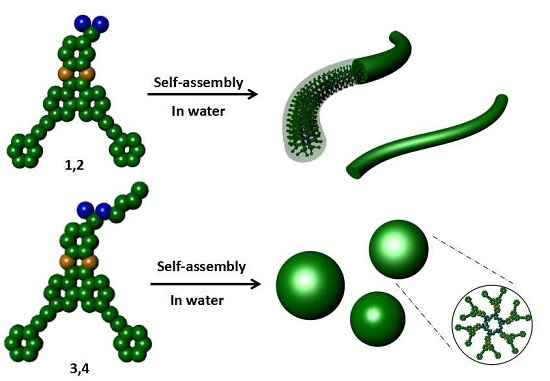
Construction of Supramolecular Nanostructures from V-Shaped Amphiphilic Rod-Coil Molecules Incorporating Phenazine Units
Abstract
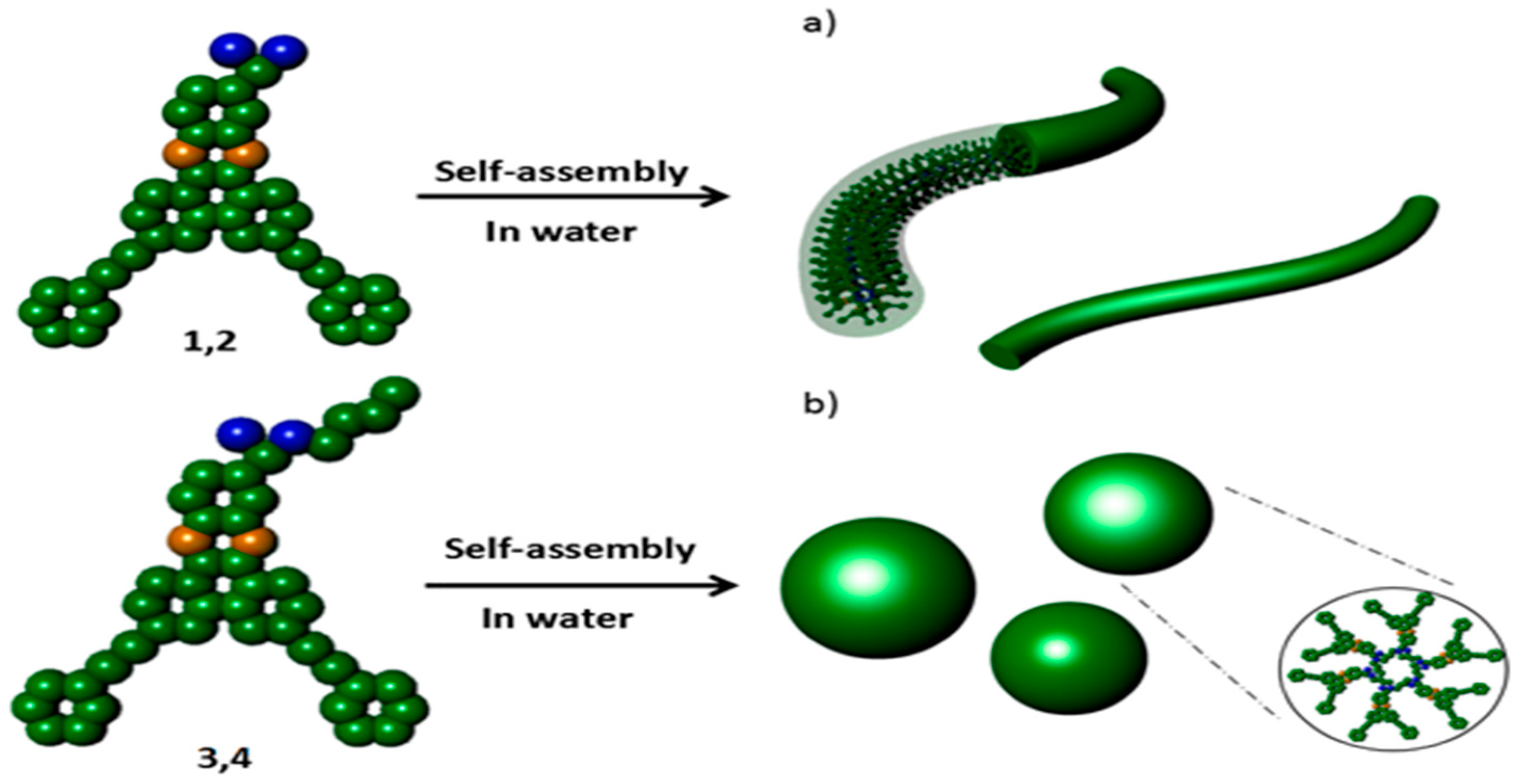
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Techniques
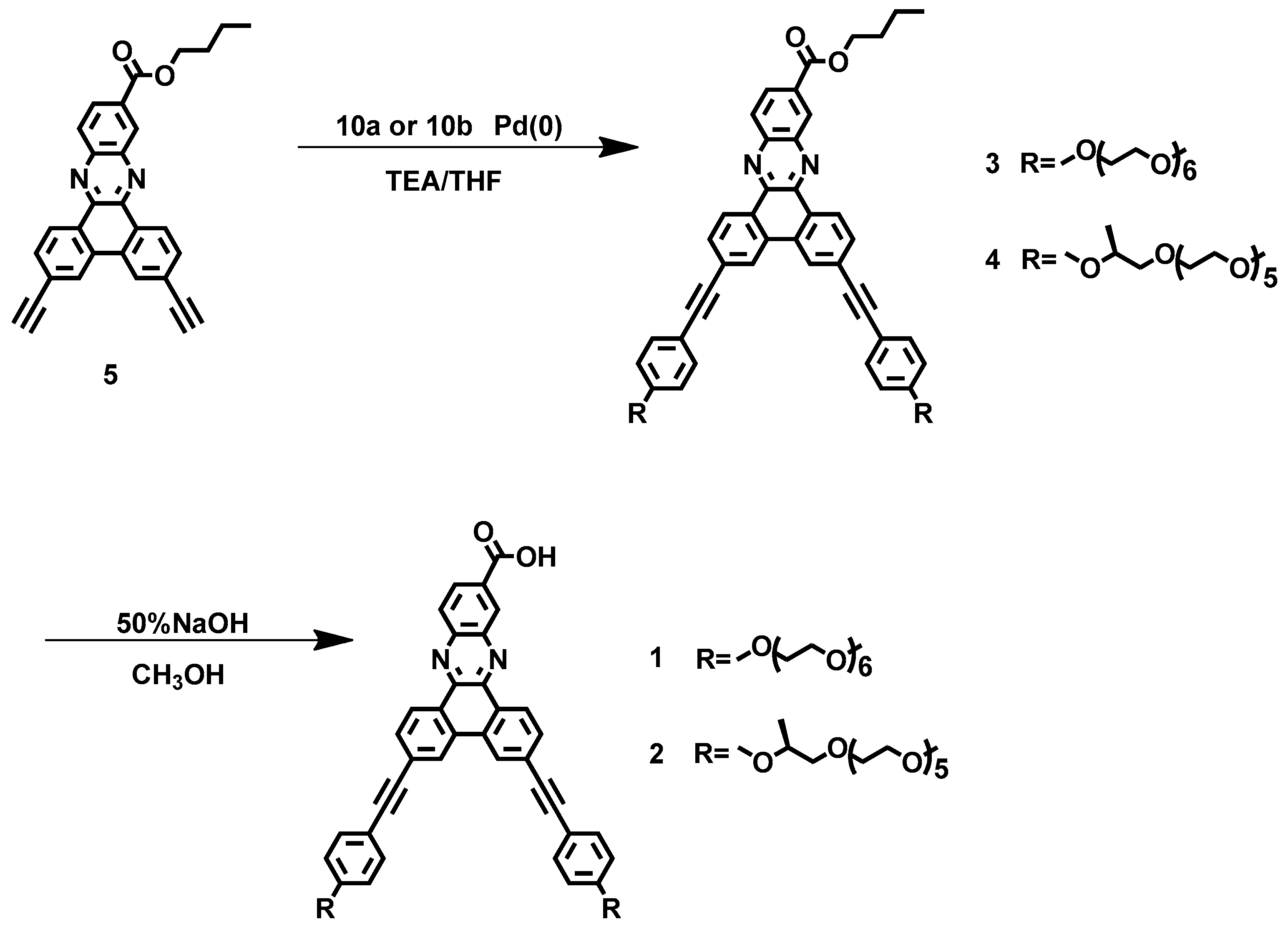
2.3. Synthesis of Molecules 3 and 4
2.4. Synthesis of Molecules 1 and 2
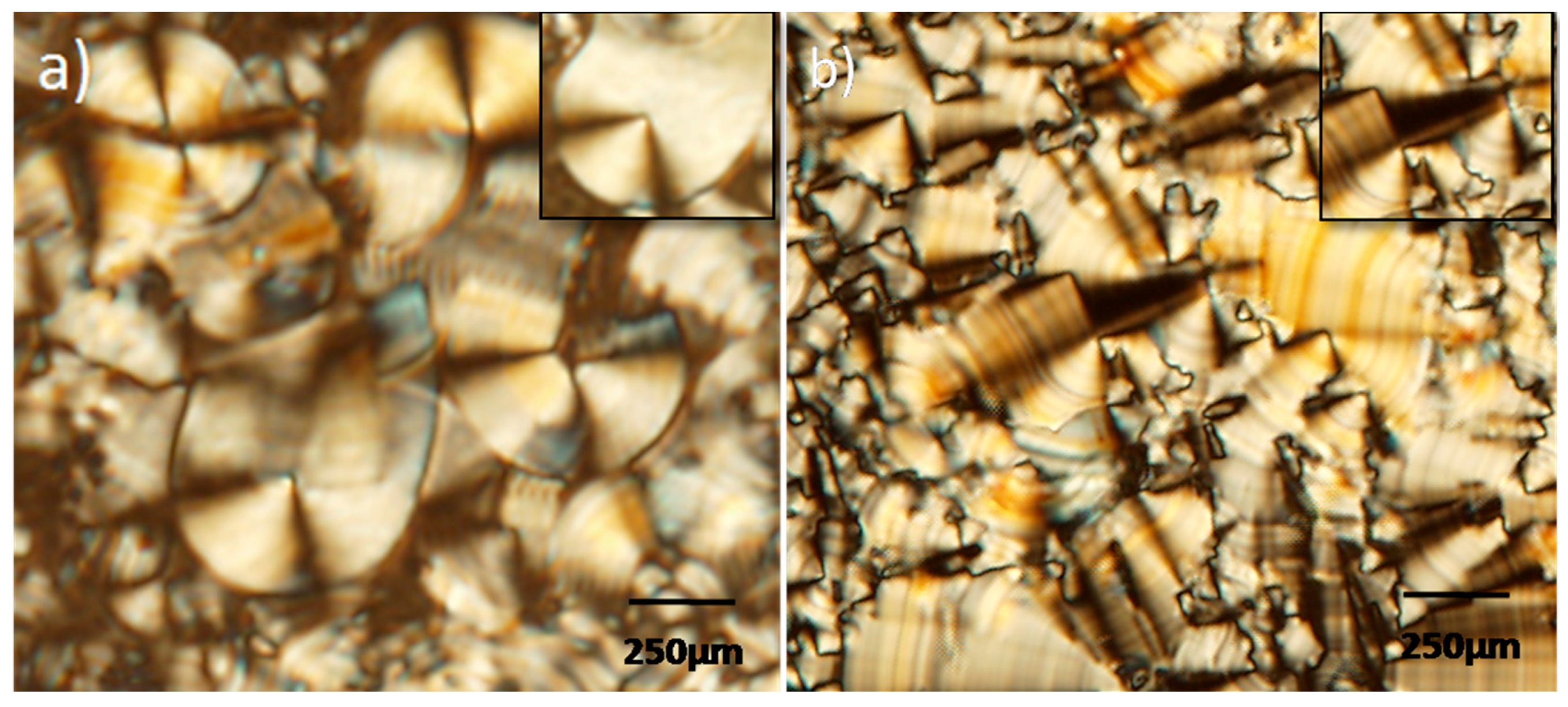
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Willersinn, J.; Schmidt, B.V.K.J. Self-Assembly of Double Hydrophilic Poly(2-ethyl-2-oxazoline)-b-poly(N-vinylpyrrolidone) Block Copolymers in Aqueous Solution. Polymers 2017, 9, 293. [Google Scholar] [CrossRef]
- Egli, S.; Schlaad, H.; Bruns, N.; Meier, W. Functionalization of Block Copolymer Vesicle Surfaces. Polymers 2011, 3, 252–280. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Su, Z.; Reynolds, N.P.; Arosio, P.; Hamley, I.W.; Gazit, E.; Mezzenga, R. Self-assembling Peptide and Protein Amyloids: From Structure to Tailored Function in Nanotechnology. Chem. Soc. Rev. 2017, 46, 4661–4708. [Google Scholar] [CrossRef] [PubMed]
- Busseron, E.; Ruff, Y.; Moulin, E.; Giuseppone, N. Supramolecular Self-assemblies as Functional Nanomaterials. Nanoscale 2013, 5, 7098–7140. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Tang, T.; Lin, C.; Shen, X.; Lu, C.; Xu, L.; Gu, Z.; Xu, Z.; Qiu, H.; Zhang, Q.; et al. Conjugated Polymers Containing BODIPY and Fluorene Units for Sensitive Detection of CN− Ions: Site-Selective Synthesis, Photo-Physical and Electrochemical Properties. Polymers 2017, 9, 512. [Google Scholar] [CrossRef]
- Kim, H.J.; Liu, F.; Ryu, J.H.; Kang, S.K.; Zeng, X.B.; Ungar, G.; Lee, J.K.; Zin, W.C.; Lee, M. Self-Organization of Bent Rod Molecules into Hexagonally Ordered Vesicular Columns. J. Am. Chem. Soc. 2012, 134, 13871–13880. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Oda, Y.; Hirata, T.; Matsuyama, R.; Matsunob, H.; Tanak, K. Surface Segregation of a Branched Polymer with Hydrophilic Poly[2-(2-ethoxy)ethoxyethyl Vinyl Ether] side Chains. Polym. Chem. 2017, 8, 505–510. [Google Scholar] [CrossRef]
- Higashihara, T.; Liu, C.L.; Chen, W.C.; Ueda, M. Synthesis of Novel π-Conjugated Rod-Rod-Rod Triblock Copolymers Containing Poly(3-hexylthiophene) and Polyacetylene Segments by Combination of Quasi-Living GRIM and Living Anionic Polymerization. Polymers 2011, 3, 236–251. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Li, Z.; Zhong, K.; Jin, L.Y.; Lee, M. Self-Assembly of n-Shaped Rod-Coil Molecules into Thermoresponsive Nanoassemblies: Construction of Reversible Helical Nanofibers in Aqueous Environment. Macromolecules 2016, 49, 5912–5920. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chien, W.C.; Tsai, C.L. Preparation and Characterization of Thermo-Responsive Rod-Coil Diblock Copolymers. Polymers 2017, 9, 340. [Google Scholar] [CrossRef]
- Yu, S.; Yang, Y.; Chen, T.; Xu, J.Z.; Jin, L.Y. Donor–Acceptor Interaction-Driven Self-Assembly of Amphiphilic Rod–Coil Molecules into Supramolecular Nanoassemblies. Nanoscale 2017, 9, 17975–17982. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Y.; Chen, T.; Jin, L.Y.; Lee, M. Construction of Supramolecular Assemblies from Self-Organization of Amphiphilic Molecular Isomers. Chem. Asian J. 2016, 2265, 1861–4728. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Moon, K.S.; Lee, M. Rod-coil Block Molecules: Their Aqueous Self-assembly and Biomaterials Applications. J. Mater. Chem. 2008, 18, 2909–2918. [Google Scholar] [CrossRef]
- Poppe, S.; Poppe, M.; Ebert, H.; Prehm, M.; Chen, C.; Liu, F.; Werner, S.; Bacia, K.; Tschierske, C. Effects of Lateral and Terminal Chains of X-Shaped Bolapolyphiles with Oligo(phenyleneethynylene) Cores on Self-Assembly Behaviour. Part 1: Transition between Amphiphilic and Polyphilic Self-Assembly in the Bulk. Polymers 2017, 9, 471. [Google Scholar] [CrossRef]
- Guan, Z.; Wang, L.; Zhua, X.; Lin, J. Striped Patterns Self-assembled from Rod–Coil Diblock Copolymers on Spherical Substrates. Mater. Chem. Front. 2017, 1, 697–708. [Google Scholar] [CrossRef]
- Takenami, K.; Uemura, S.; Funahashi, M. In situ Polymerization of Liquid-crystalline thin Films of electron-transporting Perylene Tetracarboxylic Bisimide bearing Cyclotetrasiloxane Rings. RSC Adv. 2016, 6, 5474–5484. [Google Scholar] [CrossRef]
- Oyafuso, M.H.; Carvalho, F.C.; Takeshita, T.M.; Souza, A.L.R.; Araújo, D.R.; Merino, V.; Gremião, M.P.D.; Chorilli, M. Development and In Vitro Evaluation of Lyotropic Liquid Crystals for the Controlled Release of Dexamethasone. Polymers 2017, 9, 330. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.; Jiang, L.H. Stimuli-Responsive Supramolecular Nanocapsules from Amphiphilic Calixarene Assembly. J. Am. Chem. Soc. 2004, 126, 12724–12725. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, J.K.; Lee, M. Tubular Stacking of Water-Soluble Toroids Triggered by Guest Encapsulation. J. Am. Chem. Soc. 2009, 131, 18242–18243. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, T.; Kim, T.; Lee, M. Responsive Nanostructures from Aqueous Assembly of Rigid−Flexible Block Molecules. Acc. Chem. Res. 2011, 44, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ryu, J.H.; Lee, E.; Lee, M. Tunable Columnar Organization by Twisted Stacking of End-Capped Aromatic Rods. Chem. Mater. 2007, 19, 6569–6574. [Google Scholar] [CrossRef]
- Chen, S.; Ma, C.; Huang, Z.; Lee, M. Controlled Helicity of the Rigid-Flexible Molecular Assembly Triggered by Water Addition: From Nanocrystal to Liquid Crystal Gel and Aqueous Nanofibers. J. Phys. Chem. C 2014, 118, 8181–8186. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Kim, Y.; Lee, M. Reversible, Short α-Peptide Assembly for Controlled Capture and Selective Release of Enantiomers. J. Am. Chem. Soc. 2016, 138, 5773–5776. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Cai, C.; Jiang, T.; Lin, J.; Yang, C. Self-assembly Behavior of rod–coil–rod polypeptide block copolymers. Polymer 2014, 55, 602–610. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, J.Z.; Sun, Z.Y.; Shi, T.F.; An, L.J.; Jia, Y.X. Self-assembly of linear ABC coil-coil-rod triblock copolymers. Polymer 2010, 51, 3315–3319. [Google Scholar] [CrossRef]
- Gorecka, E.; Pociecha, D.; Mieczkowski, J.; Matraszek, J.; Guillon, D.; Donnio, B. Supramolecular Self-Assembly into Biofunctional Soft Nanotubes: From Bilayers to Monolayers. J. Am. Chem. Soc. 2004, 126, 15946–15947. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, N.; Ros, M.B.; Serrano, J.L.; Fuente, M.R.D. Axially Polar Columnar Phase Made of Polycatenar Bent-Shaped Molecules. Chem. Mater. 2008, 20, 1262–1271. [Google Scholar] [CrossRef]
- Dantlgraber, G.; Shen, D.; Diele, S.; Tschierske, C. Antiferroelectric Switchable Mesophases of Nonchiral Bent-Core Liquid Crystals Containing Fluorinated Central Cores. Chem. Mater. 2002, 14, 1149–1158. [Google Scholar] [CrossRef]
- Keith, C.; Reddy, R.A.; Baumeister, U.; Tschierske, C. Banana-Shaped Liquid Crystals with Two Oligosiloxane End-Groups: Field-Induced Switching of Supramolecular Chirality. J. Am. Chem. Soc. 2004, 126, 14312–14313. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Pegenau, A.; Diele, S.; Wirth, I.; Tschierske, C. Molecular Design of Nonchiral Bent-Core Liquid Crystals with Antiferroelectric Properties. J. Am. Chem. Soc. 2000, 122, 1593–1601. [Google Scholar] [CrossRef]
- Keith, C.; Reddy, R.A.; Hauser, A.; Baumeister, U.; Tschierske, C. Silicon-Containing Polyphilic Bent-Core Molecules: The Importance of Nanosegregation for the Development of Chirality and Polar Order in Liquid Crystalline Phases Formed by Achiral Molecules. J. Am. Chem. Soc. 2006, 128, 3051–3066. [Google Scholar] [CrossRef] [PubMed]
- Keith, C.; Dantlgraber, G.; Reddy, R.A.; Baumeister, U.; Tschierske, C. Ferroelectric and Antiferroelectric Smectic and Columnar Liquid Crystalline Phases Formed by Silylated and Non-Silylated Molecules with Fluorinated Bent Cores. Chem. Mater. 2007, 19, 694–710. [Google Scholar] [CrossRef]
- Zhang, Y.; Baumeister, U.; Tschierske, C.; O’Callaghan, M.J.; Walker, C. Achiral Bent-Core Molecules with a Series of Linear or Branched Carbosilane Termini: Dark Conglomerate Phases, Supramolecular Chirality and Macroscopic Polar Order. Chem. Mater. 2010, 22, 2869–2884. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, J.; Liang, Y.; Chen, T.; Lee, M.; Yin, B.; Jin, L.Y. Supramolecular Nanostructures from Self-Assembly of T-Shaped Rod Building Block Oligomers. Polym. Chem. 2013, 51, 5021–5028. [Google Scholar] [CrossRef]
- You, S.; Zhong, K.; Jin, L.Y. Control of supramolecular nanoassemblies by tuning the interactions of bent-shaped rod–coil molecules. Soft Matter 2017, 13, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhong, K.; Liu, Y.; Li, Z.; Chen, T.; Jin, L.Y. Self-organizing p-quinquephenyl Building Blocks Incorporating Lateral Hydroxyl and Methoxyl Groups into Supramolecular Nano-assemblies. Soft Matter 2016, 12, 3860–3867. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, K.L.; Li, Z.H.; Wang, Y.Q.; Chen, T.; Lee, M.; Jin, L.Y. Synthesis and Self-assembly of Amphiphilic Bent-shaped Molecules Based on Dibenzo[a,c]phenazine and Poly(ethylene oxide) Units. Polym. Chem. 2015, 6, 7395–7401. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Mo, G.; Xing, X.; Liu, P. A Small-angle X-ray Scattering Station at Beijing Synchrotron Radiation Facility. Instrum. Sci. Technol. 2014, 42, 128–141. [Google Scholar] [CrossRef]
- Lee, M.; Cho, B.K.; Jang, Y.G.; Zin, W.C. Spontaneous Organization of Supramolecular Rod-Bundles into a Body-Centered Tetragonal Assembly in Coil–Rod–Coil Molecules. J. Am. Chem. Soc. 2000, 122, 7449–7455. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, M.; Zhu, Y.; Chen, X.F.; Li, C.Y. Tunable Supramolecular Hexagonal Columnar Structures of Hydrogen-Bonded Copolymers Containing Two Different Sized Dendritic Side Chains. ACS Macro Lett. 2017, 6, 479–484. [Google Scholar] [CrossRef]
- Cristiano, R.; Eccher, J.; Bechtold, I.H.; Tironi, C.N.; Vieira, A.A.; Molin, F.; Gallardo, H. Luminescent Columnar Liquid Crystals Based on Tristriazolotriazine. Langmuir 2012, 28, 11590–11598. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Chen, H.L. Undulating the Lamellar Interface of Polymer–Surfactant Complex by Dendrimer. Macromolecules 2017, 50, 6501–6508. [Google Scholar] [CrossRef]
- Li, Z.; Fan, S.-F.; Chen, T.; Jin, L.Y. Organization of Coil–Rod–Coil Triblock Copolymers into Columnar Aggregates. Acta Polym. Sin. 2016, 204–210. [Google Scholar] [CrossRef]
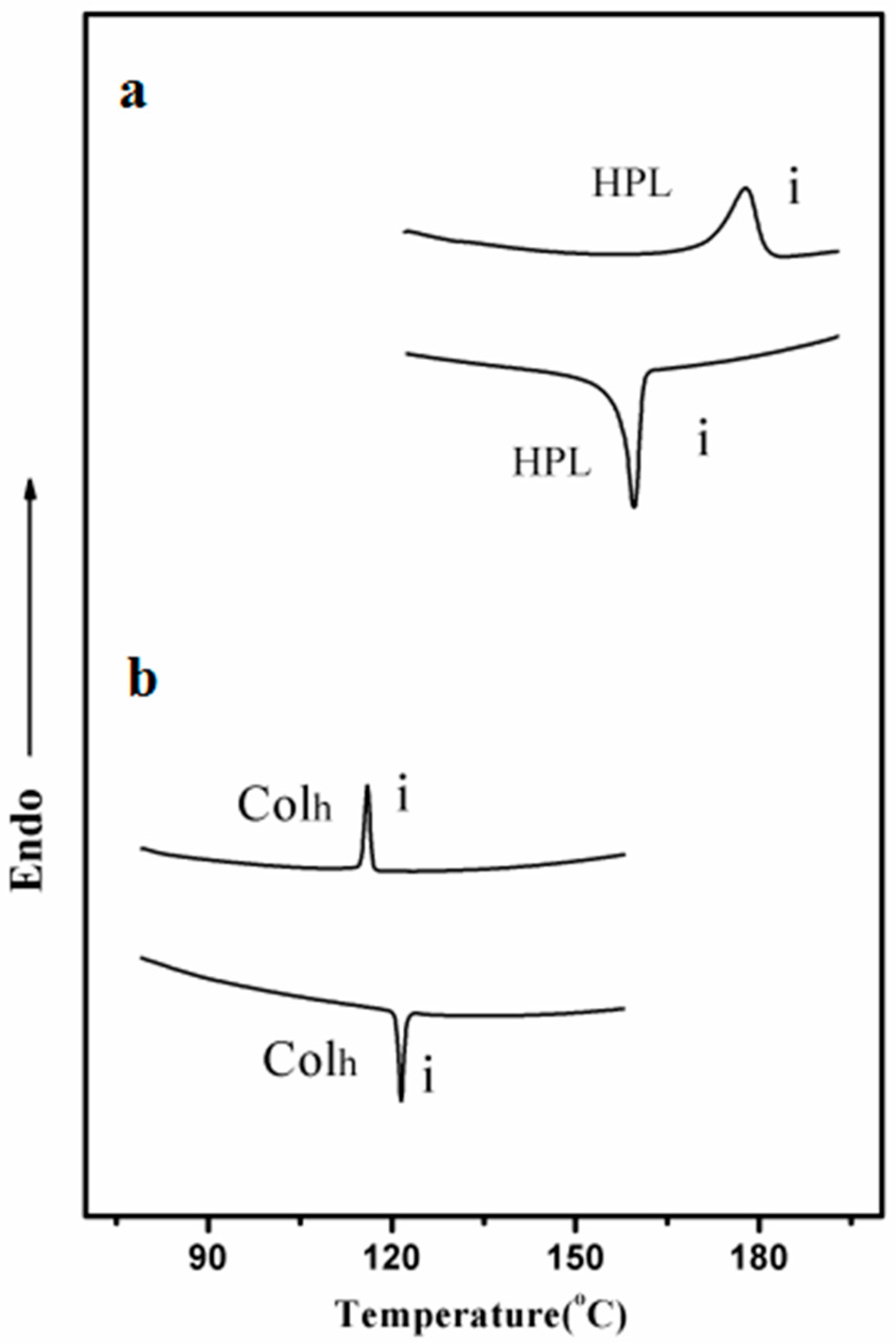
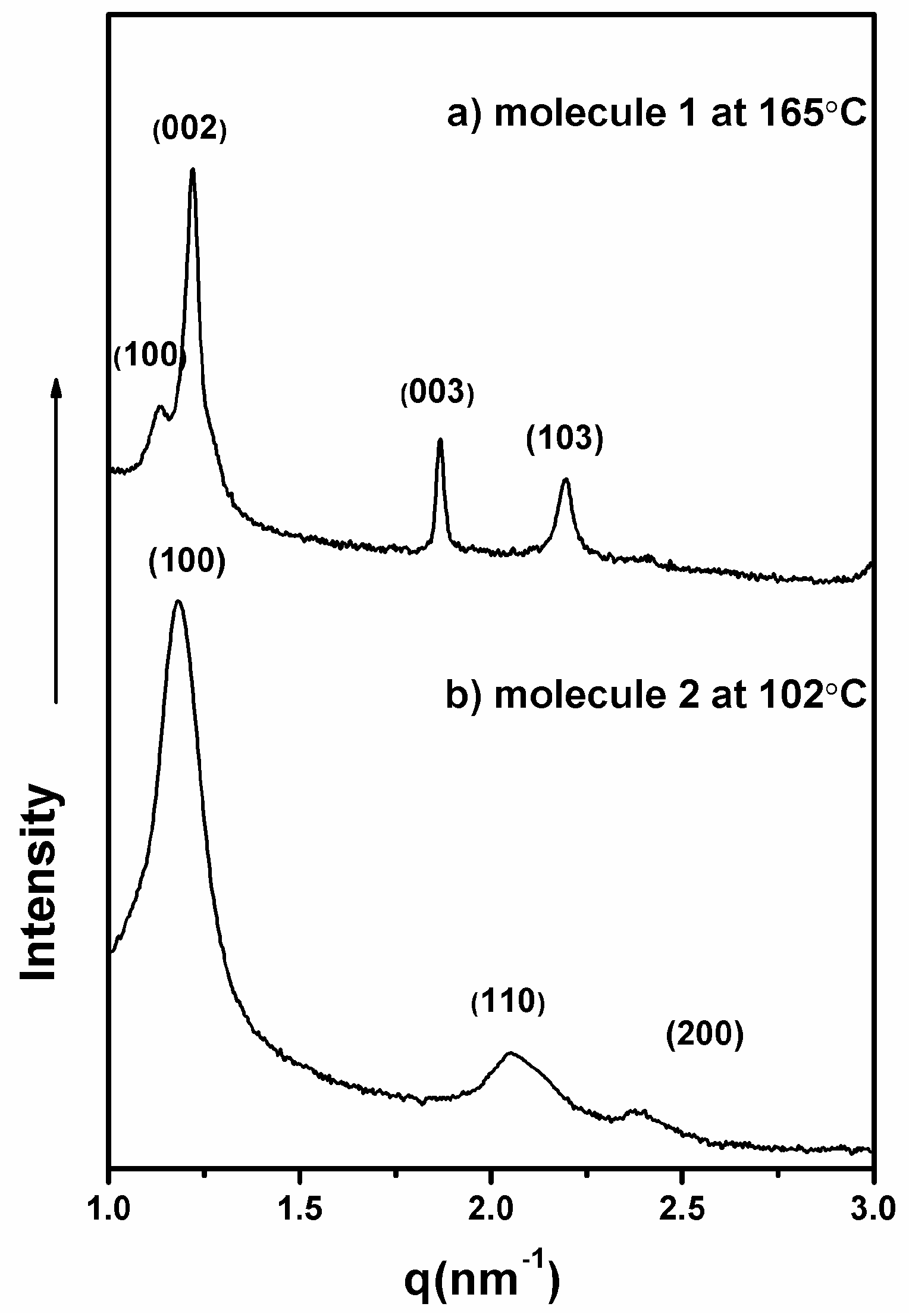
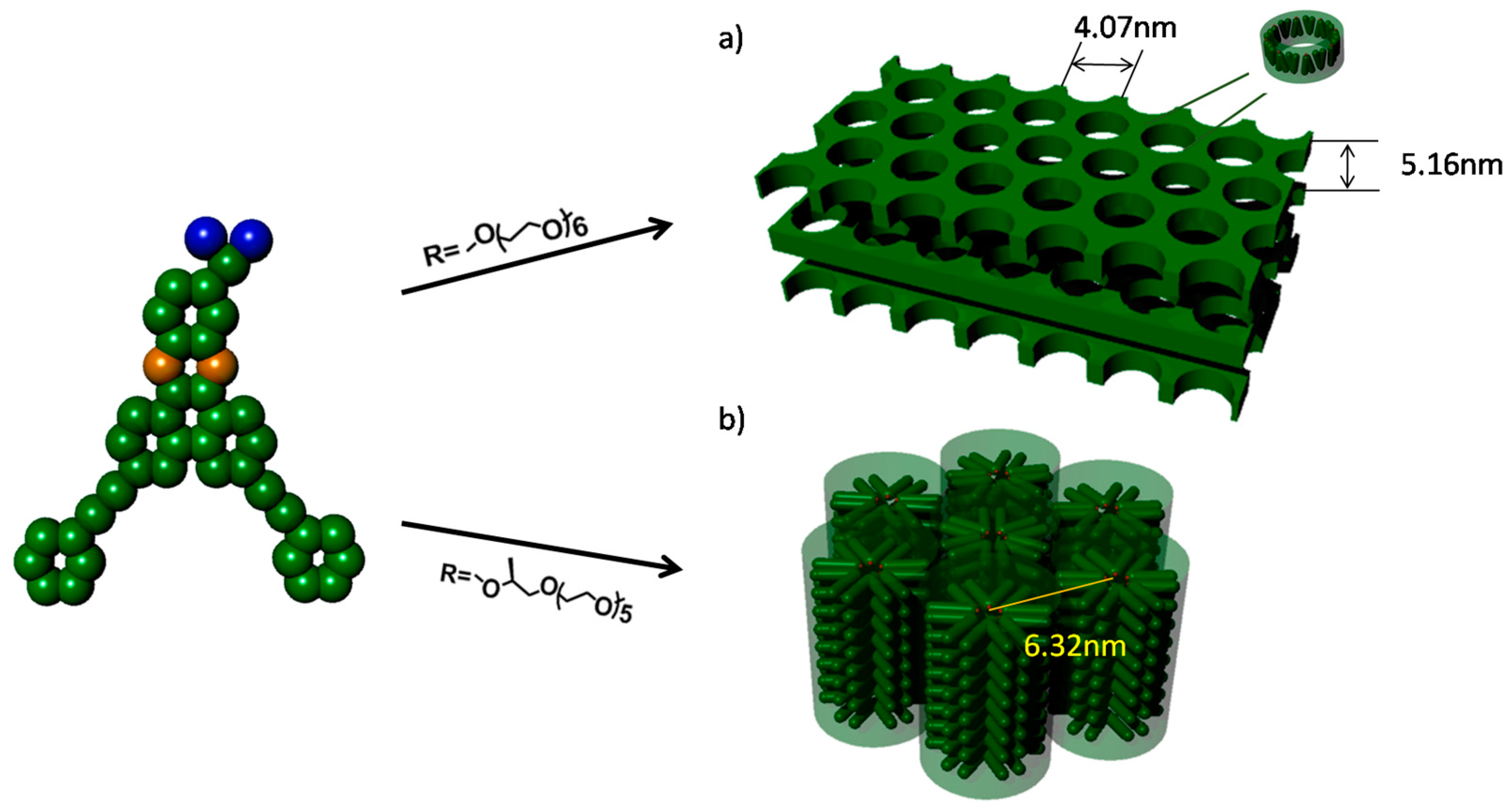
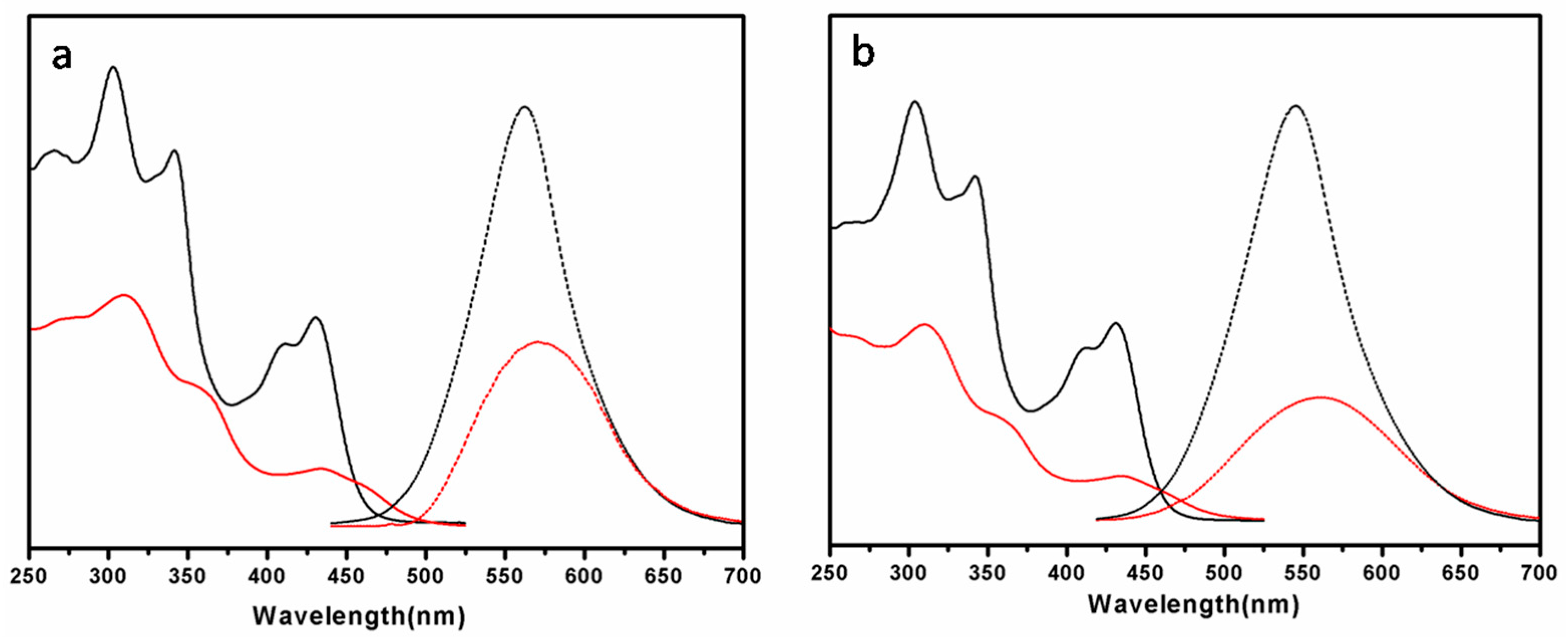
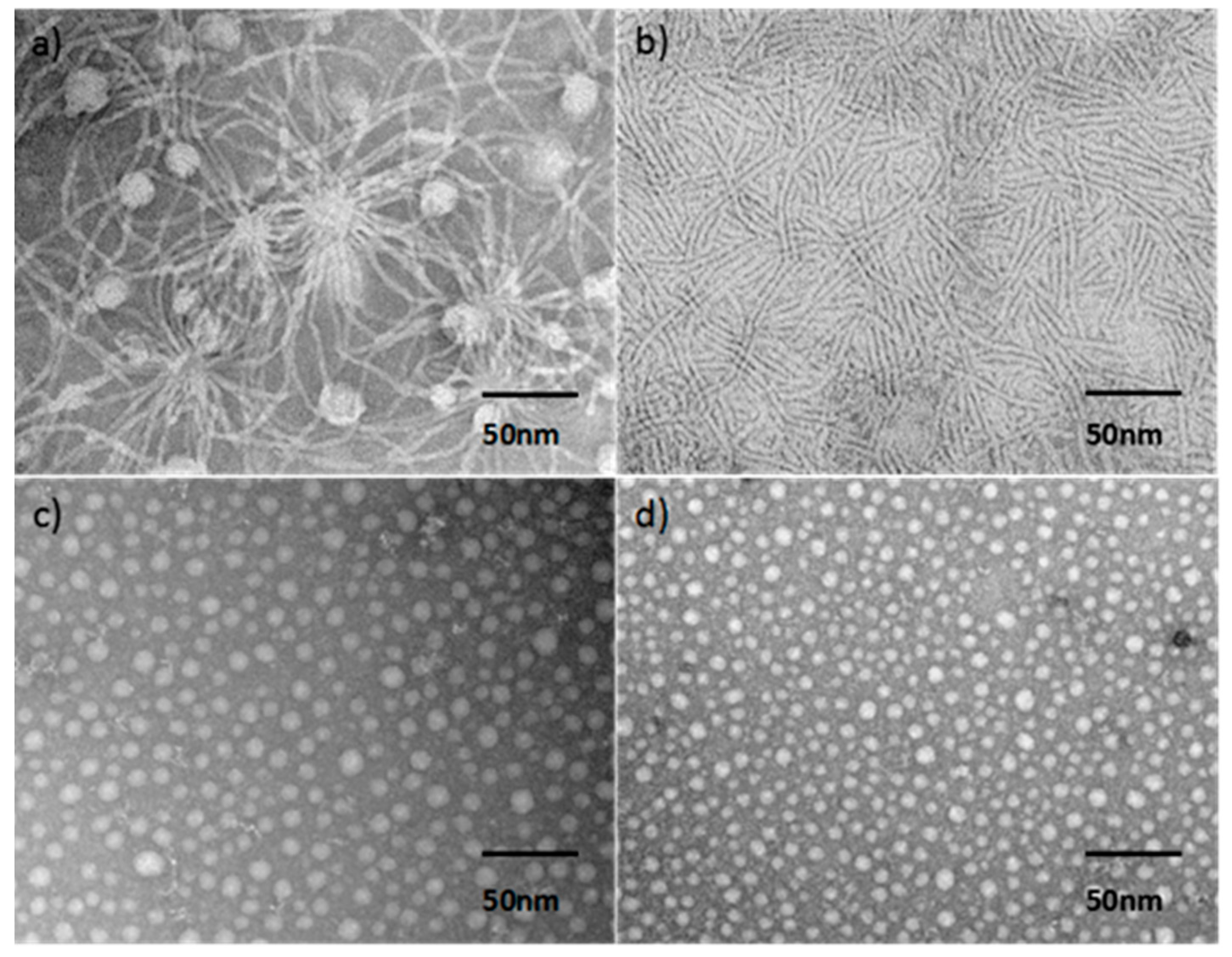
| Molecule | fcoila | Phase transition (°C) | |
|---|---|---|---|
| Heating | Cooling | ||
| 1 | 0.53 | HPL177i | i159HPL |
| 2 | 0.54 | Colh115i | i120Colh |
| 3 | 0.50 | —— | —— |
| 4 | 0.52 | —— | —— |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Yu, S.; Zhong, K.; Jin, L.Y. Construction of Supramolecular Nanostructures from V-Shaped Amphiphilic Rod-Coil Molecules Incorporating Phenazine Units. Polymers 2017, 9, 685. https://doi.org/10.3390/polym9120685
Xu J, Yu S, Zhong K, Jin LY. Construction of Supramolecular Nanostructures from V-Shaped Amphiphilic Rod-Coil Molecules Incorporating Phenazine Units. Polymers. 2017; 9(12):685. https://doi.org/10.3390/polym9120685
Chicago/Turabian StyleXu, Junying, Shengsheng Yu, Keli Zhong, and Long Yi Jin. 2017. "Construction of Supramolecular Nanostructures from V-Shaped Amphiphilic Rod-Coil Molecules Incorporating Phenazine Units" Polymers 9, no. 12: 685. https://doi.org/10.3390/polym9120685