Efficient Partitioning of Assimilates in Stress-Tolerant Groundnut Genotypes under High-Temperature Stress
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Performance of Groundnut Genotypes under Heat Stress
3.2. Heat-Tolerant and Superior Pod-Yielding Groundnut Genotypes
4. Materials and Methods
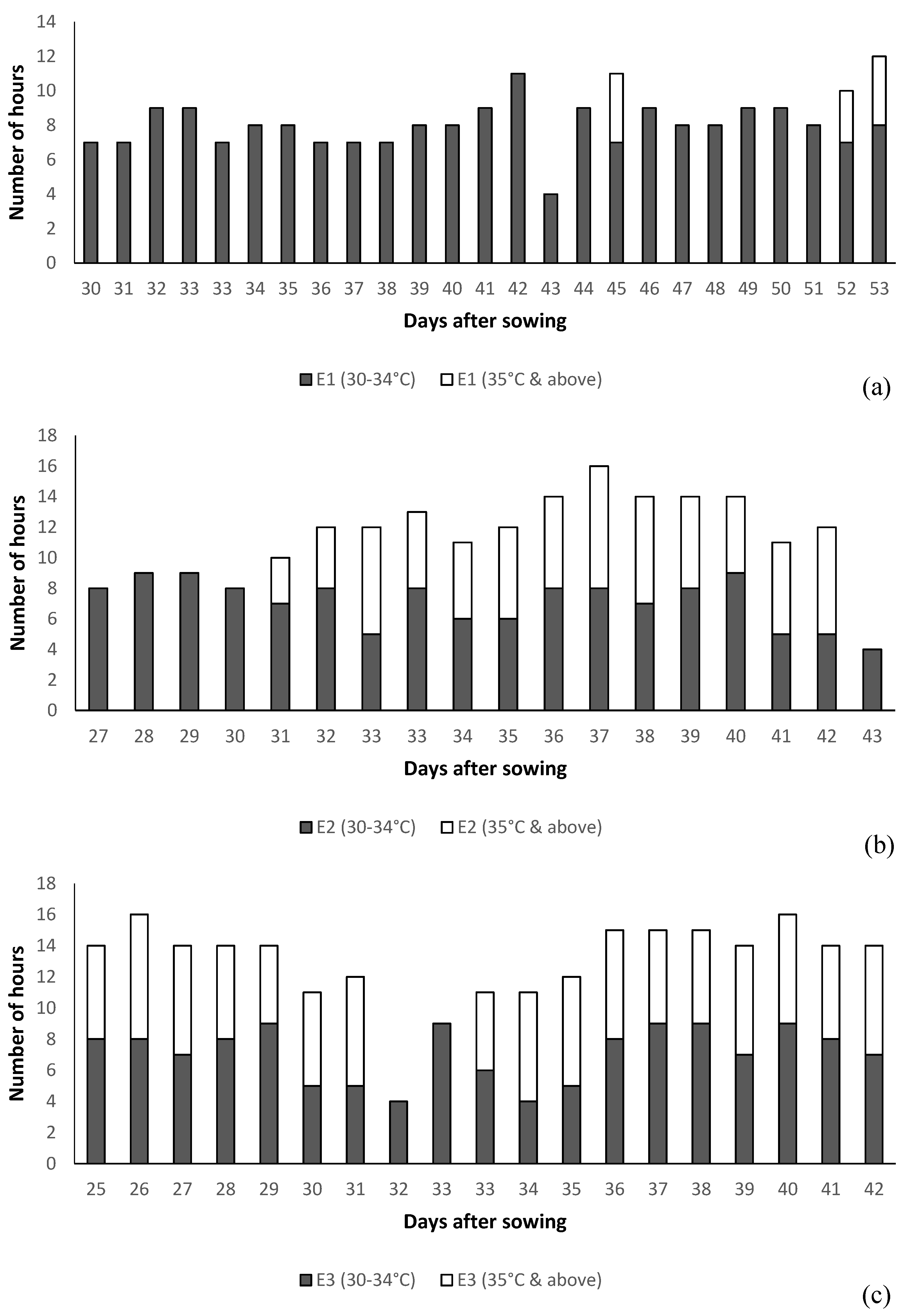
4.1. Experimental Conditions and Environments
4.2. Observations
4.3. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- FAOSTAT. 2014. Available online: http://faostat3.fao.org/ (accessed on 25 June 2016).
- Anonymous. All India Coordinated Research Project on Groundnut (Annual Report); ICAR-DGR: Sagdividi, India, 2014. [Google Scholar]
- Lobell, D.B.; Banziger, M.; Magorokosho, C.; Vivek, B. Nonlinear effects on African maize as evidenced by historical yield trials. Nat. Clim. Chang. 2011, 1, 42–45. [Google Scholar] [CrossRef]
- Kucharik, C.J.; Serbin, S.P. Impacts of recent climate change on Wisconsin corn and soybean yield trends. Environ. Res. Lett. 2008, 3, 034003. [Google Scholar] [CrossRef]
- Hundal, S.S.; Kaur, P. Climate change and its impact on crop productivity in Punjab, India. In Climate Variability and Agriculture; Abrol, Y.P., Gadgil, S., Pant, G.B., Eds.; Narosa Publishing House: New Delhi, India, 1996; pp. 377–393. [Google Scholar]
- Vara Prasad, P.V.; Craufurd, P.Q.; Summerfield, R.J. Fruit number in relation to pollen production and viability in groundnut exposed to short episodes of heat stress. Ann. Bot. 1999, 84, 381–386. [Google Scholar] [CrossRef]
- Paulsen, G.M. High temperature responses of crop plants. In Physiology and Determination of Crop Yield; Boote, K.J., Bennett, J.M., Sinclair, T.R., Paulsen, G.M., Eds.; American Society of Agronomy: Madison, WI, USA, 1994; pp. 365–389. [Google Scholar]
- Marshall, H.G. Breeding for tolerance to heat and cold. In Breeding Plants for Less Favourable Environments; Christiansen, M.N., Lewis, C.F., Eds.; John Wiley & Sons: New York, NY, USA, 1982; pp. 47–70. [Google Scholar]
- Ong, C.K. Agroclimatological factors affecting phenology of peanut. In Agrometeorology of Groundnut, Proceedings of An International Symposium, Niamey, Niger, 21–26 August 1985; International Crops Research Institute for the Semi-Arid Tropics: Patancheru, India, 1986; pp. 115–125. [Google Scholar]
- Craufurd, P.Q.; Prasad, P.V.; Kakani, G.V.; Wheeler, T.R.; Nigam, S.N. Heat tolerance in groundnut. Field Crop. Res. 2003, 80, 63–77. [Google Scholar] [CrossRef]
- Khattak, G.S.S.; Saeed, I.; Muhammad, T. Breeding for heat tolerance in mungbean (Vigna radiata (L.) Wilczek). Pak. J. Bot. 2006, 38, 1539–1550. [Google Scholar]
- Rehman, A.; Habib, I.; Ahmad, N.; Hussain, N.; Arif Khan, M.; Farooq, J.; Amjad, M. Screening wheat germplasm for heat tolerance at terminal growth stage. Plant Omics J. 2009, 2, 9–19. [Google Scholar]
- Devasirvatham, V.; Tan, D.K.Y.; Gaur, P.M.; Raju, T.N.; Trethowan, R.M. High temperature tolerance in chickpea and its implications for plant development. Crop Pasture Sci. 2012, 63, 419–428. [Google Scholar] [CrossRef]
- Moghaddam, A.; Hadizadeh, M.H. Study use of compression stress in drought stress tolerance varieties selection in maize (Zea mays L.). J. Crop Sci. 2000, 2, 25–38. [Google Scholar]
- Fernandez, G.J. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and other Food Crops in Temperature and Water Stress, Shanhua, Taiwan, 13–16 August 1992; pp. 257–270. [Google Scholar]
- Porch, T.G. Application of Stress Indices for Heat Tolerance Screening of Common Bean. J. Agron. Crop Sci. 2006, 192, 390–394. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Bell, M.J.; Bagnall, D.J.; Harch, G. Effect of photoperiod on reproductive development of peanut (Arachis hypogaea L.) in a cool subtropical environment. II. Temperature interactions. Aust. J. Agric. Res. 1991, 42, 1151–1161. [Google Scholar] [CrossRef]
- Bell, M.J.; Wright, G.C.; Harch, G. Environmental and agronomic effects on the growth of four peanut cultivars in a sub-tropical environment. II. Dry matter partitioning. Exp. Agric. 1993, 29, 491–501. [Google Scholar] [CrossRef]
- Nigam, S.N.; Nageswara Rao, R.C.; Wynne, J.C.; Williams, J.H.; Eitzner, M.; Nagabhushanam, G.V.S. Effect and interaction of temperature and photoperiod on growth and partitioning in three groundnut (Arachis hypogaea L.) genotypes. Ann. Appl. Biol. 1994, 125, 541–552. [Google Scholar] [CrossRef]
- Hamidou, F.; Halilou, O.; Vadez, V. Assessment of groundnut under Combined Heat and Drought Stress. J. Agron. Crop Sci. 2013, 199, 1–11. [Google Scholar] [CrossRef]
- Gangappa, E.; Ravi, N.; Veerakumar, G.N. Evaluation of groundnut (Arachis hypogaea L.) genotypes for temperature tolerance based on Temperature Induction Response (TIR) technique. Indian J. Genet. Plant Breed. 2006, 66, 127–130. [Google Scholar]
- Selvaraj, M.; Burow, G.B.; Burke, J.J.; Belamkar, V.; Puppala, N.; Burow, M. Heat stress screening of peanut seedlings for acquired thermotolerance. J. Plant Growth Regul. 2011, 65, 83–91. [Google Scholar] [CrossRef]
- Ntare, B.R.; Williams, J.H.; Dougbedji, J. Evaluation of groundnut genotypes for heat tolerance under field conditions in a Sahelian environment using a simple physiological model for yield. J. Agric. Sci. 2001, 136, 81–88. [Google Scholar] [CrossRef]
- Vara Prasad, P.V.; Craufurd, P.Q.; Summerfield, R.J.; Wheeler, T.R. Effect of short episodes of heat stress on flower production and fruit-set of groundnut (Arachis hypogaea L.). J. Exp. Bot. 2000a, 51, 777–784. [Google Scholar]
- Vara Prasad, P.V.; Craufurd, P.Q.; Summerfield, R.J. Sensitivity of peanut to timing of heat stress during reproductive development. Crop Sci. 1999, 39, 1352–1357. [Google Scholar] [CrossRef]
- Ketring, D.L. Temperature effects on vegetative and reproductive development of peanut. Crop Sci. 1984, 24, 877–882. [Google Scholar] [CrossRef]
- Golombek, S.D.; Johansen, C. Effect of soil temperature on vegetative and reproductive growth and development in three Spanish genotypes of peanut (Arachis hypogaea L.). Peanut Sci. 1997, 24, 67–72. [Google Scholar] [CrossRef]
- Ono, Y.; Nakayama, K.; Kubota, M. Effects of soil temperature and soil moisture in podding zone on pod development of peanut plants. Crop Sci. Soc. Jpn. 1974, 43, 247–251. [Google Scholar] [CrossRef]
- Greenberg, D.C.; Williams, J.H.; Ndunguru, B.J. Differences in yield determining processes of peanut (Arachis hypogaea) genotypes in varied drought environments. Ann. Appl. Biol. 1992, 120, 557–566. [Google Scholar] [CrossRef]
- Wheeler, T.R.; Chatzialioglou, A.; Craufurd, P.Q.; Ellis, R.H.; Summerfield, R.J. Dry matter partitioning in groundnut exposed to high temperature stress. Crop Sci. 1997, 37, 1507–1513. [Google Scholar] [CrossRef]
- Shinde, P.P.; Khanpara, M.D.; Vachhani, J.H.; Jivani, L.L.; Kachhadia, V.H. Genetic variability in virginia bunch groundnut (Arachis hypogaea L.). Plant Arch. 2010, 10, 703–706. [Google Scholar]
- Injeti, S.K.; Venkataravana, P.; Rao, M.R.G. Evaluation of new germplasm and advanced breeding lines of groundnut (Arachis hypogaea L.) under late kharif situation. Legum. Res. 2008, 31, 254–258. [Google Scholar]
- John, K.; Vasanthi, R.P.; Venkateswarlu, O. Variability and correlation studies for pod yield and its attributes in F2 generation of six Virginia × Spanish crosses of groundnut (Arachis hypogaea L.). Legume Res. 2007, 30, 292–296. [Google Scholar]
- Khote, A.C.; Bendle, V.W.; Bhave, S.G.; Patil, P.P. Genetic variability, heritability and genetic advance in some exotic genotype of groundnut (Arachis hypogaea L.). Crop Res. 2009, 37, 186–191. [Google Scholar]
- Mahalakshmi, P.; Manivannan, N.; Muralidharan, V. Variability and correlation studies in groundnut (Arachis hypogaea L.). Legume Res. 2005, 28, 194–197. [Google Scholar]
- Thirumalarao, V.; Venkanna, V.; Bhadru, D.; Bharathi, D. Studies on Variability, Character Association and Path Analysis on Groundnut (Arachis hypogaea L.). Int. J. Pure Appl. Bioscience 2014, 2, 194–197. [Google Scholar]
- Noubissie, T.J.B.; Njintang, N.Y.; Dolinassou, S. Heritability studies of Protein and oil contents in groundnut (Arachis hypogaea L.) genotypes. Int. J. Innovations Biol. Chem. Sci. 2012, 2, 162–171. [Google Scholar]
- Thakur, S.B.; Ghimire, S.K.; Chaudhary, N.K.; Shrestha, S.M.; Mishra, B. Genic variability, heritability and genic advance of pod-yield component traits of groundnut (Arachis hypogaea L.). J. Instant Agric. Anim. Sci. 2011, 32, 133–141. [Google Scholar]
- Craufurd, P.Q.; Wheeler, T.R.; Ellis, R.H.; Summerfield, J.; Vara Prasad, J.V. Escape and tolerance to high temperature at flowering in groundnut (Arachis hypogaea L.). J. Agric. Sci. 2000, 135, 371–378. [Google Scholar] [CrossRef]
- Vara Prasad, P.V.; Craufurd, P.Q.; Summerfield, R.J. Effect of high air and soil temperature on dry matter production, pod yield and yield components of groundnut. Plant Soil 2000b, 222, 231–239. [Google Scholar] [CrossRef]
- Chen, S.L.; Li, Y.R.; Xu, G.Z.; Cheng, Z.S. Simulation on Oil Accumulation characteristics in Different High-Oil Peanut Varieties. Acta Agron. Sin. 2008, 34, 142–149. [Google Scholar] [CrossRef]
- Chi, X.; Hu, R.; Zhang, H.; Chen, M.; Chen, N.; Pan, L.; Wang, T.; Wang, M.; Wang, Z.; Wang, Q.; et al. Cloning and functional analysis of three diacylglycerol acyltransferase genes from peanut (Arachis hypogaea L.). PLoS ONE 2014, 9, e105834. [Google Scholar] [CrossRef] [PubMed]
- Ndunguru, B.J.; Ntare, B.R.; Williams, J.H.; Greenberg, D.C. Assessment of groundnut cultivars for end-of-season drought tolerance in a Sahelian environment. J. Agric. Sci. 1995, 125, 79–85. [Google Scholar] [CrossRef]
- Rahmatollah, K.; Mohtasham, M.; Naser, S.; Mohammad, K.S. Using different aspects of stability concepts for interpreting genotype by environment interaction of some lentil genotypes. Aust. J. Crop Sci. 2012, 6, 1017–1023. [Google Scholar]
- Finlay, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef]
- Wood, I.M.W. The effect of temperature at early flowering on the growth and development of peanuts (Arachis hypogaea). Aust. J. Agric. Res. 1968, 19, 241–251. [Google Scholar] [CrossRef]
- Vindhiyavarman, P.; Nigam, S.N.; Janila, P.; Vaidhyalingan, M.; Manivannan, N.; Saravanan, S.; Meenakumari, B.; Gopalakrishnan, C.; Kennedy, J.S. A new high yielding Spanish bunch groundnut variety CO 7 (ICGV 00351) for the drought prone areas of Tamil Nadu. Electron. J. Plant Breed. 2014, 5, 192–196. [Google Scholar]
- Janila, P.; Nigam, S.N.; Abhishek, R.; Kumar, V.A.; Manohar, S.S.; Venuprasad, R. Iron and zinc concentrations in peanut (Arachis hypogaea L.) seeds and their relationship with other nutritional and yield parameters. J. Agric. Sci. 2014, 153, 975–994. [Google Scholar] [CrossRef]
- Duncan, W.G.; Mccloud, D.E.; Mcgraw, R.L.; Boote, K.J. Physiological aspects of peanut yield improvement. Crop Sci. 1978, 18, 1015–1020. [Google Scholar] [CrossRef]
- Sundaram, J.; Kandala, C.V.; Holser, R.A.; Butts, C.L.; Windham, W.R. Determination of in-shell peanut oil and fatty acid composition using near infrared reflectance spectroscopy. J. Am. Oil Chem. Soc. 2010, 87, 1103–1114. [Google Scholar] [CrossRef]
- Williams, J.H.; Saxena, N.P. The use of non-destructive measurement and physiological models of yield determination to investigate factors affecting differences in seed yield between genotypes of ‘desi’ chickpeas (Cicer arietum). Ann. Appl. Biol. 1991, 119, 105–112. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat (Triticum aestivum L.) cultivars. I Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Allard, R.W. Principles of Plant Breeding; John Wiley & Sons Inc.: New York, NY, USA, 1960; pp. 1–485. [Google Scholar]
- VSN International. GenStat, 15th ed.; VSN International: Indore, India, 2012. [Google Scholar]
| Source of Variation | df | HSW | PYH | SHP | HI | CGR | DF | DH | KYH | OC | PGR | SMK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Environment 1 | ||||||||||||
| Genotype | 61 | 108.4 * | 2,384,353 * | 54.4 * | 91.5 * | 58.0 * | 18.3 * | 64.5 * | 1,067,839 * | 12.8 * | 81.1 * | 24.2 * |
| Error | 61 | 24.4 | 597,880 | 31.9 | 21.7 | 13.2 | 1.6 | 4.5 | 398,066 | 2.0 | 27.9 | 13.8 |
| Environment 2 | ||||||||||||
| Genotype | 61 | 61.4 * | 2,378,533 * | 60.7 * | 106.6 * | 44.2 NS | 4.5 * | 231.1 * | 903,795 * | 12.9 * | 57.1 * | 61.8 * |
| Error | 61 | 8.7 | 512,739 | 17.8 | 28.9 | 25.4 | 0.5 | 20.4 | 229,684 | 2.3 | 19.9 | 26.3 |
| Environment 3 | ||||||||||||
| Genotype | 61 | 99.6 * | 2,380,653 * | 84.5 * | 120.0 * | 60.2 * | 3.9 * | 268.1 * | 992,383 * | 15.2 * | 88.1 * | 43.1 NS |
| Error | 61 | 18.1 | 608,927 | 28.2 | 25.1 | 20.6 | 0.9 | 47.3 | 301,551 | 2.8 | 19.3 | 24.3 |
| Source of Variation | df | HSW | PYH | SHP | HI | CGR | DF | DH | KYH | OC | PGR | SMK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | 61 | 229.9 * | 6,182,834 * | 136.5 * | 262.4 * | 114.3 * | 21.4 * | 473.6 * | 2,426,473 * | 36.4 * | 172.4 * | 53.7 * |
| Environment | 2 | 1253.2 * | 11,292,308 * | 1215.5 * | 1025.6 * | 249.1 * | 2564.2 * | 3071.1 * | 9,160,152 * | 14.2 NS | 464.4 * | 230.3 * |
| Genotype × Environment | 122 | 21.4 NS | 495,882 NS | 31.7 NS | 28.8 NS | 24.9 NS | 4.3 * | 44.5 * | 270,253 NS | 2.3 NS | 26.7 NS | 36.3 NS |
| Trait | Environment 1 (E1) | Environment 2 (E2) | Environment 3 (E3) | |||
|---|---|---|---|---|---|---|
| Range | H (%) | Range | H (%) | Range | H (%) | |
| Days to 75% flowering (days) | 35–50 | 92.5 | 31–39 | 89.9 | 29–37 | 80.5 |
| Days to maturity (days) | 123–139 | 92.9 | 105–139 | 91 | 101–139 | 95.6 |
| Pod Yield (kg ha−1) | 2205–6761 | 77.6 | 2034–6767 | 80 | 1483–6180 | 76.5 |
| Hundred Kernel Weight (g) | 27–59 | 79.8 | 21–44 | 85.1 | 17–52 | 84.5 |
| Oil Content (%) | 48.6–58.8 | 84.4 | 48.1–58.4 | 81.6 | 48.2–61.4 | 84.3 |
| Harvest Index (%) | 33.2–65.2 | 76 | 31.7–62.6 | 71.9 | 24.6–64.6 | 80.2 |
| Crop Growth Rate (g m−2 day−1) | 5.7–14.6 | 85.3 | 8.4–15.7 | 51.1 | 7.4–15.9 | 61.6 |
| Pod Growth Rate (g m−2 day−1) | 4.9–13.4 | 69.7 | 5.7–12.8 | 67.9 | 3.1–13.2 | 75.8 |
| Genotypes | CV (%) |
|---|---|
| ICGV 07356, ICGV 97183, GG 20, Abhaya, K 6, J 11, TPG 41, ICGV 06175, ICGV 06424, ICGV 99001, GPBD 4 | 20–27.5 |
| Chico, GJG 31, ICGV 03109, ICGV 05155, ICGV 86325, ICGV 91114, ICGV 02271, ICGV 00308, ICGV 96346, ICGV 93468, ICGV 92035, ICGV 01232, ICGV 07148, VRI 6, ICGS 11, ICGV 07268, ICGV 87846 , ICGV 07012, ICGV 95390, ICGV 07246, ICGV 07038, ICGV 06099, ICGV 05200, ICGV 03057, JL 24, ICGV 07273, ICGV 07217, ICGV 06040 | 10–19.9 |
| ICGV 92195, ICGV 07456, ICGV 07213, ICGV 07211, ICGV 98294, ICGV 00350, ICGV 00351, ICGV 07013, ICGV 06039, ICGV 05032, TG 37, ICGV 89280, ICGV 02266, TMV 2, ICGV 00298, TAG 24, ICGV 03042 | 5–9.9 |
| TCGS 1043, ICGV 97182, ICGV 87141, ICGV 87128, ICGV 06420 | <5 |
| Genotypes | Stress Tolerance Index (E2) | Stress Tolerance Index (E3) |
|---|---|---|
| ICGV 99001, ICGV 86325, TMV 2, JL 24, ICGV 02271, GG 20, ICGV 07456, ICGV 91114, GPBD 4, K 6, ICGV 00298, ICGV 87141, ICGV 92195, Chico, GJG 31, J 11, ICGV 00308, ICGV 07211, ICGV 87128, ICGV 98294, VRI 6, ICGV 06175, ICGV 07356, ICGV 87846, ICGV 93468, ICGV 95390, ICGV 96346, TCGS 1043, Abhaya, ICGS 11, ICGV 07217, TPG 41 | 0.2–0.9 | 0.2–0.8 |
| ICGV 92035, ICGV 97183, ICGV 05200 | 1.0–1.1 | 0.7–0.9 |
| ICGV 97182, TG 37, ICGV 01232, ICGV 07013, ICGV 07213, ICGV 89280, TAG 24, ICGV 00350, ICGV 03057, ICGV 06420, ICGV 02266, ICGV 03109, ICGV 06099, ICGV 07273, ICGV 00351, ICGV 07268, ICGV 06039, ICGV 07148, ICGV 03042, ICGV 05032, ICGV 07038, ICGV 05155, ICGV 06040, ICGV 07012, ICGV 06424, ICGV 07246 | 1.1–2.1 | 1.0–1.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, A.; Singh Manohar, S.; Tottekkaad Variath, M.; Kurapati, S.; Pasupuleti, J. Efficient Partitioning of Assimilates in Stress-Tolerant Groundnut Genotypes under High-Temperature Stress. Agronomy 2017, 7, 30. https://doi.org/10.3390/agronomy7020030
Akbar A, Singh Manohar S, Tottekkaad Variath M, Kurapati S, Pasupuleti J. Efficient Partitioning of Assimilates in Stress-Tolerant Groundnut Genotypes under High-Temperature Stress. Agronomy. 2017; 7(2):30. https://doi.org/10.3390/agronomy7020030
Chicago/Turabian StyleAkbar, Ashna, Surendra Singh Manohar, Murali Tottekkaad Variath, Sadaiah Kurapati, and Janila Pasupuleti. 2017. "Efficient Partitioning of Assimilates in Stress-Tolerant Groundnut Genotypes under High-Temperature Stress" Agronomy 7, no. 2: 30. https://doi.org/10.3390/agronomy7020030






