Blood Compatibility of ZrO2 Particle Reinforced PEEK Coatings on Ti6Al4V Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Characterization
2.3. Blood Donors
2.4. Erythrocytes Observation
2.5. Hemolysis Assay
2.6. Plasma Recalcification Time
2.7. Statistics
3. Results
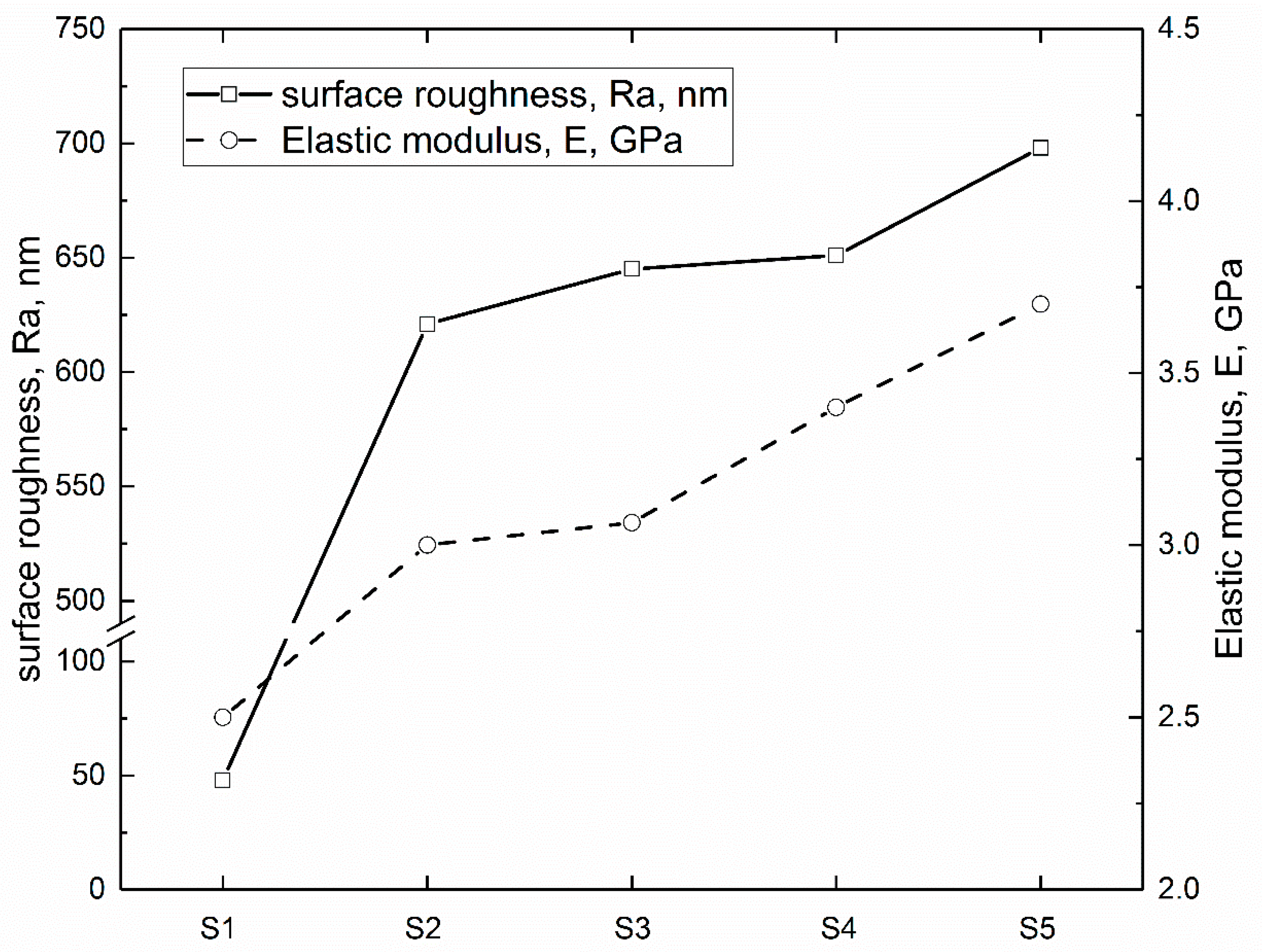
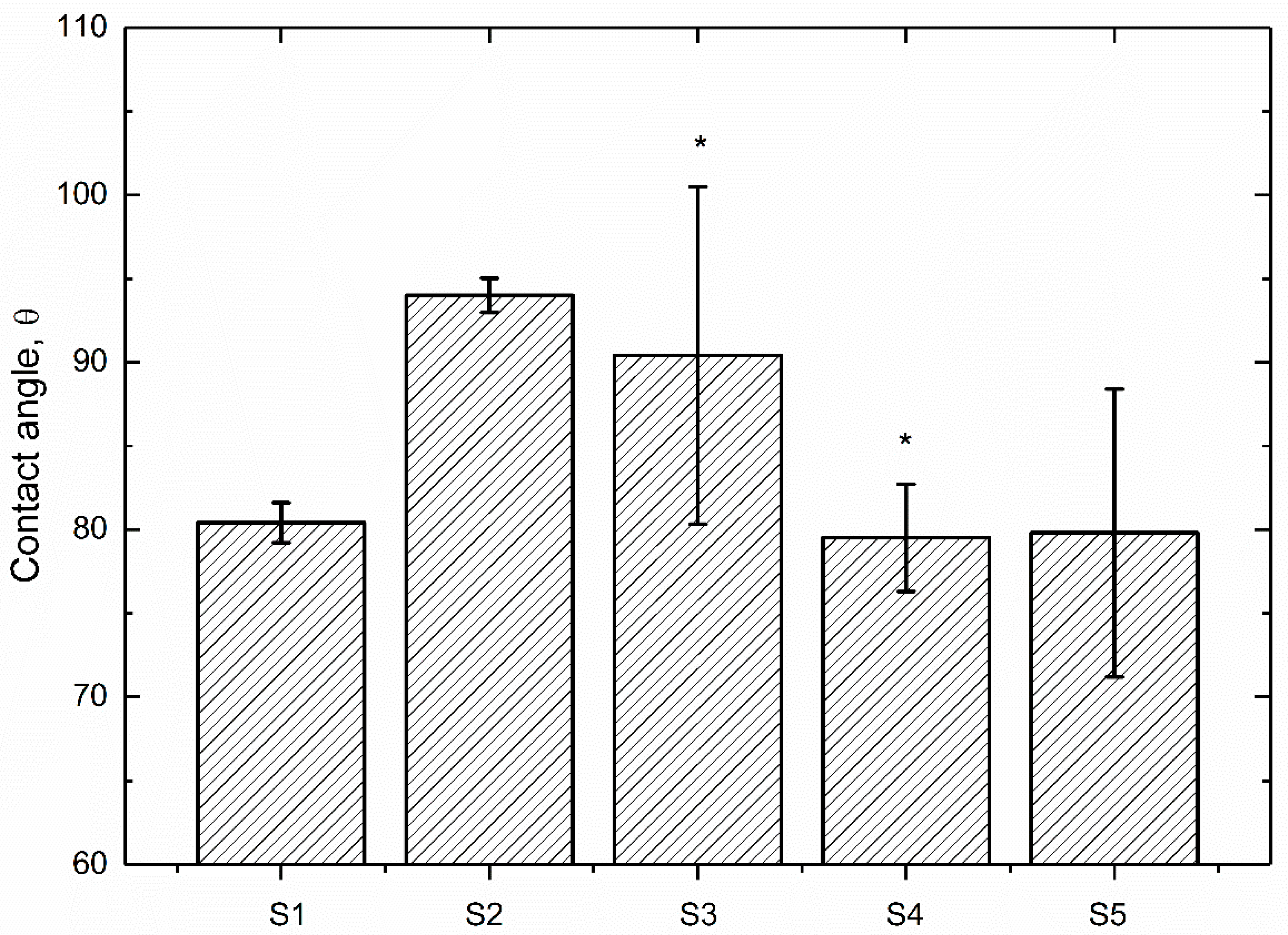
3.1. Surface Characterization
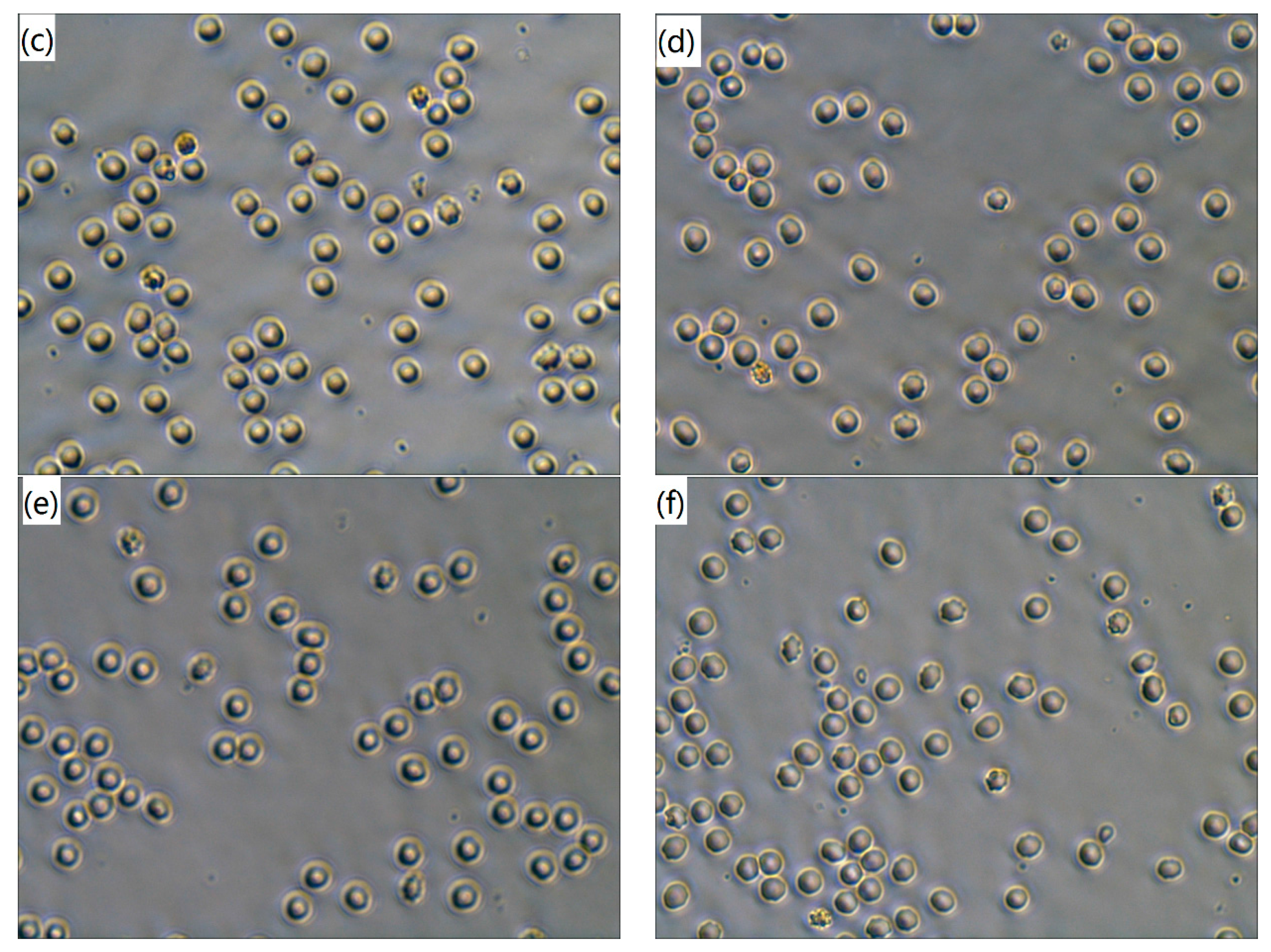
3.2. Erythrocytes Observation
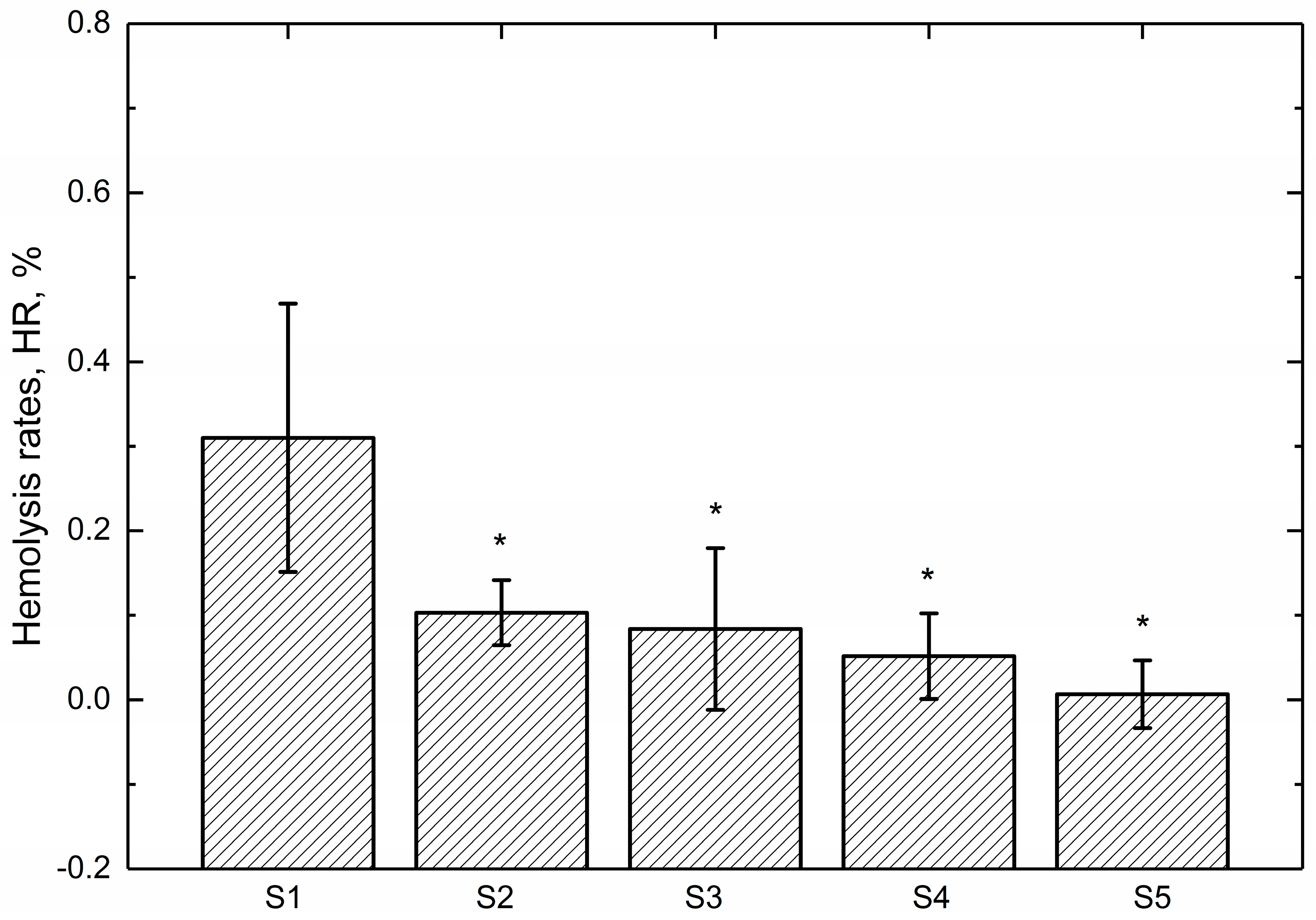
3.3. Hemolysis Assay
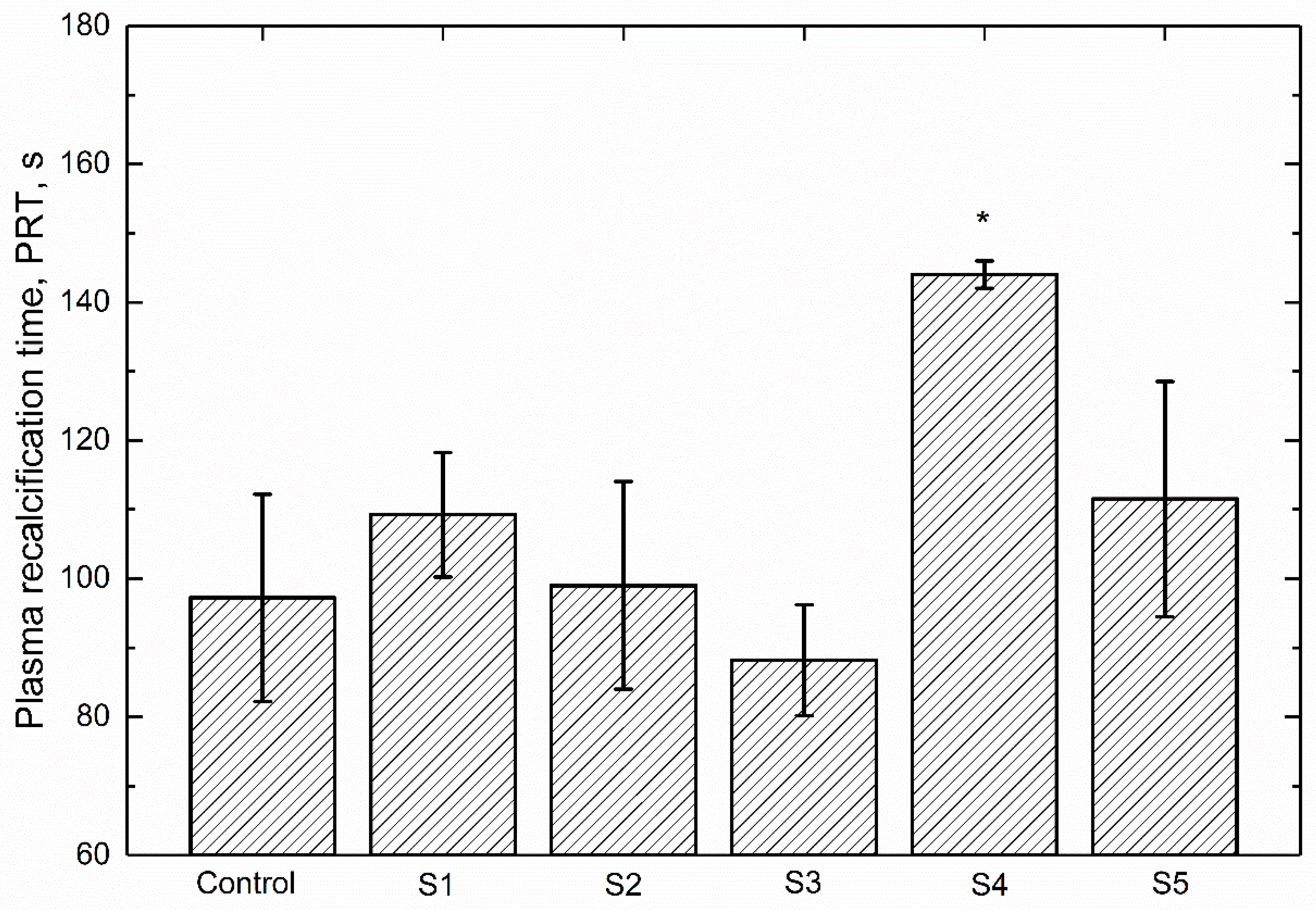
3.4. Plasma Recalcification Time
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andersen, O.Z.; Offermanns, V.; Sillassen, M.; Almtoft, K.P.; Andersen, I.H.; Sørensen, S.; Jeppesen, C.S.; Kraft, D.C.; Bøttiger, J.; Rasse, M. Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials 2013, 34, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lai, Y.; Zhang, Q.; Wu, K.; Zhang, L.; Lin, C.; Tang, P. A novel electrochemical strategy for improving blood compatibility of titanium-based biomaterials. Colloids Surf. B Biointerfaces 2010, 79, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Greisler, H.P. Biomaterials in the development and future of vascular grafts. J. Vasc. Surg. 2003, 37, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Andersson, J.; Ekdahl, K.N.; Elgue, G.; Axén, N.; Larsson, R.; Nilsson, B. Titanium is a highly thrombogenic biomaterial: Possible implications for osteogenesis. Thromb. Haemost. 1999, 82, 58–64. [Google Scholar] [PubMed]
- Faghihi, S.; Li, D.; Szpunar, J.A. Tribocorrosion behaviour of nanostructured titanium substrates processed by high-pressure torsion. Nanotechnology 2010, 2, 485703. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Hauert, R. A review of modified DLC coatings for biological applications. Diam. Relat. Mater. 2003, 12, 583–589. [Google Scholar] [CrossRef]
- Tsyganov, I.; Maitz, M.F.; Wieser, E.; Richter, E.; Reuther, H. Correlation between blood compatibility and physical surface properties of titanium-based coatings. Surf. Coat. Technol. 2005, 200, 1041–1044. [Google Scholar] [CrossRef]
- Panjwani, B.; Satyanarayana, N.; Sinha, S.K. Tribological characterization of a biocompatible thin film of UHMWPE on Ti6Al4V and the effects of PFPE as top lubricating layer. J. Mech. Behav. Biomed. Mater. 2011, 4, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Y.; Wen, S.; Wang, S. Poly(vinylphosphonic acid) (PVPA) on Titanium Alloy Acting as Effective Cartilage-like Superlubricity Coatings. ACS Appl. Mater. Interfaces 2014, 6, 17571–17578. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ge, S.; Jin, Z.; Fisher, J. Effect of surface modification on surface properties and tribological behaviours of titanium alloys. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2009, 223, 311–316. [Google Scholar] [CrossRef]
- Song, J.; Liu, Y.H.; Wang, S.; Liao, Z.H.; Liu, W.Q. Study on the wettability and tribological behaviors of glass fiber reinforced poly (ether-ether-ketone) against different polymers as bearing materials for artificial cervical disc. Biotribology 2015, 4, 18–29. [Google Scholar] [CrossRef]
- Song, J.; Liao, Z.; Shi, H.; Xiang, D.; Liu, Y.; Liu, W.; Peng, Z. Fretting Wear Study of PEEK-Based Composites for Bio-implant Application. Tribol. Lett. 2017, 65, 150. [Google Scholar] [CrossRef]
- Devine, D.M.; Hahn, J.; Richards, R.G.; Gruner, H.; Wieling, R.; Pearce, S.G. Coating of carbon fiber-reinforced polyetheretherketone implants with titanium to improve bone apposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Monich, P.R.; Henriques, B.; de Oliveira, A.P.N.; Souza, J.C.; Fredel, M.C. Mechanical and biological behavior of biomedical PEEK matrix composites: A focused review. Mater. Lett. 2016, 185, 593–597. [Google Scholar] [CrossRef]
- Song, J.; Liu, Y.; Liao, Z.; Wang, S.; Tyagi, R.; Liu, W. Wear studies on ZrO2-filled PEEK as coating bearing materials for artificial cervical discs of Ti6Al4V. Mater. Sci. Eng. C 2016, 69, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Fukumoto, K.; Iwasaki, Y.; Nakabayashi, N. Modification of polysulfone with phospholipid polymer for improvement of the blood compatibility. Part 1. Surface characterization. Biomaterials 1999, 20, 1545–1551. [Google Scholar] [CrossRef]
- Jun, L.; Xing, H.J.; Wu, M.F.; Zhu, Q.S.; Yang, X.Y. Comparison of blood compatibility between carbon fibers reinforced polyetheretherketone and titanium alloy. J. Clin. Rehabil. Tissue Eng. Res. 2009, 13, 1455–1458. [Google Scholar]
- Kawasaki, Y.; Iwasaki, Y. Surface modification of poly (ether ether ketone) with methacryloyl-functionalized phospholipid polymers via self-initiation graft polymerization. J. Biomater. Sci. Polym. Ed. 2014, 25, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Nunez, E.E.; Yeo, S.M.; Polychronopoulou, K.; Polycarpou, A.A. Tribological study of high bearing blended polymer-based coatings for air-conditioning and refrigeration compressors. Surf. Coat. Technol. 2011, 205, 2994–3005. [Google Scholar] [CrossRef]
- Song, J.; Liao, Z.; Wang, S.; Liu, Y.; Liu, W.; Tyagi, R. Study on the Tribological Behaviors of Different PEEK Composite Coatings for Use as Artificial Cervical Disk Materials. J. Mater. Eng. Perform. 2016, 25, 116–129. [Google Scholar] [CrossRef]
- Wang, S.; Song, J.; Liao, Z.; Liu, Y.; Zhang, C.; Liu, W. Study on the Wettability and Tribological Behavior of Different Polymers as Bearing Materials for Cervical Prosthesis. J. Mater. Eng. Perform. 2015, 24, 1–13. [Google Scholar] [CrossRef]
- Bober, P.; Liu, J.; Mikkonen, K.S.; Ihalainen, P.; Pesonen, M.; Plumed-Ferrer, C.; von Wright, A.; Lindfors, T.; Xu, C.; Latonen, R. Biocomposites of nanofibrillated cellulose, polypyrrole, and silver nanoparticles with electroconductive and antimicrobial properties. Biomacromolecules 2014, 15, 3655–3663. [Google Scholar] [CrossRef] [PubMed]
- Fitch, D.A.; Hoffmeister, B.K.; de Ana, J. Ultrasonic evaluation of polyether ether ketone and carbon fiber-reinforced PEEK. J. Mater. Sci. 2010, 45, 3768–3777. [Google Scholar] [CrossRef]
- Feyerabend, F.; Wendel, H.; Mihailova, B.; Heidrich, S.; Agha, N.A.; Bismayer, U.; Willumeit-Römer, R. Blood compatibility of magnesium and its alloys. Acta Biomater. 2015, 25, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Kainthan, R.K.; Gnanamani, M.; Ganguli, M.; Ghosh, T.; Brooks, D.E.; Maiti, S.; Kizhakkedathu, J.N. Blood compatibility of novel water soluble hyperbranched polyglycerol-based multivalent cationic polymers and their interaction with DNA. Biomaterials 2006, 27, 5377–5390. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Ye, J.; Yuan, J.; Xiao, Y. Fabrication of poly (ε-caprolactone)/keratin nanofibrous mats as a potential scaffold for vascular tissue engineering. Mater. Sci. Eng. C 2016, 68, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Shen, F. Blood compatibility of a ferulic acid (FA)-eluting PHBHHx system for biodegradable magnesium stent application. Mater. Sci. Eng. C 2015, 52, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Andrea, G.D.; Terrazzino, S.; Leon, A.; Fortin, D.; Perini, F.; Granella, F.; Bussone, G. Elevated levels of circulating trace amines in primary headaches. Neurol 2004, 62, 1701–1705. [Google Scholar] [CrossRef]
- Sagnella, S.; Mai-Ngam, K. Chitosan based surfactant polymers designed to improve blood compatibility on biomaterials. Colloids Surf. B Biointerfaces 2005, 42, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, D.; Shi, F.; Cai, Y. Sol–gel deposited TiO2 film on NiTi surgical alloy for biocompatibility improvement. Thin Solid Films 2003, 429, 225–230. [Google Scholar] [CrossRef]
- Jordan, J.; Jacob, K.I.; Tannenbaum, R.; Sharaf, M.A.; Jasiuk, I. Experimental trends in polymer nanocomposites—A review. Mater. Sci. Eng. A 2005, 393, 1–11. [Google Scholar] [CrossRef]
- Katti, K.S. Biomaterials in total joint replacement. Colloids Surf. B Biointerfaces 2004, 39, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, H.; Zhang, Z.; Xie, S. Processing and performance improvements of SWNT paper reinforced PEEK nanocomposites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 388–392. [Google Scholar] [CrossRef]
- Zoidis, P.; Papathanasiou, I. Modified PEEK resin-bonded fixed dental prosthesis as an interim restoration after implant placement. J. Prosthet. Dent. 2016, 116, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [PubMed]
- Rochford, E.; Poulsson, A.; Varela, J.S.; Lezuo, P.; Richards, R.G.; Moriarty, T.F. Bacterial adhesion to orthopaedic implant materials and a novel oxygen plasma modified PEEK surface. Colloids Surf. B Biointerfaces 2014, 113, 213–222. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Shi, Z.; Sun, W.; Ma, J.; Xia, J.; Zhang, X.; Chen, W.; Huang, J. Hemocompatibility of folic-acid-conjugated amphiphilic PEG-PLGA copolymer nanoparticles for co-delivery of cisplatin and paclitaxel: Treatment effects for non-small-cell lung cancer. Tumor Biol. 2016, 37, 7809–7821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Yin, D.; Xu, L.; Yang, L.; Yang, K. Microstructure, mechanical and corrosion properties and biocompatibility of Mg–Zn–Mn alloys for biomedical application. Mater. Sci. Eng. C 2009, 29, 987–993. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Nicolae, C. Micro-and nano-mechanical characterization of polyamide 11 and its composites containing cellulose nanofibers. Eur. Polym. J. 2013, 49, 3857–3866. [Google Scholar] [CrossRef]
- Hansson, K.M.; Tosatti, S.; Isaksson, J.; Wetterö, J.; Textor, M.; Lindahl, T.L.; Tengvall, P. Whole blood coagulation on protein adsorption-resistant PEG and peptide functionalised PEG-coated titanium surfaces. Biomaterials 2005, 26, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Kainthan, R.K.; Hester, S.R.; Levin, E.; Devine, D.V.; Brooks, D.E. In vitro biological evaluation of high molecular weight hyperbranched polyglycerols. Biomaterials 2007, 28, 4581–4590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liao, J.; Shi, X.; Qiu, Y.; Chen, H. Surface biocompatible construction of polyurethane by heparinization. J. Polym. Res. 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Yang, S.; Wu, S.; Zeng, R.; Wu, H.; Zhang, J.; Zha, Z.; Tu, M. Improving blood-compatibility via surface heparin-immobilization based on a liquid crystalline matrix. Mater. Sci. Eng. C 2016, 58, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Y.; Chen, K.C.; Zhang, G.; Shi, X.; Chen, H. Surface biocompatible modification of polyurethane by entrapment of a macromolecular modifier. Colloids Surf. B Biointerfaces 2013, 102, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Chen, H.; Shen, F. Biocorrosion properties and blood and cell compatibility of pure iron as a biodegradable biomaterial. J. Mater. Sci. Mater. Med. 2010, 21, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Y.; Xu, J.L.; Liu, Z.H.; Deng, L.; Sun, B.; Liu, S.D.; Wang, L.; Liu, H.Y. Preparation, corrosion resistance and hemocompatibility of the superhydrophobic TiO2 coatings on biomedical Ti-6Al-4V alloys. Appl. Surf. Sci. 2015, 347, 591–595. [Google Scholar] [CrossRef]
- Chu, C.L.; Wang, R.M.; Yin, L.H.; Pu, Y.P.; Lin, P.H.; Dong, Y.S.; Chung, C.Y.; Yeung, K.; Chu, P.K. Effects of anodic oxidation in H2SO4 electrolyte on the biocompatibility of NiTi shape memory alloy. Mater. Lett. 2008, 62, 3512–3514. [Google Scholar] [CrossRef]
- Polaschegg, H.D. Red blood cell damage from extracorporeal circulation in hemodialysis. Semin. Dial. 2009, 22, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, G.; Suriyaprabha, R.; Manivasakan, P.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Screening of in vitro cytotoxicity, antioxidant potential and bioactivity of nano-and micro-ZrO2 and-TiO2 particles. Ecotoxicol. Environ. Saf. 2013, 93, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, P. Surface modification of polyethylene by plasma pretreatment and UV-induced graft polymerization for improvement of antithrombogenicity. J. Appl. Polym. Sci. 2004, 93, 2014–2018. [Google Scholar] [CrossRef]
- Yahyapour, N.; Eriksson, C.; Malmberg, P.; Nygren, H. Thrombin, kallikrein and complement C5b-9 adsorption on hydrophilic and hydrophobic titanium and glass after short time exposure to whole blood. Biomaterials 2004, 25, 3171–3176. [Google Scholar] [CrossRef] [PubMed]

| Sample Code | Coating |
|---|---|
| S1 | Pure PEEK |
| S2 | PEEK + 2 wt % ZrO2 |
| S3 | PEEK + 5 wt % ZrO2 |
| S4 | PEEK + 10 wt % ZrO2 |
| S5 | PEEK + 15 wt % ZrO2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Liao, Z.; Shi, H.; Xiang, D.; Xu, L.; Liu, Y.; Mu, X.; Liu, W. Blood Compatibility of ZrO2 Particle Reinforced PEEK Coatings on Ti6Al4V Substrates. Polymers 2017, 9, 589. https://doi.org/10.3390/polym9110589
Song J, Liao Z, Shi H, Xiang D, Xu L, Liu Y, Mu X, Liu W. Blood Compatibility of ZrO2 Particle Reinforced PEEK Coatings on Ti6Al4V Substrates. Polymers. 2017; 9(11):589. https://doi.org/10.3390/polym9110589
Chicago/Turabian StyleSong, Jian, Zhenhua Liao, Hongyu Shi, Dingding Xiang, Lin Xu, Yuhong Liu, Xiaohong Mu, and Weiqiang Liu. 2017. "Blood Compatibility of ZrO2 Particle Reinforced PEEK Coatings on Ti6Al4V Substrates" Polymers 9, no. 11: 589. https://doi.org/10.3390/polym9110589