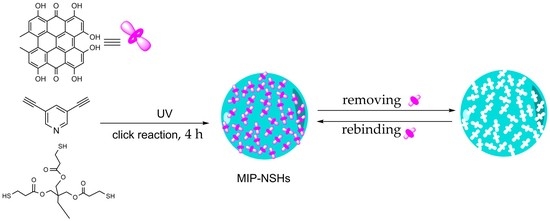
Fabrication of Hypericin Imprinted Polymer Nanospheres via Thiol-Yne Click Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Instrumentation
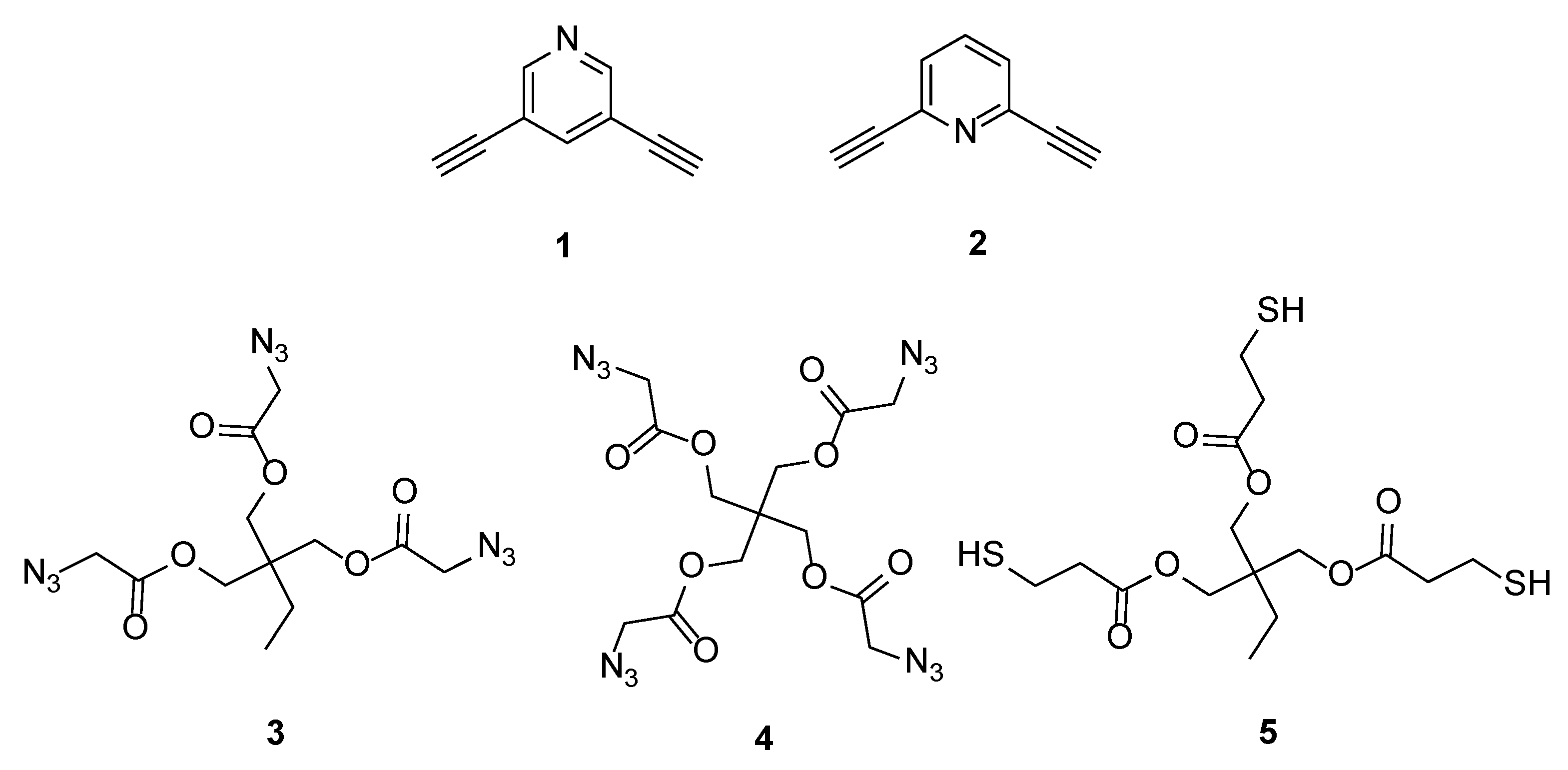
2.2. Synthesis of Monomers and Crosslinkers
2.3. Preparation of Imprinted Polymer Nanospheres towards Hypericin and Non-Imprinted Polymer Nanospheres
2.4. Determination of Static Adsorption Capacity
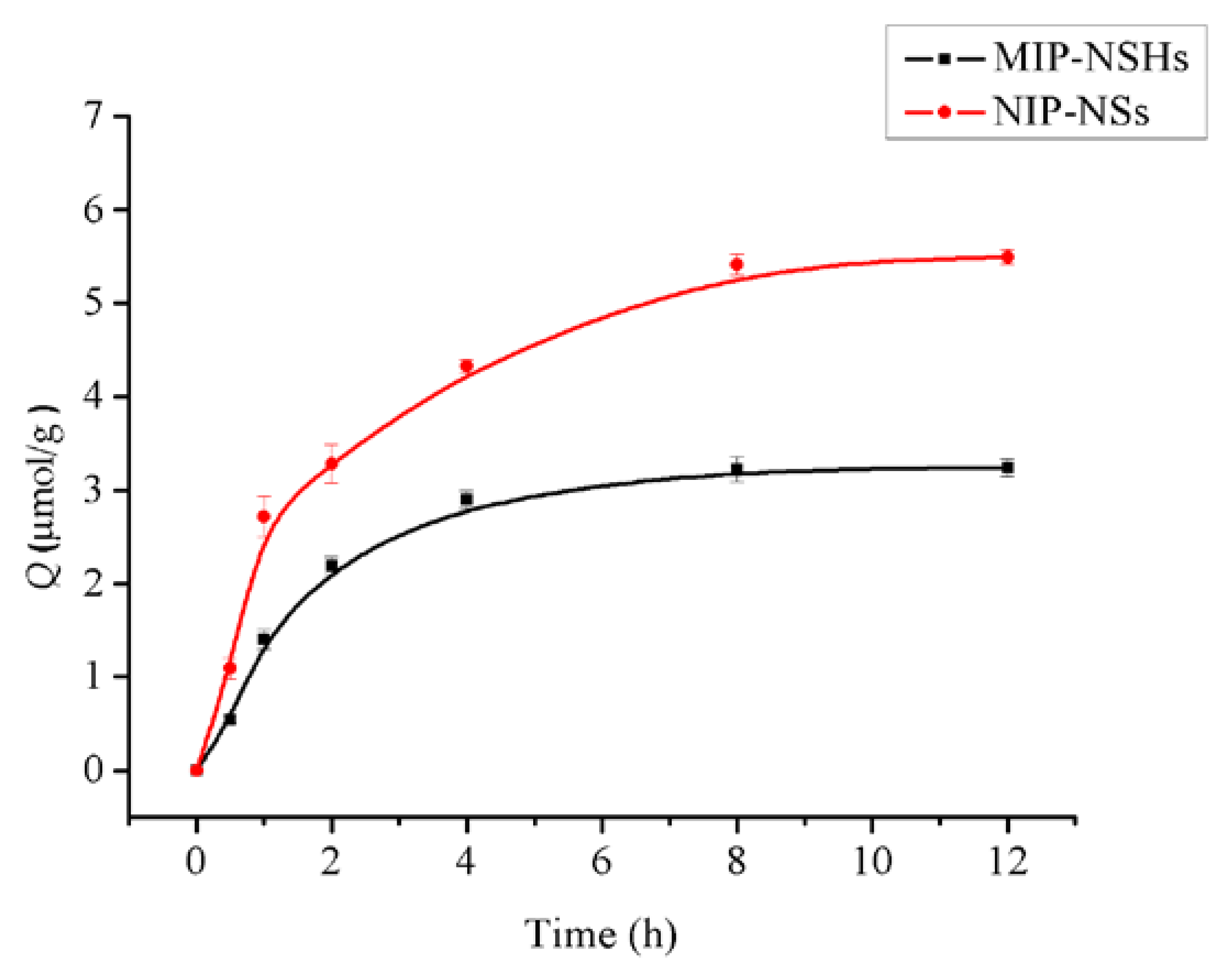
2.5. Kinetic of Template Adsorption
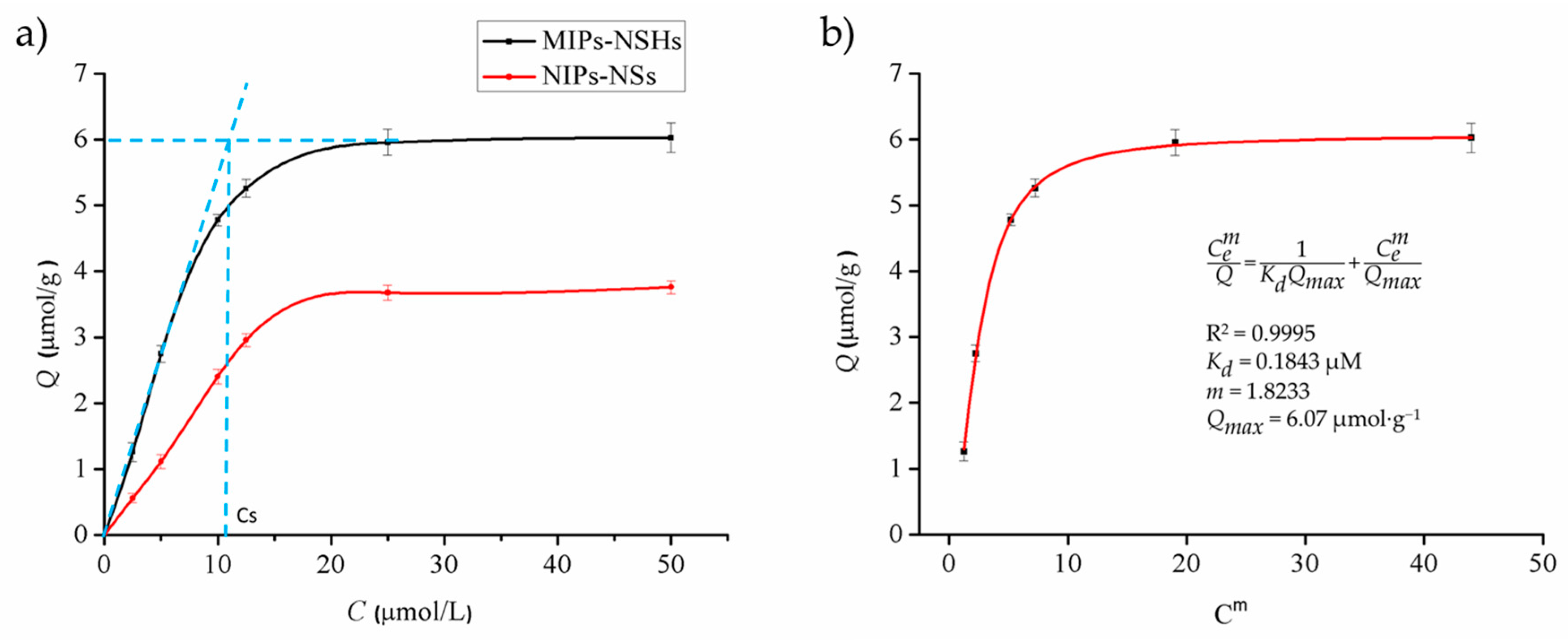
2.6. Isotherm Adsorption
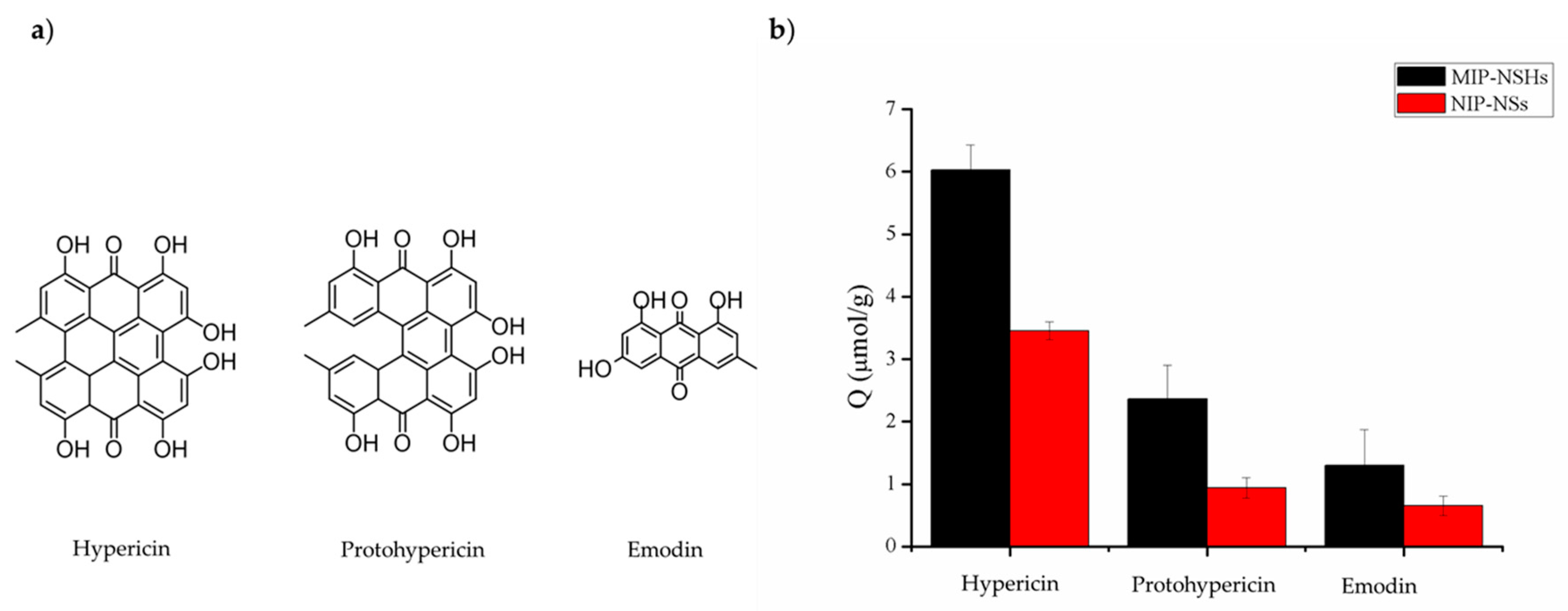
2.7. Selectivity of MIP–NSHs and NIP–NSs for Hypericin
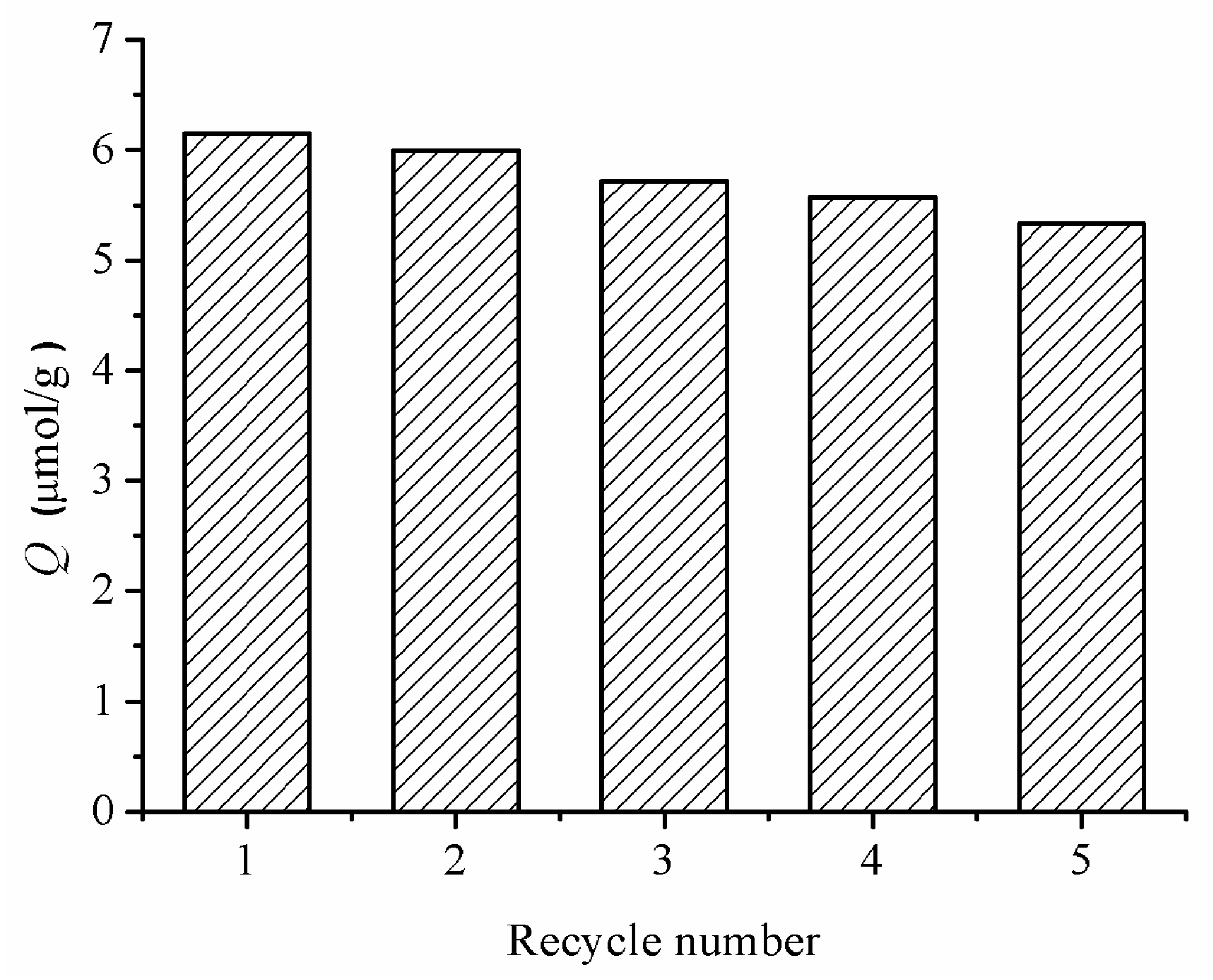
2.8. The Reusability of MIP–NSHs
3. Results and Discussion
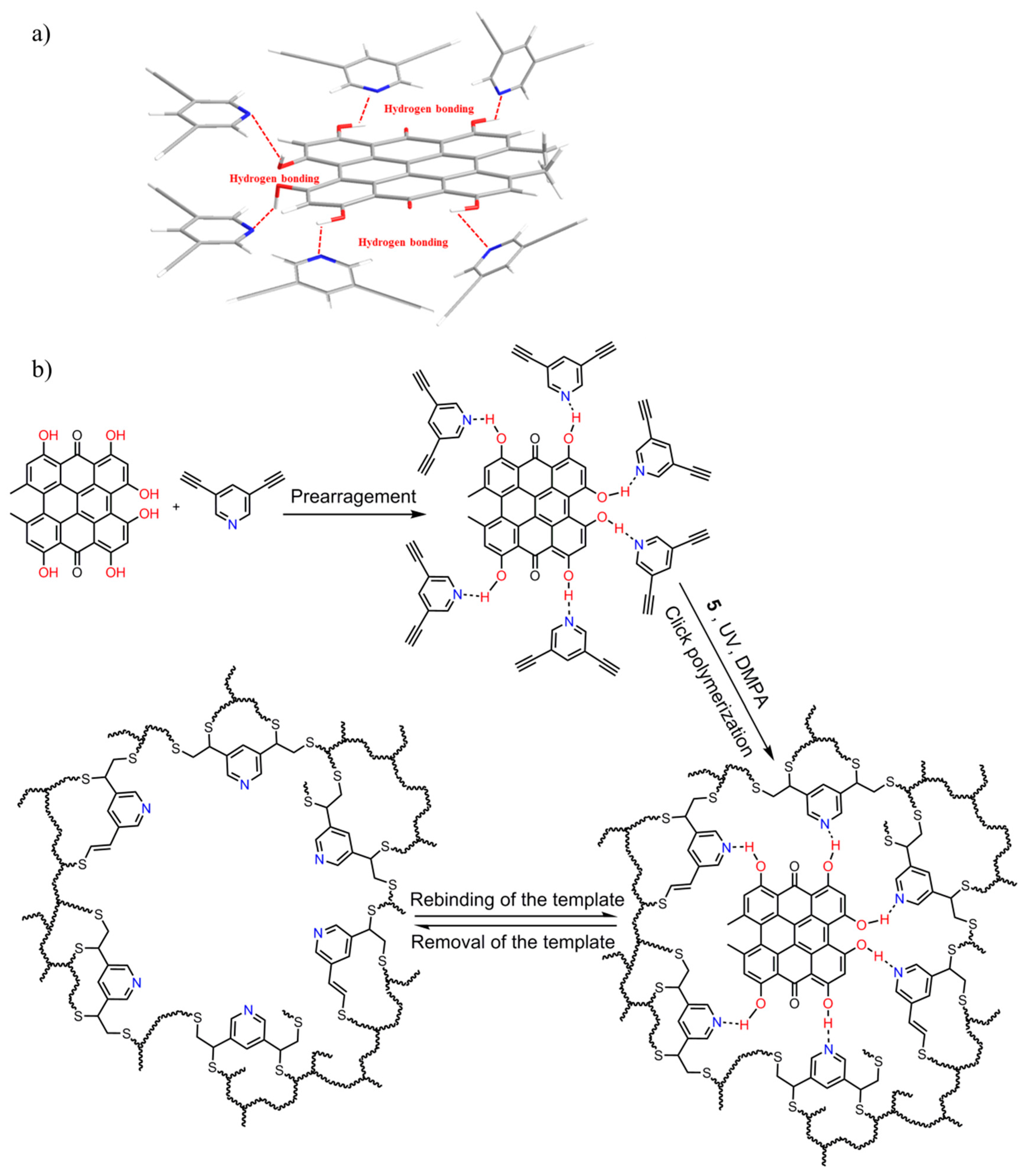
3.1. Synthesis of MIP–NSHs
3.1.1. Screening of Monomers and Crosslinkers
3.1.2. Characterization of MIP–NSHs
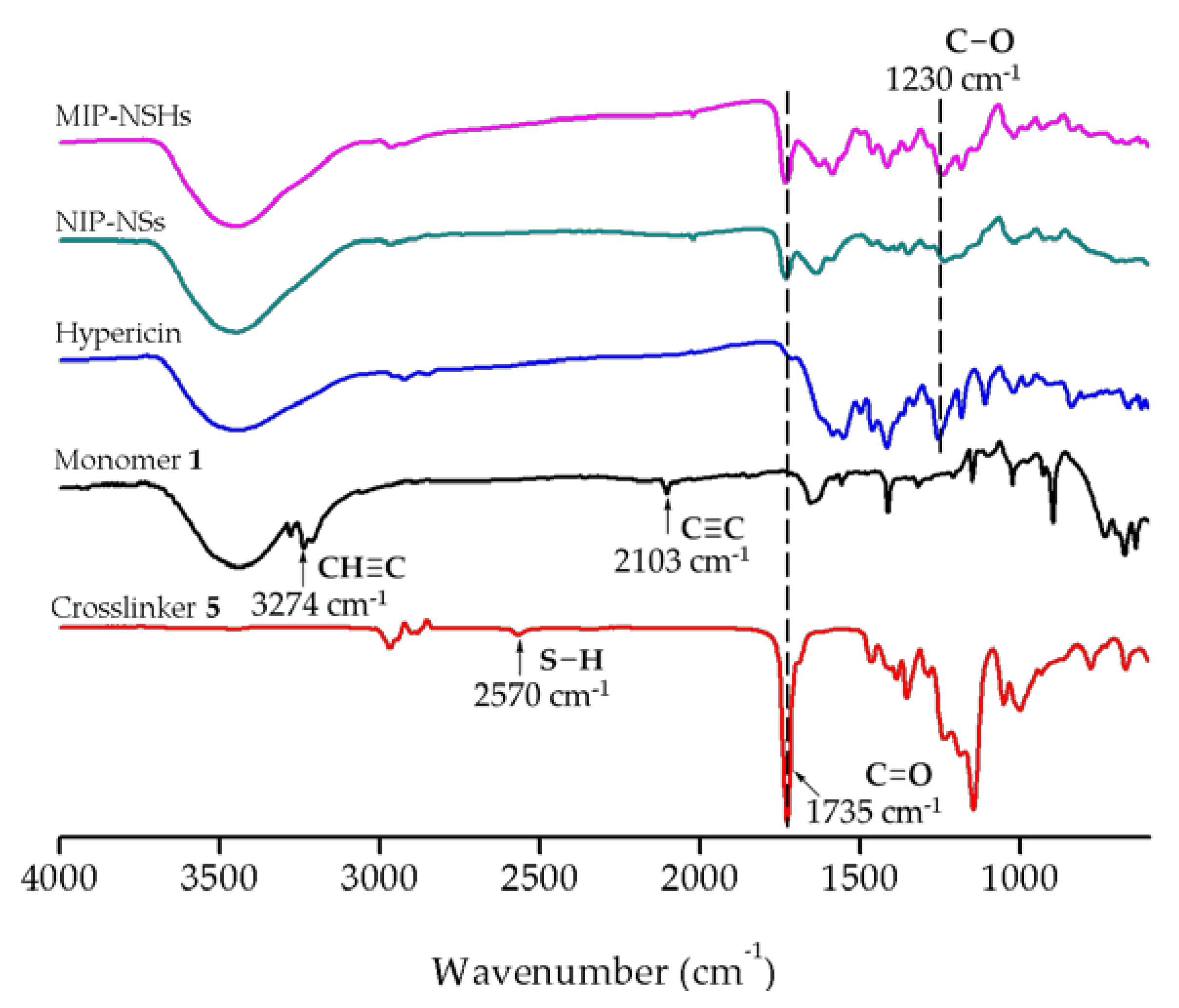
FTIR Analysis
SEM and DLS Analysis
BET Analysis
3.1.3. Optimization of Preparation Conditions for Specific Adsorption Capacity of MIP–NSHs
Effect of Photoinitiator Concentration on Qs
Effect of Solvent Composition on Qs
Effect of Template Concentration and the Ratio of Monomer to Crosslinker on Qs
3.2. Kinetic of Template Adsorption
3.3. Affinity Analysis
3.4. Binding Selectivity
3.5. Reusability of MIP–NSHs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wulff, G. The use of polymers with enzyme-analogous structures for the resolution of racemates. Angew. Chem. Int. Ed. 1972, 11, 341. [Google Scholar]
- Andersson, L.; Sellergren, B.; Mosbach, K. Imprinting of amino acid derivatives in macroporous polymers. Tetrahedron Lett. 1984, 25, 5211–5214. [Google Scholar] [CrossRef]
- Pardeshi, S.; Singh, S.K. Precipitation polymerization: A versatile tool for preparing molecularly imprinted polymer beads for chromatography applications. RSC Adv. 2016, 6, 23525–23536. [Google Scholar] [CrossRef]
- Sun, G.; Liu, Y.; Ahat, H.; Shen, A.; Liang, X.; Xue, X.; Luo, Y.; Yang, J.; Liu, Z.-S.; Aisa, H.A. “Two-dimensional” molecularly imprinted solid-phase extraction coupled with crystallization and high performance liquid chromatography for fast semi-preparative purification of tannins from pomegranate husk extract. J. Chromatogr. A 2017, 1505, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Walshe, M.; Howarth, J.; Kelly, M.T.; OKennedy, R.; Smyth, M.R. The preparation of a molecular imprinted polymer to 7-hydroxycoumarin and its use as a solid-phase extraction material. J. Pharm. Biomed. Anal. 1997, 16, 319–325. [Google Scholar] [CrossRef]
- Li, P.; Wang, T.; Lei, F.; Peng, X.; Wang, H.; Qin, L.; Jiang, J. Preparation and evaluation of paclitaxel-imprinted polymers with a rosin-based crosslinker as the stationary phase in high-performance liquid chromatography. J. Chromatogr. A 2017, 1502, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, M. Application of molecularly-imprinted polymers for rapid sample cleanup: Immunochemical and hplc analysis. Arab. J. Sci. Eng. 2014, 39, 2561–2572. [Google Scholar]
- Piletsky, S.A.; Parhometz, Y.P.; Lavryk, N.V.; Panasyuk, T.L.; El’Skaya, A.V. Sensors for low-weight organic molecules based on molecular imprinting technique. Sens. Actuat. B-Chem. 1994, 19, 629–631. [Google Scholar] [CrossRef]
- Mirata, F.; Resmini, M. Molecularly Imprinted Polymers for Catalysis and Synthesis; Springer International Publishing: Cham, Switzerland, 2015; pp. 107–129. [Google Scholar]
- Norell, M.C.; Andersson, H.S.; Nicholls, I.A. Theophylline molecularly imprinted polymer dissociation kinetics: A novel sustained release drug dosage mechanism. J. Mol. Recognit. 1998, 11, 98–102. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Molecularly imprinted polymers for drug delivery. J. Chromatogr. B 2004, 804, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, D.; Kirby, A.; Alexander, C. Molecularly imprinted drug delivery systems. Adv. Drug. Deliv. Rev. 2005, 57, 1836–1853. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, K.; Reimhult, K.; Krozer, A.; Mosbach, K.; Sode, K.; Ye, L. Uniform molecularly imprinted microspheres and nanoparticles prepared by precipitation polymerization: The control of particle size suitable for different analytical applications. Anal. Chim. Acta 2007, 584, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Cederfur, J.; Pei, Y.X.; Meng, Z.H.; Kempe, M. Synthesis and screening of a molecularly imprinted polymer library targeted for penicilling. J. Comb. Chem. 2003, 5, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Cormack, P.A.G.; Sherrington, D.C.; Khoshdel, E. Monodisperse, molecularly imprinted polymer microspheres prepared by precipitation polymerization for affinity separation applications. Angew. Chem. Int. Ed. 2003, 42, 5336–5338. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.G.; Li, L.; He, X.W. Comparison of trimethoprim molecularly imprinted polymers in bulk and in sphere as the sorbent for solid-phase extraction and extraction of trimethoprim from human urine and pharmaceutical tablet and their determination by high-performance liquid chromatography. Anal. Chim. Acta 2005, 537, 215–222. [Google Scholar]
- Lai, J.P.; Yang, M.L.; Niessner, R.; Knopp, D. Molecularly imprinted microspheres and nanospheres for di(2-ethylhexyl)phthalate prepared by precipitation polymerization. Anal. Bioanal. Chem. 2007, 389, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S.; Ihara, H.; Honbo, J.; Hirayama, C.; Kurisaki, H.; Ikegami, S. Spherical carbon packings prepared from spherical cellulose particles for high-performance liquid chromatography. Anal. Sci. 1994, 10, 543–551. [Google Scholar] [CrossRef]
- Wu, N.; Clausen, A.M. Fundamental and practical aspects of ultrahigh pressure liquid chromatography for fast separations. J. Sep. Sci. 2007, 30, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Meouche, W.; Laatikainen, K.; Margaillan, A.; Silvonen, T.; Siren, H.; Sainio, T.; Beurroies, I.; Denoyel, R.; Branger, C. Effect of porogen solvent on the properties of nickel ion imprinted polymer materials prepared by inverse suspension polymerization. Eur. Polym. J. 2017, 87, 124–135. [Google Scholar] [CrossRef]
- Zhang, N.; Hu, X.; Guan, P.; Du, C.; Li, J.; Qian, L.; Zhang, X.; Ding, S.; Li, B. Preparation of protein imprinted microspheres using amphiphilic ionic liquid as stabilizer and emulsifier via miniemulsion polymerization. Biochem. Eng. J. 2017, 317, 356–367. [Google Scholar] [CrossRef]
- Guan, X.W.; Li, X.Y.; Chai, S.G.; Zhang, X.H.; Zou, Q.C.; Zhang, J.Z. A sensitive electrochemical sensor based on solution polymerized molecularly imprinted polymers for procaine detection. Electroanalysis 2016, 28, 2007–2015. [Google Scholar] [CrossRef]
- Sambe, H.; Hoshina, K.; Haginaka, J. Molecularly imprinted polymers for triazine herbicides prepared by multi-step swelling and polymerization method: Their application to the determination of methylthiotriazine herbicides in river water. J. Chromatogr. A 2007, 1152, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Zhao, Q.; Zhang, Z.; Wang, Z.; Zhu, J.J. Molecularly imprinted polymers microsphere prepared by precipitation polymerization for hydroquinone recognition. Talanta 2008, 75, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Hizal, G.; Tunca, U.; Sanyal, A. Discrete macromolecular constructs via the diels–alder “click” reaction. J. Polym. Sci. Pol. Chem. 2011, 49, 4103–4120. [Google Scholar] [CrossRef]
- Xu, C.; Ye, L. Clickable molecularly imprinted nanoparticles. Chem. Commun. 2011, 47, 6096–6098. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Deng, P.; Tang, S.; Kuang, D.; Zhang, F.; Li, J. Preparation of 2d molecularly imprinted materials based on mesoporous silicas via click reaction. J. Mater. Chem. 2014, 2, 8418–8426. [Google Scholar] [CrossRef]
- Stephenson-Brown, A.; Acton, A.L.; Preece, J.A.; Fossey, J.S.; Mendes, P.M. Selective glycoprotein detection through covalent templating and allosteric click-imprinting. Chem. Sci. 2015, 6, 5114–5119. [Google Scholar] [CrossRef]
- Hou, Y.; Cao, S.; Li, X.; Wang, B.; Pei, Y.; Wang, L.; Pei, Z. One-step synthesis of dual clickable nanospheres via ultrasonic-assisted click polymerization for biological applications. ACS Appl. Mater. Interfaces 2014, 6, 16909–16917. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Cao, S.; Wang, L.; Pei, Y.; Zhang, G.; Zhang, S.; Pei, Z. Morphology-controlled dual clickable nanoparticles via ultrasonic- assisted click polymerization. Polym. Chem. 2014, 6, 223–227. [Google Scholar] [CrossRef]
- Falk, H. From the photosensitizer hypericin to the photoreceptor stentorin- the chemistry of phenanthroperylene quinones. Angew. Chem. Int. Ed. 1999, 38, 3116–3136. [Google Scholar] [CrossRef]
- Pei, Y.-X.; Li, Z.-B.; Pei, Z.-C.; Hou, Y. An Efficient Method for the Synthesis of Hypericin by Monochromatic Light. CN 103274920 A, 17 December 2014. [Google Scholar]
- Sun, S.S.; Lees, A.J. Synthesis and photophysical properties of dinuclear organometallic rhenium(i) diimine complexes linked by pyridine-containing macrocyclic: Phenylacetylene ligands. Organometallics 2001, 20, 2353–2358. [Google Scholar] [CrossRef]
- Dana, B.H.; Robinson, B.H.; Simpson, J. Intramolecular interactions in 2,6-pyridylacetylenes and their Co2(CO)4dppm complexes. J. Organomet. Chem. 2002, 648, 251–269. [Google Scholar] [CrossRef]
- Pant, C.S.; Wagh, R.M.; Nair, J.K.; Gore, G.M.; Venugopalan, S. Synthesis and characterization of two potential energetic azido esters. Propellant Explos. Pyrotech. 2006, 31, 477–481. [Google Scholar] [CrossRef]
- Jin, F.; Zheng, M.L.; Zhang, M.L.; Zhao, Z.S.; Duan, X.M. A facile layer-by-layer assembly method for the fabrication of fluorescent polymer/quantum dot nanocomposite thin films. RSC Adv. 2014, 4, 33206–33214. [Google Scholar] [CrossRef]
- Cheng, W.X.; Fan, F.F.; Zhang, Y.; Pei, Z.C.; Wang, W.J.; Pei, Y.X. A facile approach for fabrication of core-shell magnetic molecularly imprinted nanospheres towards hypericin. Polymers 2017, 9. [Google Scholar] [CrossRef]
- Ansell, R.J. Characterization of the Binding Properties of Molecularly Imprinted Polymers. Adv. Biochem. Eng. Biotechnol. 2015, 150, 51–93. [Google Scholar] [PubMed]
- Wu, X. Molecular imprinting for anion recognition in aqueous media. Microchim. Acta 2012, 176, 23–47. [Google Scholar] [CrossRef]
- Cheong, W.J.; Yang, S.H.; Ali, F. Molecular imprinted polymers for separation science: A review of reviews. J. Sep. Sci. 2013, 36, 609–628. [Google Scholar] [CrossRef] [PubMed]
- Nematollahzadeh, A.; Lindemann, P.; Sun, W.; Stute, J.; Lütkemeyer, D.; Sellergren, B. Robust and selective nano cavities for protein separation: An interpenetrating polymer network modified hierarchically protein imprinted hydrogel. J. Chromatogr. A 2014, 1345, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Qin, C.L.; Li, D.M.; Hou, Y.Z.; Li, S.B.; Sun, J.J. Molecularly imprinted polymer for specific extraction of hypericin from hypericum perforatum l. Herbal extract. J. Pharm. Biomed. Anal. 2014, 98, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Senhadjikebiche, O.; Belaid, T.; Benamor, M. Preparation and characterization of molecularly imprinted polymer as spe sorbent for melamine isolation. Polymers 2013, 5, 1215–1228. [Google Scholar]
- Kupai, J.; Razali, M.; Buyuktiryaki, S.; Kecili, R.; Szekely, G. Long-term stability and reusability of molecularly imprinted polymers. Polym. Chem. 2017, 8, 666–673. [Google Scholar] [CrossRef] [PubMed]

| Polymer | Combination | Qs (μmol·g−1 ) | RSD (n = 3) |
|---|---|---|---|
| P1 | 1 + 3 | 1.911 | 0.022 |
| P2 | 2 + 3 | 0.331 | 0.070 |
| P3 | 1 + 4 | 0.452 | 0.069 |
| P4 | 2 + 4 | 0.745 | 0.038 |
| P5 | 1 + 5 | 2.204 | 0.032 |
| NPs | Paticle Size (nm) | Polydispersity Index | ζ Potential (mV) |
|---|---|---|---|
| MIP–NSHs | 677 ± 68 | 1.137 | 0.95 ± 0.90 |
| NIP–NSs | 497 ± 80 | 0.994 | −2.86 ± 0.52 |
| MIP–NSHs1 | 661 ± 98 | 0.609 | −13.51 ± 0.17 |
| NIP–NSs1 | 464 ± 75 | 0.874 | −1.42 ± 0.56 |
| NPs | Average Pore Diameter (nm) | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) |
|---|---|---|---|
| MIP–NSHs | 52.839 ± 0.393 | 5.274 ± 0.517 | 0.0330 ± 0.0299 |
| NIP–NPs | 4.139 ±0.0522 | 2.083 ± 0.266 | 0.00392 ± 0.0726 |
| Polymer | Concentration of DMPA (mg·mL−1) | Qs (μmol·g−1) | RSD (n = 3) |
|---|---|---|---|
| P6 | 2.0 | 0.64 | 0.035 |
| P7 | 4.0 | 2.16 | 0.095 |
| P8 | 8.0 | 1.09 | 0.046 |
| P9 | 16.0 | 0.57 | 0.089 |
| Polymer | Acetone/Acetonitrile (v/v) | Qs (μmol·g−1) | RSD (n = 3) |
|---|---|---|---|
| P7 | 4:0 | 2.16 | 0.095 |
| P10 | 3:1 | 2.23 | 0.036 |
| P11 | 1:1 | 1.47 | 0.048 |
| P12 | 1:3 | 0.28 | 0.079 |
| Polymer | Template concentration (mg·mL−1) | Ratio of 1 to 5 (in equiv.) | Qs (μmol·g−1) | RSD (n = 3) |
|---|---|---|---|---|
| P13 | 0.625 | 4:3 | 1.35 | 0.059 |
| P7 | 1.25 | 4:3 | 2.17 | 0.095 |
| P14 | 2.5 | 4:3 | 2.57 | 0.052 |
| P15 | 5.0 | 4:3 | 1.52 | 0.039 |
| P16 | 2.5 | 4:1 | 0.59 | 0.069 |
| P17 | 2.5 | 3:1 | 1.10 | 0.093 |
| P18 | 2.5 | 2:1 | 2.23 | 0.058 |
| P19 | 2.5 | 1:1 | 1.94 | 0.049 |
| P20 | 2.5 | 1:2 | 0.45 | 0.084 |
| Factor | Hypericin | Protohypericin | Emodin |
|---|---|---|---|
| SF | / | 3.34 | 8.04 |
| IF | 2.44 | 2.88 | 2.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Fan, F.; Wang, X.; Feng, W.; Hou, Y.; Pei, Z. Fabrication of Hypericin Imprinted Polymer Nanospheres via Thiol-Yne Click Reaction. Polymers 2017, 9, 469. https://doi.org/10.3390/polym9100469
Pei Y, Fan F, Wang X, Feng W, Hou Y, Pei Z. Fabrication of Hypericin Imprinted Polymer Nanospheres via Thiol-Yne Click Reaction. Polymers. 2017; 9(10):469. https://doi.org/10.3390/polym9100469
Chicago/Turabian StylePei, Yuxin, Fengfeng Fan, Xinxin Wang, Weiwei Feng, Yong Hou, and Zhichao Pei. 2017. "Fabrication of Hypericin Imprinted Polymer Nanospheres via Thiol-Yne Click Reaction" Polymers 9, no. 10: 469. https://doi.org/10.3390/polym9100469