Barrier Properties of Polylactic Acid in Cellulose Based Packages Using Montmorillonite as Filler
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Coating Solutions
2.3. Coating Application
2.4. Paper Thickness Measurement
2.5. Contact Angle (CA)
2.6. Water Vapor Permeability (WVP)
2.7. Grease Permeability (GP)
2.8. Scanning Electron Microscopy (SEM)
2.9. Confocal Laser Scanning Microscopy (CLSM)
2.10. Differential Scanning Calorimetry (DSC)
2.11. Gel Permeation Chromatography (GPC)
2.12. Statistical Analyses
3. Results and Discussion
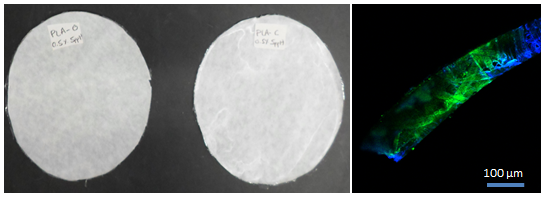
3.1. Scanning Electron Microscopy (SEM) and Confocal Laser Scanning Microscopy (CLSM)

3.2. Paper Thickness Measurement
| PLA Type | PLA (%) | BC (pph) | Thickness (µm) | CA (°) | GP (seg) |
|---|---|---|---|---|---|
| Semicrystalline | 0.1 | 0 | 59 ± 3 | 78.9 ± 3.4 | 583 |
| 5 | 60 ± 0 | 76.1 ± 2.9 | 1800+ | ||
| 10 | 61 ± 3 | 71.9 ± 1.5 | 900 | ||
| 0.25 | 0 | 60 ± 0 | 73.9 ± 2.4 | 1800+ | |
| 5 | 60 ± 0 | 71.1 ± 5.9 | 1633 | ||
| 10 | 60 ± 0 | 77.1 ± 3.2 | 1800+ | ||
| 0.5 | 0 | 68 ± 4 | 75.0 ± 0.5 | 1800+ | |
| 5 | 69 ± 3 | 70.5 ± 1.5 | 1800+ | ||
| 10 | 69 ± 3 | 73.1 ± 3.5 | 654 | ||
| 0.75 | 0 | 63 ± 5 | 74.0 ± 1.4 | 1800+ | |
| 5 | 64 ± 5 | 71.3 ± 3.9 | 270 | ||
| 10 | 65 ± 13 | 74.8 ± 1.2 | ND | ||
| 1 | 0 | 73 ± 7 | 72.1 ± 2.1 | 1800+ | |
| 5 | 81 ± 10 | 71.3 ± 1.2 | 1452 | ||
| 10 | 77 ± 9 | 73.1 ± 1.6 | 1282 | ||
| 1.5 | 0 | 72 ± 4 | 76.4 ± 2.4 | 1800+ | |
| 5 | 70 ± 5 | ND | 1800+ | ||
| 10 | 72 ± 8 | 72.3 ± 0.9 | ND | ||
| Amorphous | 0.1 | 0 | 58 ± 4 | 76.6 ± 2.7 | 1064 |
| 5 | 56 ± 5 | 73.2 ± 6.5 | 1553 | ||
| 10 | 58 ± 4 | 74.6 ± 3.4 | 360 | ||
| 0.25 | 0 | 60 ± 0 | 75.2 ± 1.4 | 1800+ | |
| 5 | 61 ± 6 | 74.1 ± 2.7 | 1800+ | ||
| 10 | 60 ± 0 | 73.5 ± 2.2 | 1800+ | ||
| 0.5 | 0 | 69 ± 7 | 74.5 ± 2.1 | 1800+ | |
| 5 | 69 ± 6 | 74.5 ± 1.6 | 1800+ | ||
| 10 | 73 ± 7 | 75.3 ± 2.3 | 1800+ | ||
| 0.75 | 0 | 68 ± 4 | 73.0 ± 6.4 | 1800+ | |
| 5 | 68 ± 6 | 75.1 ± 1.9 | 1800+ | ||
| 10 | 75 ± 14 | 74.8 ± 3.7 | 1800+ | ||
| 1 | 0 | 73 ± 7 | 72.5 ± 3.3 | 1053 | |
| 5 | 78 ± 8 | 72.9 ± 2.3 | 1800+ | ||
| 10 | 74 ± 5 | 73.6 ± 2.2 | 1800+ | ||
| 1.5 | 0 | 84 ± 18 | 73.0 ± 2.7 | 1800+ | |
| 5 | 80 ± 19 | 73.0 ± 2.6 | 1800+ | ||
| 10 | 74 ± 7 | 73.8 ± 2.3 | 1800+ | ||
| Glassine Paper | 40 ± 0 | 66.1 ± 3.2 | 95 | ||
| Grease Proof Paper 1 | 60 ± 0 | 65.7 ± 3.5 | 135 | ||
| Grease Proof Paper 2 | 60 ± 0 | 80.7 ± 2.4 | 1095 | ||
| Uncoated paper | 60 ± 2 | 11.9 ± 3.7 | 2 | ||

3.3. Contact Angle (CA)
3.4. Grease Permeability
3.5. Water Vapor Permeability (WVP)

| Parameter/Sample | PLA–AM | PLA–AM (F) | PLA–SC | PLA–SC (F) |
|---|---|---|---|---|
| Tg (°C) | 57.4 | 56.0 | 59.0 | 59.5 |
| Tcc (°C) | na | na | 132.0 | 130.0 |
| ΔH crystallization (J·g−1) | na | na | 3.7 | 14.0 * |
| Xc (%) | na | Na | 3.1 | 11.0 |
| Mn (g·mol−1) | 9.1 × 104 | 8.14 × 104 | 1.4 × 105 | 1.3 ×105 |
| Mw (g·mol−1) | 2.7 × 105 | 2.74 × 105 | 3.3 × 105 | 3.1 × 105 |
3.6. Fourier Transformed Infrared Spectrometry

3.7. Differential Scanning Calorimetry (DSC) and Gel Permeation Chromatography (GPC)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ham-Pichavant, F.; Sèbe, G.; Pardon, P.; Coma, V. Fat resistance properties of chitosan-based paper packaging for food applications. Carbohydr. Polym. 2005, 61, 259–265. [Google Scholar] [CrossRef]
- Begley, T.H.; White, K.; Honigfort, P.; Twaroski, M.L.; Neches, R.; Walker, R.A. Perfluorochemicals: Potential sources of and migration from food packaging. Food Addit. Contam. 2005, 22, 1023–1031. [Google Scholar]
- Yoo, S.; Lau, S.H.; Krochta, J.M. Grease penetration and browning resistance of pulpboard and paperboard coated with whey protein. Packag. Technol. Sci. 2012, 25, 259–270. [Google Scholar] [CrossRef]
- Trezza, T.A.; Vergano, P.J. Grease resistance of corn zein coated paper. J. Food Sci. 1994, 59, 912–915. [Google Scholar] [CrossRef]
- Parris, N.; Vergano, P.J.; Dickey, L.C.; Cooke, P.H.; Craig, J.C. Enzymatic hydrolysis of zein-wax-coated paper. J. Agric. Food Chem. 1998, 46, 4056–4059. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qian, L. Enhanced water vapour barrier and grease resistance of paper bilayer-coated with chitosan and beeswax. Carbohydr. Polym. 2014, 101, 401–406. [Google Scholar] [CrossRef]
- Bordenave, N.; Grelier, S.; Coma, V. Hydrophobization and antimicrobial activity of chitosan and paper-based packaging material. Biomacromolecules 2009, 11, 88–96. [Google Scholar] [CrossRef]
- Reis, A.B.; Yoshida, C.M.; Reis, A.P.C.; Franco, T.T. Application of chitosan emulsion as a coating on Kraft paper. Polym. Int. 2011, 60, 963–969. [Google Scholar] [CrossRef]
- Khwaldia, K. Physical and mechanical properties of hydroxypropyl methylcellulose-coated paper as affected by coating weight and coating composition. Bioresources 2013, 8, 3438–3452. [Google Scholar] [CrossRef]
- Hansen, N.M.; Plackett, D. Sustainable films and coatings from hemicelluloses: A review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar]
- Vink, E.T.; Rabago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Gruber, P.; O’Brien, M. Polylactides “Natureworks® PLA”. In Biopolymers Online; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2002. [Google Scholar]
- Fang, Q.; Hanna, M.A. Rheological properties of amorphous and semicrystalline polylactic acid polymers. Ind. Crops Prod. 1999, 10, 47–53. [Google Scholar] [CrossRef]
- Petersson, L.; Kvien, I.; Oksman, K. Structure and thermal properties of poly (lactic acid)/cellulose whiskers nanocomposite materials. Compos. Sci. Technol. 2007, 67, 2535–2544. [Google Scholar] [CrossRef]
- Svagan, A.J.; Åkesson, A.; Cárdenas, M.; Bulut, S.; Knudsen, J.C.; Risbo, J.; Plackett, D. Transparent films based on PLA and montmorillonite with tunable oxygen barrier properties. Biomacromolecules 2012, 13, 397–405. [Google Scholar]
- Molinaro, S.; Cruz Romero, M.; Boaro, M.; Sensidoni, A.; Lagazio, C.; Morris, M.; Kerry, J. Effect of nanoclay-type and PLA optical purity on the characteristics of PLA-based nanocomposite films. J. Food Eng. 2013, 117, 113–123. [Google Scholar] [CrossRef]
- Balakrishnan, H.; Hassan, A.; Wahit, M.U.; Yussuf, A.A.; Razak, S.B.A. Novel toughened polylactic acid nanocomposite: mechanical, thermal and morphological properties. Mater. Des. 2010, 31, 3289–3298. [Google Scholar] [CrossRef]
- Hu, R.; Lim, J.K. Fabrication and mechanical properties of completely biodegradable hemp fiber reinforced polylactic acid composites. J. Compos. Mater. 2007, 41, 1655–1669. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Jaszkiewicz, A.; Scherzer, D. Mechanical properties of PLA composites with man-made cellulose and abaca fibres. Compos. A Appl. Sci. Manuf. 2009, 40, 404–412. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Kenny, J.M. Multifunctional bionanocomposite films of poly (lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Hughes, J.; Thomas, R.; Byun, Y.; Whiteside, S. Improved flexibility of thermally stable poly-lactic acid (PLA). Carbohydr. Polym. 2012, 88, 165–172. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Kopinke, F.D.; Remmler, M.; Mackenzie, K. Thermal decomposition of biodegradable polyesters—I: Poly (β-hydroxybutyric acid). Polym. Degrad. Stab. 1996, 52, 25–38. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Ha, C.S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Review article: Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Carrasco, F.; Gámez Pérez, J.; Santana, O.O.; Maspoch, M.L. Procesado del ácido poliláctico (PLA) y de nanocompuestos PLA/montmorillonita en planta piloto: Estudio de sus cambios estructurales y de su estabilidad térmica. Afinidad 2010, 66, 14–20. (In Spanish) [Google Scholar]
- Krikorian, V.; Pochan, D.J. Poly (l-lactic acid)/layered silicate nanocomposite: Fabrication, characterization, and properties. Chem. Mater. 2003, 15, 4317–4324. [Google Scholar] [CrossRef]
- Leephakpreeda, T. Control of Crystallinity Distribution in Polymer Extrusion Process. Ph.D. Thesis, The University of Akron, Akron, OH, USA, 1996. [Google Scholar]
- Kaur, J.; Lee, J.H.; Shofner, M.L. Influence of polymer matrix crystallinity on nanocomposite morphology and properties. Polymer 2011, 52, 4337–4344. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J.N. The measurement of the crystallinity of polymers by DSC. Polymer 2002, 43, 3873–3878. [Google Scholar] [CrossRef]
- ASTM. Standard test methods for water vapor transmission of materials, E 96–80. In Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 1989; pp. 745–754. [Google Scholar]
- TAPPI T 454 om-10 Turpentine test for voids in glassine and greaseproof Papers; Technical Association of the Pulp & Paper Industry: Peachtree Corners, GA, USA, 2010.
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly (lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Rhim, J.W.; Kim, J.H. Properties of poly (lactide)-coated paperboard for the use of 1-way paper cup. J. Food Sci. 2009, 74, E105–E111. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Rhim, J.W.; Lee, J.H.; Hong, S.I. Increase in water resistance of paperboard by coating with poly (lactide). Packag. Technol. Sci. 2007, 20, 393–402. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Dominici, F.; Santulli, C.; Kenny, J.M.; Torre, L. Investigation of thermo-mechanical, chemical and degradative properties of PLA-limonene films reinforced with cellulose nanocrystals extracted from Phormium tenax leaves. Eur. Polym. J. 2014, 56, 77–91. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Park, H.M.; Ng, P.K. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef]
- Magalhães, N.F.; Andrade, C.T. Thermoplastic corn starch/clay hybrids: Effect of clay type and content on physical properties. Carbohydr. Polym. 2009, 75, 712–718. [Google Scholar] [CrossRef]
- Weilbacher, R.; Fuss, R. Process for the Curtain-Coating of Substrates without the Use of Tensides. U.S. Patent 20100112226 A1, 6 May 2010. [Google Scholar]
- Shogren, R. Water vapor permeability of biodegradable polymers. J. Environ. Polym. Degrad. 1997, 5, 91–95. [Google Scholar] [CrossRef]
- Ashley, R.J. Permeability and plastics packaging. In Polymer Permeability; Comyn, J., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 269–308. [Google Scholar]
- Tsuji, H.; Okino, R.; Daimon, H.; Fujie, K. Water vapor permeability of poly (lactide)s: Effects of molecular characteristics and crystallinity. J. Appl. Polym. Sci. 2006, 99, 2245–2252. [Google Scholar] [CrossRef]
- Barrie, J.A. Water in polymers. In Diffusion in Polymers; Crank, J., Park, G.S., Eds.; Academic: London, UK, 1968; pp. 259–308. [Google Scholar]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Pilla, S. Handbook of Bioplastics and Biocomposites Engineering Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Sato, S.; Nyuui, T.; Matsuba, G.; Nagai, K. Correlation between interlamellar amorphous structure and gas permeability in poly(lactic acid) films. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Campbell, D.; Pethrick, R.A.; White, J.R. Polymer Characterization: Physical Techniques; Stanley Thornes (Publishers) Ltd.: Cheltenham, UK, 2000; p. 38. [Google Scholar]
- Farmahini-Farahani, M.; Xiao, H.; Zhao, Y. Poly lactic acid nanocomposites containing modified nanoclay with synergistic barrier to water vapor for coated paper. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Pluta, M.; Galeski, A.; Alexandre, M.; Paul, M.A.; Dubois, P. Polylactide/montmorillonite nanocomposites and microcomposites prepared by melt blending: Structure and some physical properties. J. Appl. Polym. Sci. 2002, 86, 1497–1506. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Lagaron, J.M. Novel clay-based nanobiocomposites of biopolyesters with synergistic barrier to UV light, gas, and vapour. J. Appl. Polym. Sci. 2010, 118, 188–199. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Maiti, P.; Okamoto, M.; Yamada, K.; Ueda, K. New polylactide/layered silicate nanocomposites. 1. Preparation, characterization, and properties. Macromolecules 2002, 35, 3104–3110. [Google Scholar]
- Gårdebjer, S.; Larsson, A.; Lofgren, C.; Strom, A. Controlling water permeability of composite films of polylactide acid, cellulose, and xyloglucan. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aldana, D.S.; Villa, E.D.; De Dios Hernández, M.; Sánchez, G.G.; Cruz, Q.R.; Gallardo, S.F.; Castillo, H.P.; Casarrubias, L.B. Barrier Properties of Polylactic Acid in Cellulose Based Packages Using Montmorillonite as Filler. Polymers 2014, 6, 2386-2403. https://doi.org/10.3390/polym6092386
Aldana DS, Villa ED, De Dios Hernández M, Sánchez GG, Cruz QR, Gallardo SF, Castillo HP, Casarrubias LB. Barrier Properties of Polylactic Acid in Cellulose Based Packages Using Montmorillonite as Filler. Polymers. 2014; 6(9):2386-2403. https://doi.org/10.3390/polym6092386
Chicago/Turabian StyleAldana, Daniela Sánchez, Eduardo Duarte Villa, Miguel De Dios Hernández, Guillermo González Sánchez, Quintín Rascón Cruz, Sergio Flores Gallardo, Hilda Piñon Castillo, and Lourdes Ballinas Casarrubias. 2014. "Barrier Properties of Polylactic Acid in Cellulose Based Packages Using Montmorillonite as Filler" Polymers 6, no. 9: 2386-2403. https://doi.org/10.3390/polym6092386