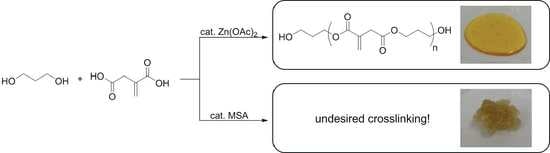
Catalyst Influence on Undesired Side Reactions in the Polycondensation of Fully Bio-Based Polyester Itaconates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
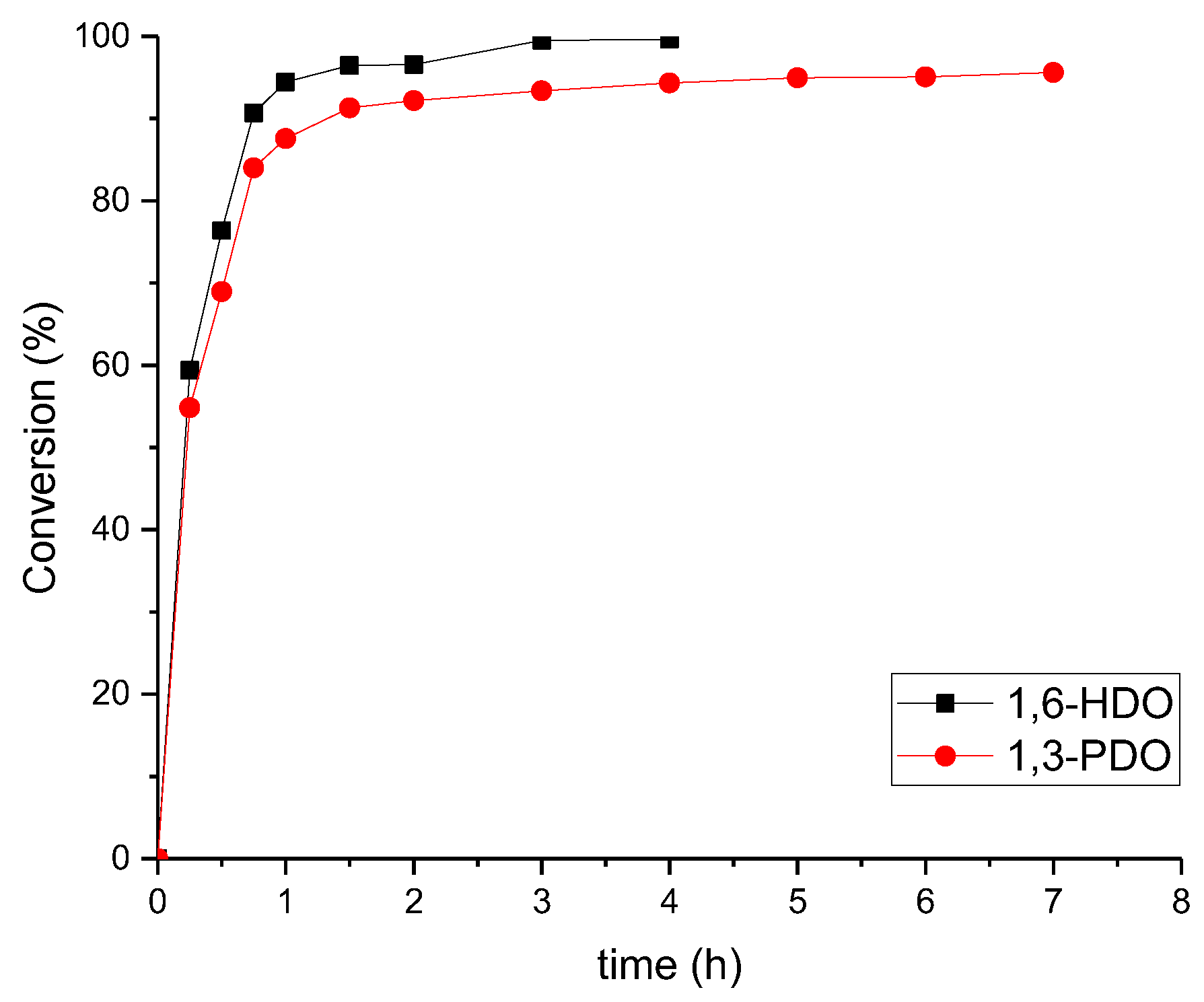
2.3. Conversion
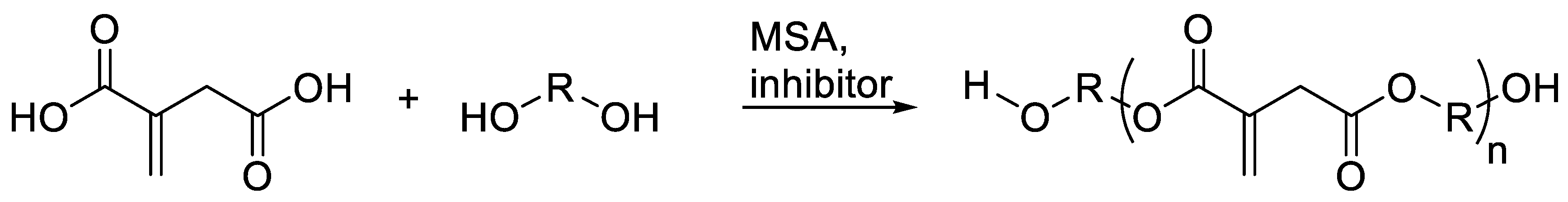
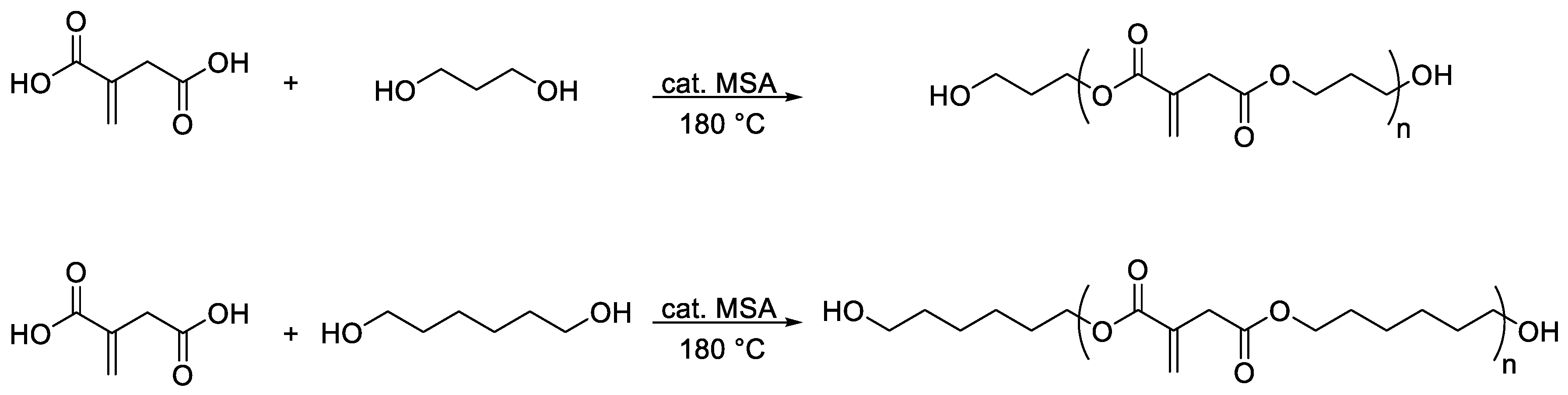
2.4. Synthesis of Polyester Itaconates
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ulber, R.; Muffler, K.; Tippkötter, N.; Hirth, T.; Sell, D. Introduction to renewable resources in the chemical industry. In Renewable Raw Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 1–5. [Google Scholar]
- Belgacem, M.N.; Gandini, A. Monomer, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfutzenreuter, R.; Rose, M.; Palkovits, R. Alternative monomers based on lignocellulose and their use for polymer production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef] [PubMed]
- Cavani, F.; Albonetti, S.; Basile, F.; Gandini, A. Chemicals and Fuels from Bio-Based Building Blocks; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Schneiderman, D.K.; Hillmyer, M.A. 50th anniversary perspective: There is a great future in sustainable polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US department of energy’s “top 10” revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Hernandez, N.; Williams, R.C.; Cochran, E.W. The battle for the “green” polymer. Different approaches for biopolymer synthesis: Bioadvantaged vs. Bioreplacement. Org. Biomol. Chem. 2014, 12, 2834–2849. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K. Controlled/living polymerization of renewable vinyl monomers into bio-based polymers. Polym. J. 2015, 47, 527–536. [Google Scholar] [CrossRef]
- Ishida, S.I.; Saito, S. Polymerization of itaconic acid derivatives. J. Polym. Sci. A 1967, 5, 689–705. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Ferguson, R. Glass and subglass transitions in a series of poly(itaconate ester)s with methyl-terminated poly(ethylene oxide) side-chains. J. Polym. Sci. B 1985, 23, 2181–2191. [Google Scholar] [CrossRef]
- Okada, S.; Matyjaszewski, K. Synthesis of bio-based poly(n-phenylitaconimide) by atom transfer radical polymerization. J. Polym. Sci. A 2015, 53, 822–827. [Google Scholar] [CrossRef]
- Bednarz, S.; Blaszczyk, A.; Blazejewska, D.; Bogdal, D. Free-radical polymerization of itaconic acid in the presence of choline salts: Mechanism of persulfate decomposition. Catal. Today 2015, 257, 297–304. [Google Scholar] [CrossRef]
- Lopez-Carrasquero, F.; de Ilarduya, A.M.; Cardenas, M.; Carrillo, M.; Arnal, M.L.; Laredo, E.; Torres, C.; Mendez, B.; Muller, A.J. New comb-like poly(n-alkyl itaconate)s with crystalizable side chains. Polymer 2003, 44, 4969–4979. [Google Scholar] [CrossRef]
- Tomić, S.L.; Dimitrijević, S.I.; Marinković, A.D.; Najman, S.; Filipović, J.M. Synthesis and characterization of poly(2-hydroxyethyl methacrylate/itaconic acid) copolymeric hydrogels. Polym. Bull. 2009, 63, 837–851. [Google Scholar] [CrossRef]
- Robert, T.; Friebel, S. Itaconic acid—A versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar] [CrossRef]
- Patil, D.M.; Phalak, G.A.; Mhaske, S.T. Design and synthesis of bio-based UV curable pu acrylate resin from itaconic acid for coating applications. Des. Monomers Polym. 2017, 20, 269–282. [Google Scholar] [CrossRef]
- Mehtiö, T.; Anghelescu-Hakala, A.; Hartman, J.; Kunnari, V.; Harlin, A. Crosslinkable poly(lactic acid)-based materials: Biomass-derived solution for barrier coatings. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Costa, C.S.M.F.; Marques, T.M.P.; Coelho, J.F.J.; Serra, A.C. The impact of a designed lactic acid-based crosslinker in the thermochemical properties of unsaturated polyester resins/nanoprecipitated calcium carbonate composites. J. Mater. Sci. 2017, 52, 1272–1284. [Google Scholar] [CrossRef]
- Brännström, S.; Malmström, E.; Johansson, M. Biobased UV-curable coatings based on itaconic acid. J. Coat. Technol. Res. 2017, 14, 851–861. [Google Scholar] [CrossRef]
- Hu, X.; Kang, H.; Li, Y.; Geng, Y.; Wang, R.; Zhang, L. Preparation, morphology and superior performances of biobased thermoplastic elastomer by in situ dynamical vulcanization for 3D-printed materials. Polymer 2017, 108, 11–20. [Google Scholar] [CrossRef]
- Friebel, S.; Biemans, T. Synthesis of Wood Coatings with Binder Resins Derived from Itaconic Acid; FKZ: 22020408; German Federal Ministry of Food and Agriculture through the Fachagentur für nachwachsende Rohstoffe e.V.: Bonn, Germany, 2013.
- Robert, T. Itaconic acid as renewable building block for UV-curing printing inks. In Proceedings of the European Coatings Show Conference, Nuremberg, Germany, 3 April 2017. [Google Scholar]
- Corici, L.; Pellis, A.; Ferrario, V.; Ebert, C.; Cantone, S.; Gardossi, L. Understanding potentials and restrictions of solvent-free enzymatic polycondensation of itaconic acid: An experimental and computational analysis. Adv. Synth. Catal. 2015, 357, 1763–1774. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; van Ekenstein, G.O.R.A.; Woortman, A.J.J.; Loos, K. Fully biobased unsaturated aliphatic polyesters from renewable resources: Enzymatic synthesis, characterization, and properties. Macromol. Chem. Phys. 2014, 215, 2185–2197. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Loos, K. Environmentally benign synthesis of saturated and unsaturated aliphatic polyesters via enzymatic polymerization of biobased monomers derived from renewable resources. Polym. Chem. 2015, 6, 5451–5463. [Google Scholar] [CrossRef]
- Pellis, A.; Corici, L.; Sinigoi, L.; D’Amelio, N.; Fattor, D.; Ferrario, V.; Ebert, C.; Gardossi, L. Towards feasible and scalable solvent-free enzymatic polycondensations: Integrating robust biocatalysts with thin film reactions. Green Chem. 2015, 17, 1756–1766. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Chen, Y.; Lei, Y.; Zhang, L.; Zhou, W.Y.; Rabie, A.B.M.; Zhao, J. Biobased poly(propylene sebacate) as shape memory polymer with tunable switching temperature for potential biomedical applications. Biomacromolecules 2011, 12, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, X.-M. Syntheses and physical characterization of biodegradable poly(butylene succinate-co-butylene itaconate) copolymers. J. Macromol. Sci. A 2015, 52, 745–751. [Google Scholar] [CrossRef]
- Farmer, T.; Castle, R.; Clark, J.; Macquarrie, D. Synthesis of unsaturated polyester resins from various bio-derived platform molecules. Int. J. Mol. Sci. 2015, 16, 14912. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Ma, S.; Liu, X.; Han, L.; Wu, Y.; Dai, X.; Zhu, J. Synthesis of bio-based unsaturated polyester resins and their application in waterborne UV-curable coatings. Prog. Org. Coat. 2015, 78, 49–54. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Wu, Y.; Han, L.; Zhang, L.; Zhu, J.; Liu, X. Polyesters derived from itaconic acid for the properties and bio-based content enhancement of soybean oil-based thermosets. Green Chem. 2015, 17, 2383–2392. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Wu, Y.; Zhu, J.; Liu, X. High bio-based content waterborne UV-curable coatings with excellent adhesion and flexibility. Prog. Org. Coat. 2015, 87, 197–203. [Google Scholar] [CrossRef]
- Ordelt, Z.; Krátký, B. Aufgabe der reaktionssteuerung bei der synthese von ungesättigten polyestern aus maleinsäureanhydrid. Farbe Lack 1969, 6, 523–531. [Google Scholar]
- Lehtonen, J.; Salmi, T.; Immonen, K.; Paatero, E.; Nyholm, P. Kinetic model for the homogeneously catalyzed polyesterification of dicarboxylic acids with diols. Ind. Eng. Chem. Res. 1996, 35, 3951–3963. [Google Scholar] [CrossRef]
- Ordelt, V.Z. Über die reaktion von glykolen mit der olefinischen doppelbindung bei der darstellung von ungesättigten polyestern durch schmelzkondensation. Die Makromol. Chem. 1963, 63, 153–161. [Google Scholar] [CrossRef]
- Malik, M.; Choudhary, V.; Varma, I.K. Current status of unsaturated polyester resins. J. Macromol. Sci. 2000, 40, 139–165. [Google Scholar] [CrossRef]
- Yang, Y.S.; Pascault, J.P. Modeling of unsaturated polyester prepolymer structures. I. Chain branches and overall chain end numbers. J. Appl. Polym. Sci. 1997, 64, 133–145. [Google Scholar] [CrossRef]
- Kharas, G.B.; Kamenetsky, M.; Simantirakis, J.; Beinlich, K.C.; Rizzo, A.-M.T.; Caywood, G.A.; Watson, K. Synthesis and characterization of fumarate-based polyesters for use in bioresorbable bone cement composites. J. Appl. Polym. Sci. 1997, 66, 1123–1137. [Google Scholar] [CrossRef]
- Carothers, W.H. Studies on polymerization and ring formation. I. An introduction to the general theory of condensation polymers. J. Am. Chem. Soc. 1929, 51, 2548–2559. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular size distribution in three dimensional polymers. I. Gelation1. J. Am. Chem. Soc. 1941, 63, 3083–3090. [Google Scholar] [CrossRef]
- Stauffer, D.; Coniglio, A.; Adam, M. Gelation and critical phenomena. In Polymer Networks; Dušek, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 103–158. [Google Scholar]
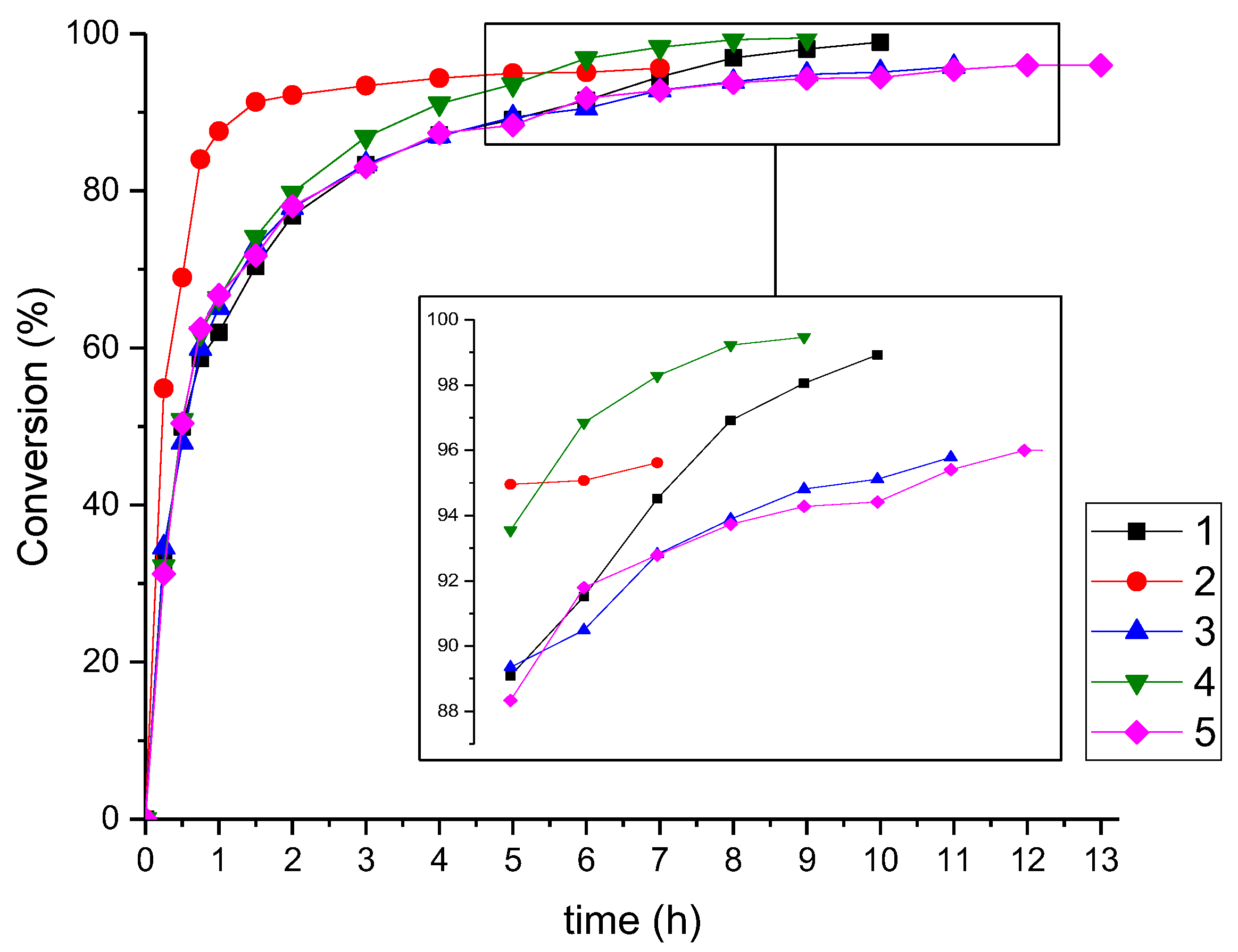


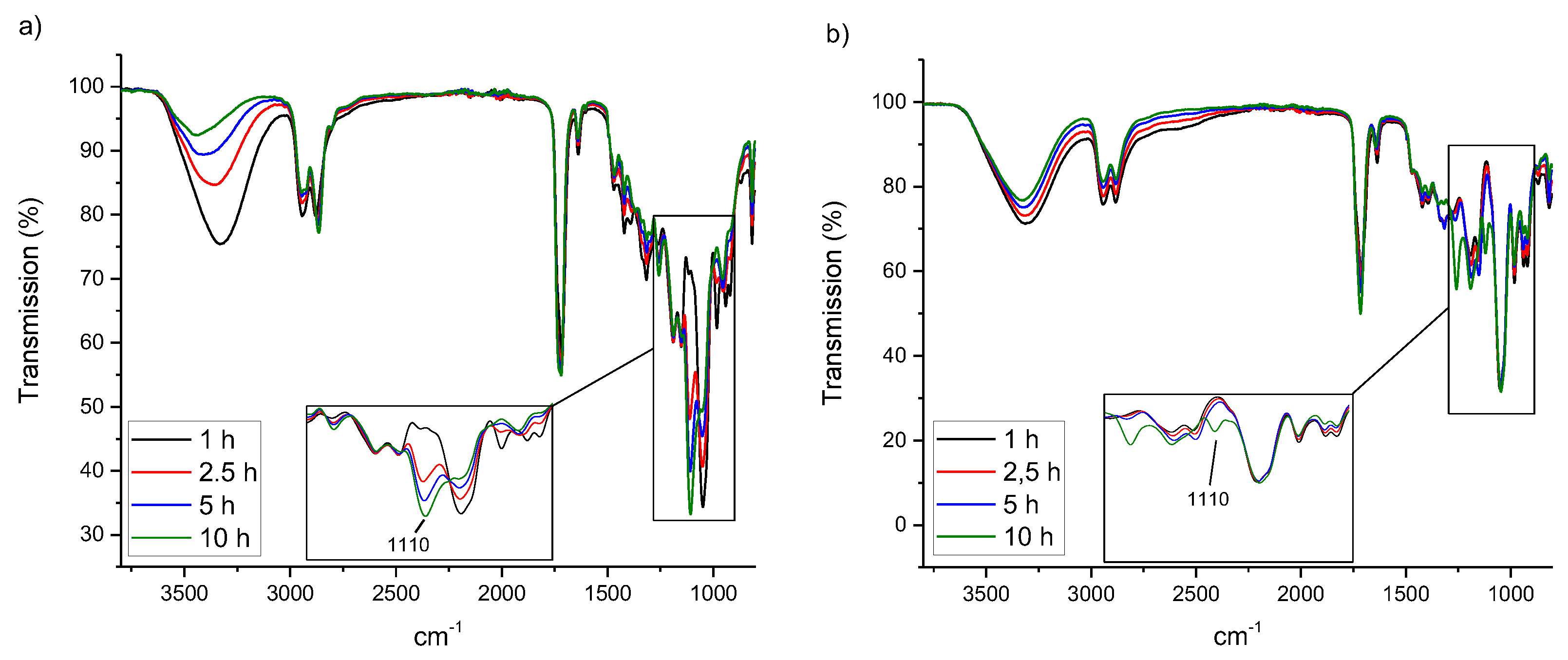
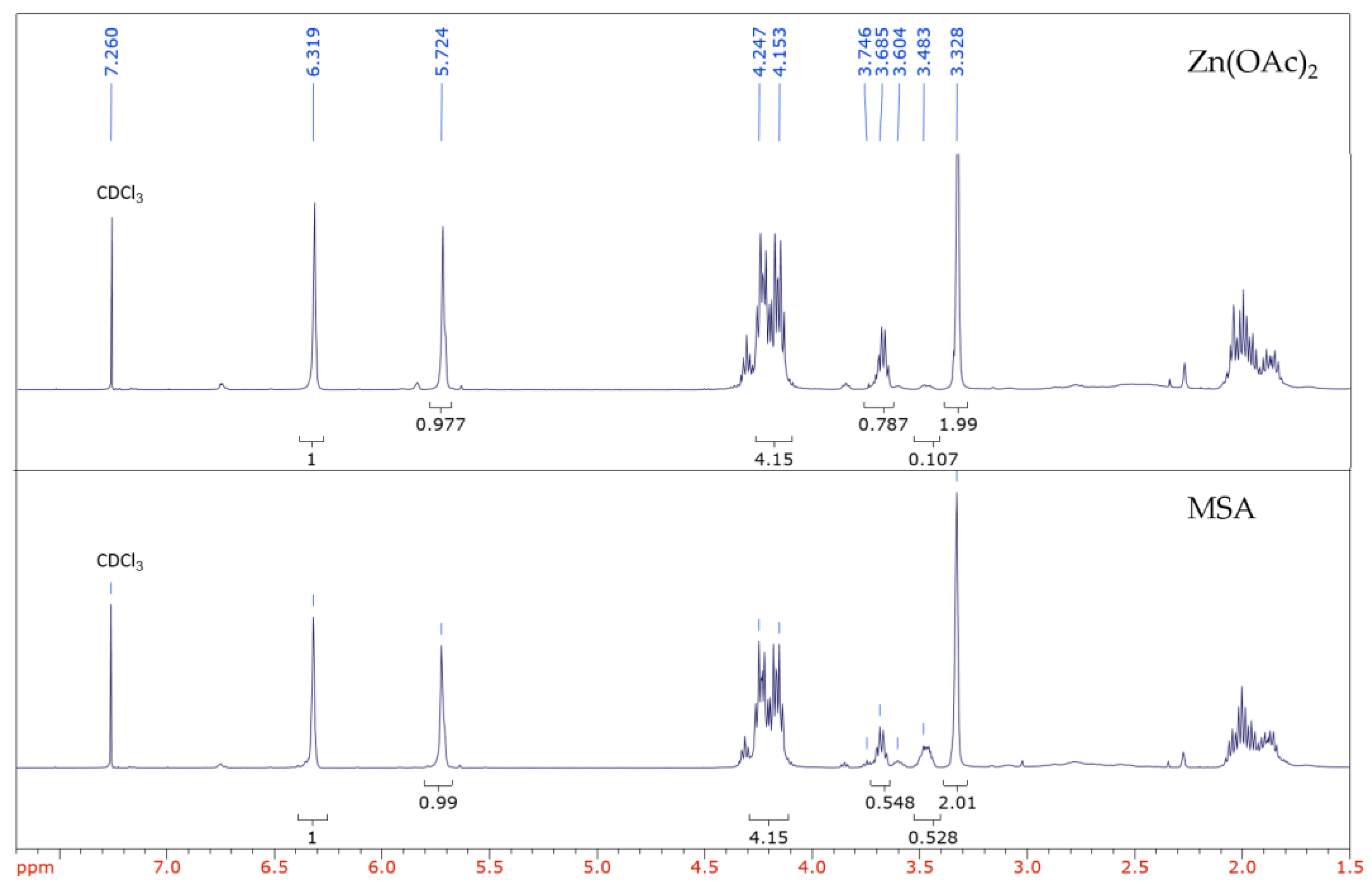
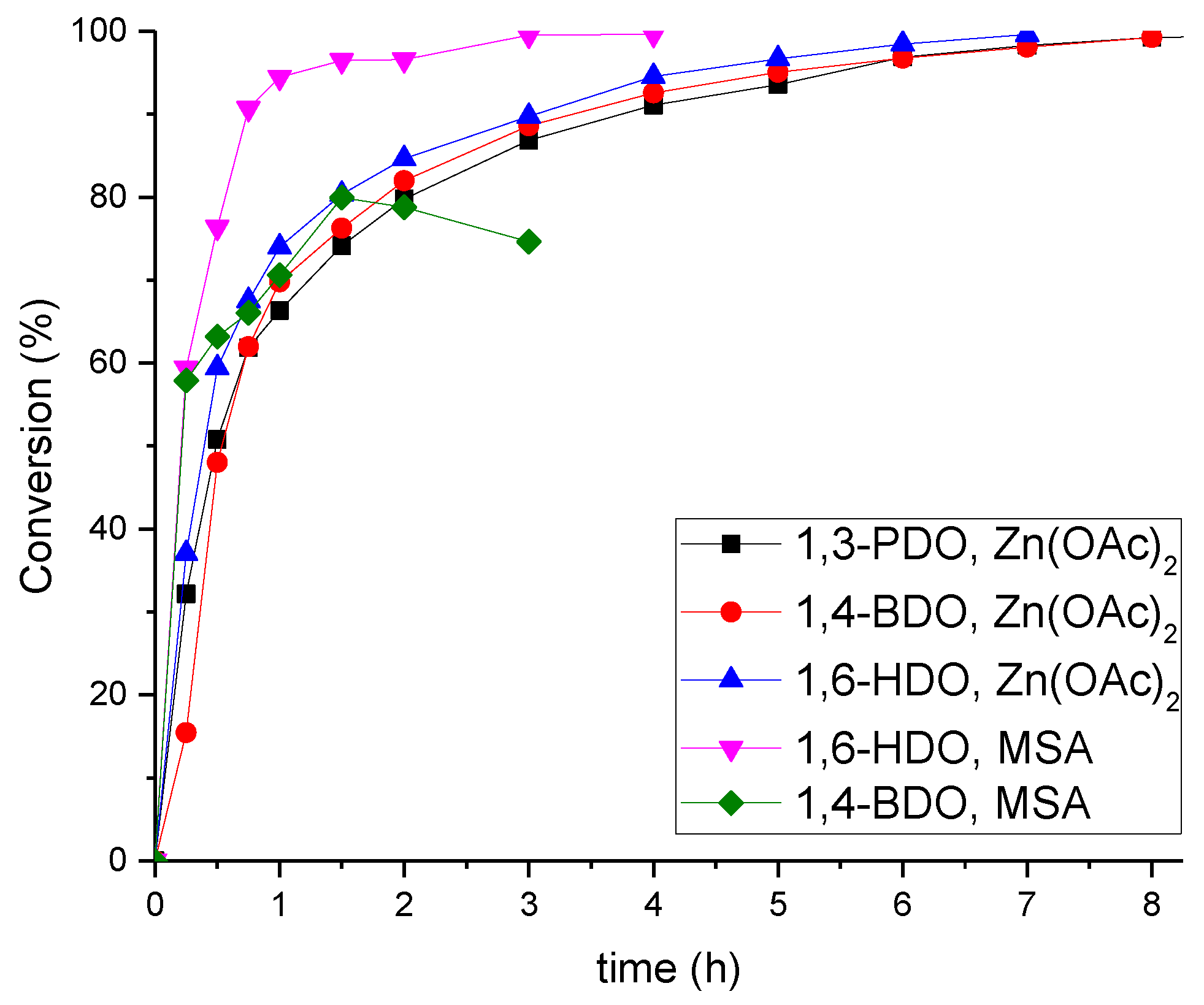
| Polyester | Catalyst | Conversion after 9 h |
|---|---|---|
| 1 | Ti(OBu)4 a | 98% |
| 2 | MSA | 96% b |
| 3 | MSA a | 96% c |
| 4 | Zn(OAc)2 | >99% |
| 5 | no catalyst | 94% |
| Polyester | Catalyst | t/h | Theoretical OHV [mg KOH/g] | Experimental OHV [mg KOH/g] | Δ OHV [mg KOH/g] |
|---|---|---|---|---|---|
| 1 | Ti(OBu)4 a | 10 | 153 | 101 | 52 |
| 2 | MSA | 7 | 169 | - b | - |
| 3 | MSA a | 11 | 168 | 68 | 100 |
| 4 | Zn(OAc)2 | 9 | 151 | 115 | 36 |
| 5 | No catalyst | 13 | 166 | 147 | 19 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoon, I.; Kluge, M.; Eschig, S.; Robert, T. Catalyst Influence on Undesired Side Reactions in the Polycondensation of Fully Bio-Based Polyester Itaconates. Polymers 2017, 9, 693. https://doi.org/10.3390/polym9120693
Schoon I, Kluge M, Eschig S, Robert T. Catalyst Influence on Undesired Side Reactions in the Polycondensation of Fully Bio-Based Polyester Itaconates. Polymers. 2017; 9(12):693. https://doi.org/10.3390/polym9120693
Chicago/Turabian StyleSchoon, Ina, Marcel Kluge, Steven Eschig, and Tobias Robert. 2017. "Catalyst Influence on Undesired Side Reactions in the Polycondensation of Fully Bio-Based Polyester Itaconates" Polymers 9, no. 12: 693. https://doi.org/10.3390/polym9120693