0.0% | |
| Beam delivery time (s) | 29.7 | 30.0 | −1.0% | |
| 30 | Number of layers | 8 | 8 | 0.0% |
| Post-processed spots | 2890 | 2890 | 0.0% | |
| Beam delivery time (s) | 29.8 | 29.8 | 0.0% |
| Range (cm) | Lateral Penumbra (cm) | Distal Penumbra (cm) | ||
|---|---|---|---|---|
| Without IMRF | With IMRF | Without IMRF | With IMRF | |
| 8 | 0.77 | 0.89 | 0.26 | 0.26 |
| 10 | 0.73 | 0.83 | 0.25 | 0.28 |
| 14 | 0.71 | 0.78 | 0.30 | 0.34 |
| 18 | 0.75 | 0.80 | 0.35 | 0.39 |
| 22 | 0.83 | 0.87 | 0.42 | 0.44 |
| 26 | 0.93 | 0.96 | 0.44 | 0.50 |
| 30 | 1.05 | 1.07 | 0.51 | 0.54 |
| Case | Parameters | Without IMRF | With IMRF | Reduction |
|---|---|---|---|---|
| Scalp (2 beams) | Number of layers | 50 + 33 = 83 | 27 + 14 = 41 | 50.6% |
| Raw spots | 5681 + 5268 = 10,949 | 2227 + 2089 = 4316 | 60.6% | |
| Post-processed spots | 5417 + 4654 = 10,071 | 2622 + 2461 = 5083 | 49.5% | |
| Beam delivery time (s) | 111.2 + 77.3 = 188.5 | 62.4 + 37.3 = 99.7 | 47.1% | |
| Brain (3 beams) | Number of layers | 36 + 39 + 30 = 105 | 27 + 23 + 27 = 77 | 26.7% |
| Raw spots | 3831 + 5442 + 2830 = 12,103 | 3038 + 2937 + 2733 = 8708 | 28.1% | |
| Post-processed spots | 2947 + 3385 + 2174 = 8506 | 2665 + 2498 + 2148 = 7311 | 14.0% | |
| Beam delivery time (s) | 76.5 + 83.2 + 63.1 = 222.8 | 58.9 + 51.2 + 57.4 = 167.5 | 24.8% | |
| Lung (3 beams) | Number of layers | 43 + 39 + 50 = 132 | 38 + 31 + 35 = 104 | 21.2% |
| Raw spots | 7182 + 7991 + 8188 = 23,361 | 6244 + 6363 + 6351 = 18,958 | 18.8% | |
| Post-processed spots | 6440 + 6740 + 7023 = 20,203 | 5866 + 5978 + 6249 = 18,093 | 10.4% | |
| Beam delivery time (s) | 100.4 + 92.8 + 114.7 = 307.9 | 89.6 + 76.3 + 84.9 = 250.8 | 18.5% | |
| Prostate (2 beams) | Number of layers | 19 + 18 = 37 | 19 + 18 = 37 | 0.0% |
| Raw spots | 1686 + 1639 = 3325 | 1767 + 1694 = 3461 | −4.1% | |
| Post-processed spots | 1566 + 1536 = 3102 | 1635 + 1595 = 3230 | −4.1% | |
| Beam delivery time (s) | 41.8 + 39.8 = 81.6 | 41.9 + 39.9 = 81.8 | −0.2% | |
| H & N (3 beams) | Number of layers | 74 + 71 + 71 = 216 | 48 + 52 + 53 = 153 | 29.2% |
| Raw spots | 19,688 + 19,506 + 22,670 = 61,864 | 14,144 + 14,678 + 15,230 = 44,052 | 28.8% | |
| Post-processed spots | 13,193 + 12,560 + 15,342 = 41,095 | 11,426 + 9960 + 11995 = 33,381 | 18.8% | |
| Beam delivery time (s) | 177.8 + 170.0 + 178.5 = 526.3 | 125.5 + 129.0 + 137.8 = 392.3 | 25.5% | |
| BOS (3 beams) | Number of layers | 46 + 41 + 39 = 126 | 41 + 37 + 35 = 113 | 10.3% |
| Raw spots | 4878 + 6171 + 6443 = 17,492 | 4735 + 5755 + 5897 = 16,387 | 6.3% | |
| Post-processed spots | 3086 + 4026 + 4165 = 11,277 | 2935 + 3915 + 4073 = 10,923 | 3.1% | |
| Beam delivery time (s) | 96.2 + 89.6 + 86.0 = 271.8 | 86.5 + 81.8 + 78.3 = 246.6 | 9.3% |
| Case | Parameters | Without IMRF | With IMRF |
|---|---|---|---|
| Scalp | HI | 96.8 | 97.0 |
| CI | 84.5 | 81.1 | |
| Brain Dmean (Gy) | 0.1 | 0.1 | |
| Brain | HI | 93.7 | 96.4 |
| CI | 92.6 | 93.7 | |
| Brain Dmean (Gy) | 9.0 | 9.1 | |
| Lung | HI | 95.6 | 96.2 |
| CI | 93.0 | 93.0 | |
| Lungs V20Gy (%) | 14.5 | 15 | |
| Spinal cord Dmax (Gy) | 20.5 | 20.5 | |
| Esophagus V60Gy (%) | 16.2 | 16.3 | |
| Prostate | HI | 97.8 | 97.9 |
| CI | 91.9 | 92.4 | |
| Bladder V70Gy | 9.2 | 9.0 | |
| Rectum V70Gy | 6.9 | 7.0 | |
| Femoral heads Dmean (Gy) | 21.0 | 21.1 | |
| H & N | HI | 94.7 | 96.5 |
| CI | 76.1 | 83.6 | |
| Oral cavity Dmean (Gy) | 5.5 | 5.4 | |
| Spinal cord Dmax (Gy) | 17.0 | 16.7 | |
| Brainstem Dmax (Gy) | 8.8 | 8.9 | |
| Parotids Dmean (Gy) | 22.4 | 23.0 | |
| BOS | HI | 95.0 | 94.9 |
| CI | 85.3 | 84.4 | |
| Left optic nerve Dmax (Gy) | 52.1 | 52.6 | |
| Right optic nerve Dmax (Gy) | 54.6 | 54.8 | |
| Optic chiasm Dmax (Gy) | 52.1 | 52.7 |
| Case | Target Volume (cc) | Number of Beams | Optimization Method | Prescription (Gy (RBE)/Fraction) |
|---|---|---|---|---|
| Scalp | CTV, 47.3 | 2 | SFO | 48/24 |
| Brain | CTV, 132.7 | 3 | SFO | 50.4/28 |
| Lung | CTV, 582 | 3 | MFO | 66/33 |
| Prostate | CTV, 88.8 | 2 | SFO | 78/39 |
| H & N | CTV1, 161.0 | 3 | MFO | 70/30 |
| CTV2, 83.5 | 63/30 | |||
| CTV3, 225.1 | 57/30 | |||
| BOS | CTV1, 142.0 | 3 | MFO | 70/30 |
| CTV2, 42.5 | 63/30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, Y.; Zhang, X.; Li, H.; Miyazaki, K.; Fujimoto, R.; Akiyama, H.; Gillin, M.T.; Poenisch, F.; Sahoo, N.; et al. Synchrotron-Based Pencil Beam Scanning Nozzle with an Integrated Mini-Ridge Filter: A Dosimetric Study to Optimize Treatment Delivery. Cancers 2017, 9, 170. https://doi.org/10.3390/cancers9120170
Wang X, Li Y, Zhang X, Li H, Miyazaki K, Fujimoto R, Akiyama H, Gillin MT, Poenisch F, Sahoo N, et al. Synchrotron-Based Pencil Beam Scanning Nozzle with an Integrated Mini-Ridge Filter: A Dosimetric Study to Optimize Treatment Delivery. Cancers. 2017; 9(12):170. https://doi.org/10.3390/cancers9120170
Chicago/Turabian StyleWang, Xianliang, Yupeng Li, Xiaodong Zhang, Heng Li, Koichi Miyazaki, Rintaro Fujimoto, Hiroshi Akiyama, Michael T. Gillin, Falk Poenisch, Narayan Sahoo, and et al. 2017. "Synchrotron-Based Pencil Beam Scanning Nozzle with an Integrated Mini-Ridge Filter: A Dosimetric Study to Optimize Treatment Delivery" Cancers 9, no. 12: 170. https://doi.org/10.3390/cancers9120170






