The Effect of Landscape Composition on the Abundance of Laodelphax striatellus Fallén in Fragmented Agricultural Landscapes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Survey of Laodelphax striatellus
2.3. Assessment of Host Patches
2.4. Landscape Metrics
2.5. Data Analysis
3. Results
3.1. Density of Laodelphax striatellus
3.2. Statistical Measures of Crop-Habitat
3.3. Correlation between Landscape Metrics and L. striatellus Population
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burel, F.; Baudry, J. Landscape Ecology: Concepts, Methods, and Applications; Science Publishers: Enfield, UK, 2003. [Google Scholar]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2011, 14, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Puech, C.; Poggi, S.; Baudry, J.; Aviron, S. Do farming practices affect natural enemies at the landscape scale? Landsc. Ecol. 2015, 30, 125–140. [Google Scholar] [CrossRef]
- Lemessa, D.; Hambäck, P.A.; Hylander, K. The effect of local and landscape level land-use composition on predatory arthropods in a tropical agricultural landscape. Landsc. Ecol. 2015, 30, 167–180. [Google Scholar] [CrossRef]
- Bennett, A.F.; Radford, J.Q.; Haslem, A. Properties of land mosaics: Implications for nature conservation in agricultural environments. Biol. Conserv. 2006, 133, 250–264. [Google Scholar] [CrossRef]
- Loos, J.; Kuussaari, M.; Ekroos, J.; Hanspach, J.; Fust, P.; Jackson, L.; Fischer, J. Changes in butterfly movements along a gradient of land use in farmlands of Transylvania (Romania). Landsc. Ecol. 2015, 30, 625–635. [Google Scholar] [CrossRef]
- Duelli, P.; Obrist, M.K. Regional biodiversity in an agricultural landscape: The contribution of seminatural habitat islands. Basic Appl. Ecol. 2003, 4, 129–138. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.S.; Agarwal, M.L. Elements of Insect Ecology; Narosa Publishing House: London, UK, 1997. [Google Scholar]
- Overgaard, H.J.; Tsuda, Y.; Suwonkerd, W.; Takagi, M. Characteristics of Anopheles minimus (Diptera: Culicidae) larval habitats in northern Thailand. Environ. Entomol. 2002, 31, 134–141. [Google Scholar] [CrossRef]
- Overgaard, H.J.; Ekbom, B.; Suwonkerd, W.; Takagi, M. Effect of landscape structure on anopheline mosquito density and diversity in northern Thailand: Implications for malaria transmission and control. Landsc. Ecol. 2003, 18, 605–619. [Google Scholar] [CrossRef]
- Cheng, J.A. Rice planthopper problems and relevant causes in China. In Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; Heong, K.L., Hardy, B., Eds.; International Rice Research Institute: Los Baños, The Philippines, 2009; pp. 157–178. [Google Scholar]
- Bottrell, D.G.; Schoenly, K.G. Resurrecting the ghost of green revolution past: The brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J. Asia Pac. Entomol. 2012, 15, 122–140. [Google Scholar] [CrossRef]
- Cheng, J.A. Rice planthoppers in the past half century in China. In Rice Planthoppers: Ecology, Management, Social Economics and Policy; Heong, K.L., Cheng, J.A., Escalada, M.M., Eds.; Zhejiang University Press: Hangzhou, China, 2014; pp. 1–32. [Google Scholar]
- Sogawa, K. Planthopper outbreaks in different paddy ecosystems in Asia: Man-made hopper plagues that threatened the Green Revolution in rice. In Rice Planthoppers: Ecology, Management, Social Economics and Policy; Heong, K.L., Cheng, J.A., Escalada, M.M., Eds.; Zhejiang University Press: Hangzhou, China, 2014; pp. 33–64. [Google Scholar]
- Li, H.; Reynolds, J.F. A simulation experiment to quantify spatial heterogeneity in categorical map. Ecology 2015, 75, 2446–2455. [Google Scholar] [CrossRef]
- Riitters, K.H.; O’Neil, R.V.; Hunsaker, C.T.; Wickham, J.D.; Yankee, D.H.; Timmins, S.P.; Jones, K.B.; Jackson, B.L. A factor analysis of landscape pattern and structure metrics. Landsc. Ecol. 1995, 10, 23–39. [Google Scholar] [CrossRef]
- Hargis, C.D.; Bissonette, H.A.; David, J.L. The behavior of landscape metrics commonly used in the study of habitat fragmentation. Landsc. Ecol. 1998, 13, 167–186. [Google Scholar] [CrossRef]
- Grilli, M.P. The role of landscape structure on the abundance of a disease vector planthopper: A quantitative approach. Landsc. Ecol. 2010, 25, 383–394. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.; Ene, E. FRAGSTATS V4: Spatial Pattern Analysis Program for Categorical and Continuous Maps; University of Massachusetts: Amherst, MA, USA, 2012. [Google Scholar]
- Jiaxing Weather. Available online: http://www.jxqx.net/weather (accessed on 15 January 2011).
- Huang, J.K.; Deng, X.Z.; Rozelle, S. Cultivated land conversion and bioproductivity in China. Proc. SPIE 2004, 5544. [Google Scholar] [CrossRef]
- Cheng, X.N.; Wu, J.C.; Ma, F. Brown Planthopper: Occurrence and Control; Chinese Agriculture Press: Beijing, China, 2003. [Google Scholar]
- Anderson, J.R.; Hardy, E.E.; Roach, J.T.; Witmer, R.E. A Land-Use and Land-Cover Classification System for Use with Remote Sensor Data; USGS paper #964; The United States Geological Survey: Washington, DC, USA, 1976; p. 28. [Google Scholar]
- Eastman, R.J. IDRISI Andes Guide to GIS and Image Processing; Clark Labs Clark University: Worcester, MA, USA, 2006. [Google Scholar]
- Jensen, J.R. Introductory Digital Image Processing: A Remote Sensing Perspective, 3rd ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Ricci, B.; Franck, P.; Toubon, J.F.; Bouvier, J.C.; Sauphanor, B.; Lavigne, C. The influence of landscape on insect pest dynamics: A case study in southeastern France. Landsc. Ecol. 2009, 24, 337–349. [Google Scholar] [CrossRef]
- Grilli, M.; Gorla, D. The spatio-temporal pattern of Delphacodes kuscheli (Homoptera: Delphacidae) abundance in central Argentina. Bull. Entomol. Res. 1997, 87, 45–53. [Google Scholar] [CrossRef]
- Grilli, M.P. Effect of local land use on populations of a disease vector planthopper. Environ. Entomol. 2006, 35, 1254–1263. [Google Scholar] [CrossRef]
- Grilli, M.P.; Bruno, M.A. Regional abundance of a planthopper pest: The effect of host patch area and configuration. Entomol. Exp. Appl. 2007, 122, 133–143. [Google Scholar] [CrossRef]
- Grilli, M.P. Spatial synchrony of planthopper species with contrasting outbreak behavior. Agric. For. Entomol. 2008, 10, 307–314. [Google Scholar] [CrossRef]
- Forman, R.T.T.; Godron, M. Landscape Ecology; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Hanski, I.; Gilpin, M.E. Metapopulation Biology. Ecology, Genetics, and Evolution; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Chefaoui, R.M. Landscape metrics as indicators of coastal morphology: A multi-scale approach. Ecol. Indic. 2014, 45, 139–147. [Google Scholar] [CrossRef]
- Fahrig, L.; Jonsen, I. Effect of habitat patch characteristics on abundance and diversity of insects in an agricultural landscape. Ecosystems 1998, 1, 197–205. [Google Scholar] [CrossRef]
- Jackson, C.R.; Marnewick, K.; Lindsey, P.A.; Roskaft, E.; Robertson, M.P. Evaluating habitat connectivity methodologies: A case study with endangered African wild dogs in South Africa. Landsc. Ecol. 2016, 31, 1433–1447. [Google Scholar] [CrossRef]
- Lou, Y.G.; Hu, L.F.; Li, J.C. Herbivore-induced defenses in dice and their potential application in rice planthopper management. In Rice Planthoppers: Ecology, Management, Social Economics and Policy; Heong, K.L., Cheng, J.A., Escalada, M.M., Eds.; Zhejiang University Press: Hangzhou, China, 2014; pp. 91–116. [Google Scholar]
- Mõisja, K.; Uuemaa, E.; Oja, T. Integrating small-scale landscape elements into land use/cover: The impact on landscape metrics’ values. Ecol. Indic. 2014, 67, 714–722. [Google Scholar] [CrossRef]
- Heong, K.L.; Wong, L.; Reyes, J.H.D. Addressing planthopper threats to Asian rice farming and food security: Fixing insecticide misuse. In Rice Planthoppers: Ecology, Management, Social Economics and Policy; Heong, K.L., Cheng, J.A., Escalada, M.M., Eds.; Zhejiang University Press: Hangzhou, China, 2014; pp. 65–76. [Google Scholar]
- Liu, Z.W.; Wu, J.C.; Zhang, Y.X.; Liu, F.; Xu, J.X.; Bao, H.B. Mechanisms of Rice Planthopper Resistance to Insecticides. In Rice Planthoppers: Ecology, Management, Social Economics and Policy; Heong, K.L., Cheng, J.A., Escalada, M.M., Eds.; Zhejiang University Press: Hangzhou, China, 2014; pp. 117–142. [Google Scholar]
- Maisonhaute, J.É.; Peres-Neto, P.; Lucas, É. Influence of agronomic practices, local environment and landscape structure on predatory beetle assemblage. Agric. Ecosyst. Environ. 2010, 139, 500–507. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Thies, C.; Nentwig, W.; Tscharntke, T. Contrasting responses of arable spiders to the landscape matrix at different spatial scales. J. Biogeogr. 2008, 35, 157–166. [Google Scholar] [CrossRef]
- Karp, D.S.; Mendenhall, C.D.; Sand, R.F.; Chaumont, N.; Ehrlich, P.R.; Hadly, E.A.; Daily, G.C. Forest bolsters bird abundance, pest control and coffee yield. Ecol. Lett. 2013, 16, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
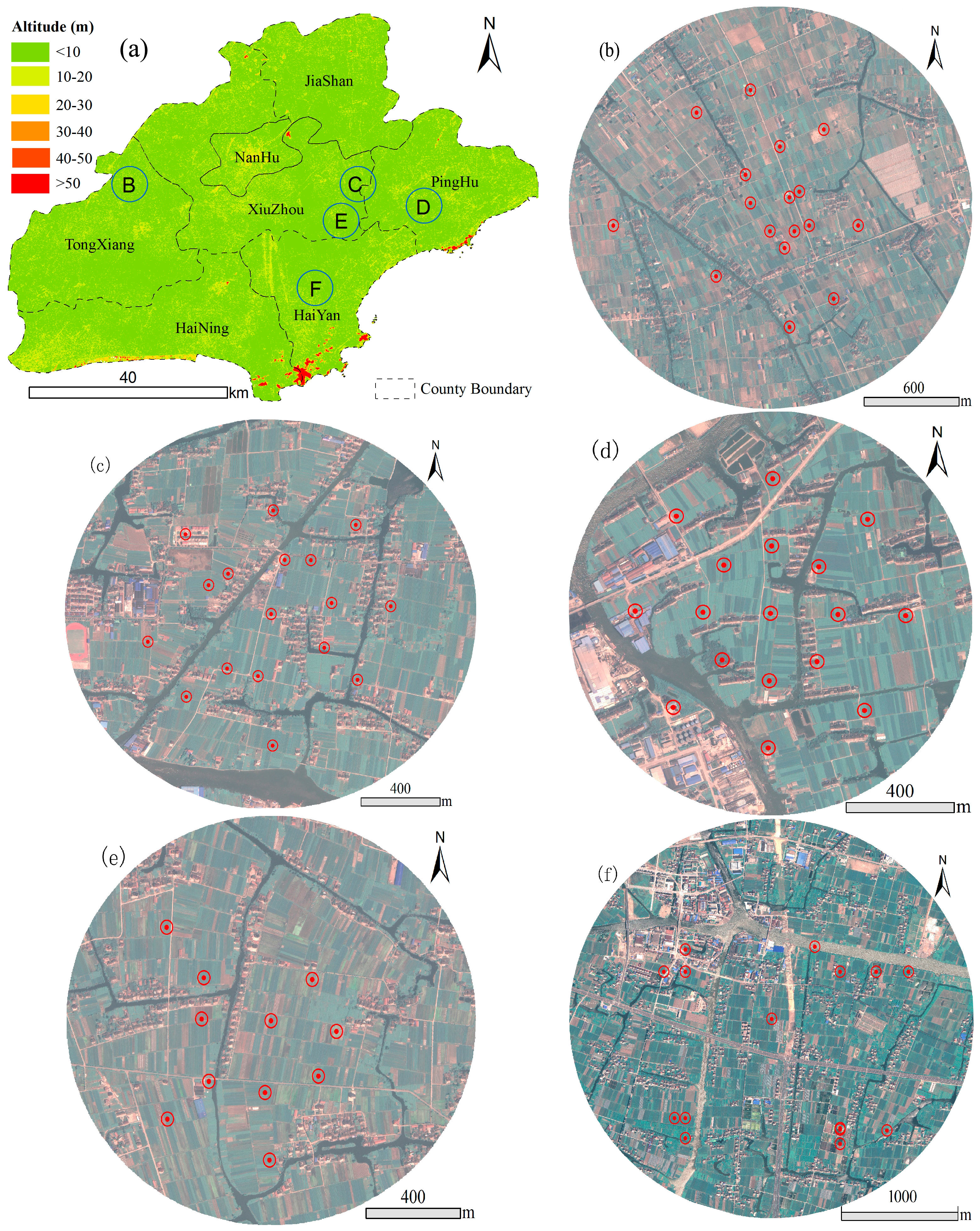
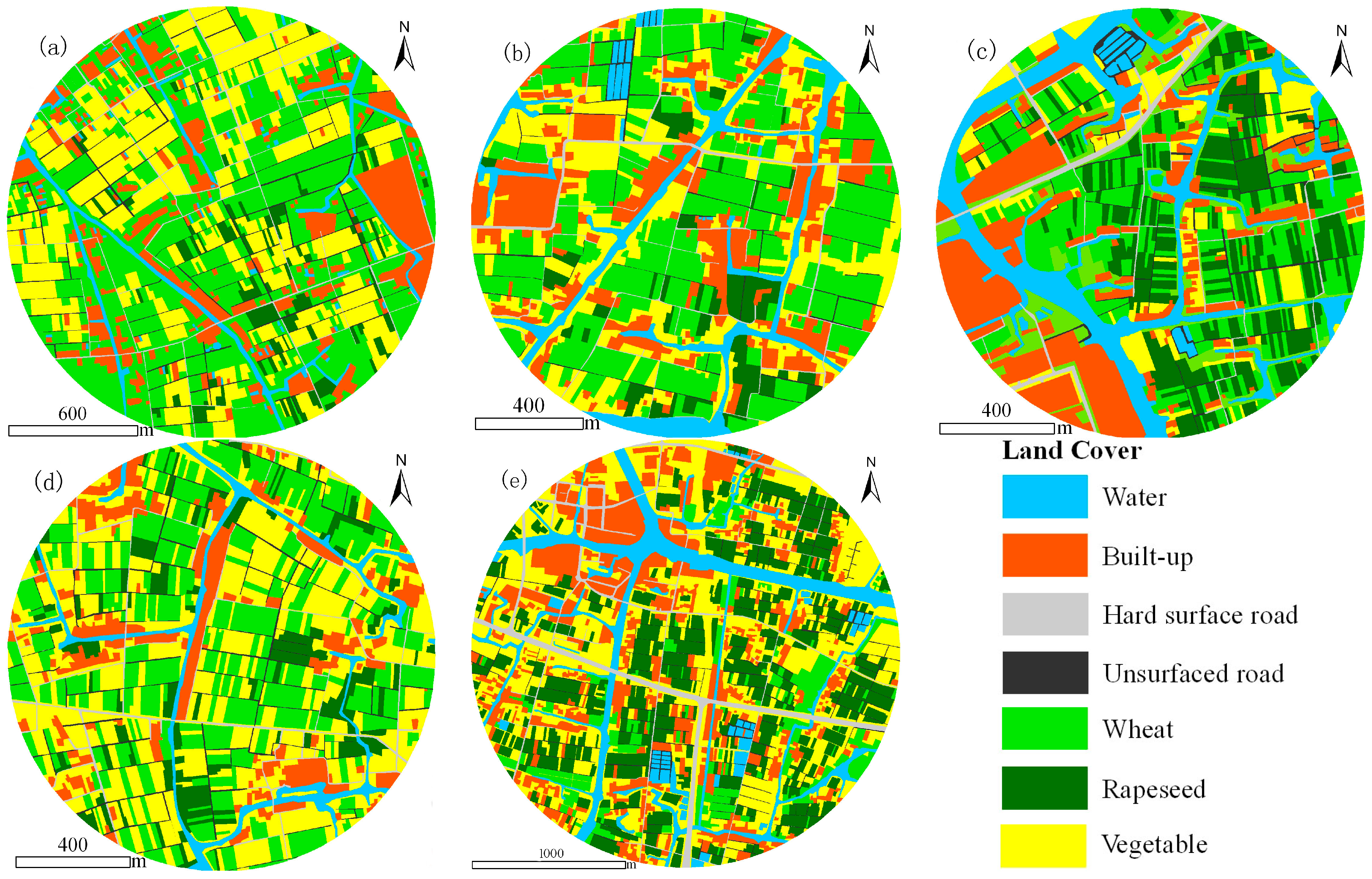

| Study Site | TongXiang (17) | XinFeng (17) | PingHu (17) | XinHuang (11) | HaiYan (15) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Life cycle | Nymph | Adult | Nymph | Adult | Nymph | Adult | Nymph | Adult | Nymph | Adult |
| Min | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 14 | 0 | 0 |
| Max | 13 | 6 | 283 | 8 | 199 | 75 | 4 | 318 | 292 | 4 |
| Mean | 4.8 | 2.2 | 76.4 | 1.6 | 54.9 | 24.2 | 0.4 | 65.5 | 53.9 | 0.9 |
| SD | 3.7 | 2.2 | 80.1 | 2.5 | 60.5 | 26.4 | 1.2 | 88.4 | 73.5 | 1.4 |
| Sum | 82 | 38 | 1299 | 28 | 933 | 411 | 4 | 720 | 809 | 14 |
| Sig. value | 0.003 | 0.001 | 0.004 | 0.035 | 0.014 | |||||
| Study Site | Radii (m) | 50 m | 100 m | 150 m | 200 m |
|---|---|---|---|---|---|
| TongXiang (17) | Min | 58.7 | 66.0 | 66.3 | 67.4 |
| Max | 97.0 | 91.3 | 92.0 | 90.3 | |
| Mean | 83.7d | 82.5b | 82.7b | 81.2a | |
| SD | 10.2 | 8.4 | 7.9 | 7.2 | |
| XinFeng (17) | Min | 4.2 | 42.9 | 51.2 | 57.8 |
| Max | 100.0 | 90.6 | 85.9 | 77.6 | |
| Mean | 73.2d | 71.7c | 68.1b | 65.8a | |
| SD | 26.4 | 16.0 | 9.7 | 5.2 | |
| PingHu (17) | Min | 39.2 | 47.5 | 31.0 | 31.7 |
| Max | 96.7 | 83.8 | 82.6 | 81.0 | |
| Mean | 76.5d | 69.3c | 62.4a | 62.9b | |
| SD | 15.7 | 10.9 | 16.4 | 15.5 | |
| XinHuang (11) | Min | 57.5 | 66.5 | 64.3 | 67.6 |
| Max | 97.0 | 94.9 | 91.9 | 90.3 | |
| Mean | 89.0d | 85.3c | 82.3b | 80.4a | |
| SD | 11.2 | 8.9 | 8.7 | 7.0 | |
| HaiYan (15) | Min | 14.4 | 29.9 | 21.8 | 23.3 |
| Max | 97.5 | 95.1 | 92.7 | 87.4 | |
| Mean | 73.1d | 70.3c | 66.5b | 65.6a | |
| SD | 24.9 | 20.9 | 20.8 | 18.3 |
| Study Site | Radii | 50 m | 100 m | 150 m | 200 m |
|---|---|---|---|---|---|
| TongXiang (17) | 50 m | 1 | |||
| 100 m | 0.651 ** | 1 | |||
| 150 m | 0.359 | 0.875 *** | 1 | ||
| 200 m | 0.147 | 0.633 ** | 0.884 *** | 1 | |
| XinFeng (17) | 50 m | 1 | |||
| 100 m | 0.878 *** | 1 | |||
| 150 m | 0.758 *** | 0.905 *** | 1 | ||
| 200 m | 0.508 * | 0.674 ** | 0.762 *** | 1 | |
| PingHu (17) | 50 m | 1 | |||
| 100 m | 0.779 *** | 1 | |||
| 150 m | 0.665 ** | 0.755 *** | 1 | ||
| 200 m | 0.634 ** | 0.755 *** | 0.810 *** | 1 | |
| XinHuang (11) | 50 m | 1 | |||
| 100 m | 0.661 * | 1 | |||
| 150 m | 0.108 | 0.774 ** | 1 | ||
| 200 m | −0.255 | 0.476 | 0.895 *** | 1 | |
| HaiYan (15) | 50 m | 1 | |||
| 100 m | 0.857 *** | 1 | |||
| 150 m | 0.687 ** | 0.945 *** | 1 | ||
| 200 m | 0.625 ** | 0.896 *** | 0.983 *** | 1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Chen, J.; Qi, J.; John, R.; Cheng, J.; Zhu, Z. The Effect of Landscape Composition on the Abundance of Laodelphax striatellus Fallén in Fragmented Agricultural Landscapes. Land 2016, 5, 36. https://doi.org/10.3390/land5040036
Liu Z, Chen J, Qi J, John R, Cheng J, Zhu Z. The Effect of Landscape Composition on the Abundance of Laodelphax striatellus Fallén in Fragmented Agricultural Landscapes. Land. 2016; 5(4):36. https://doi.org/10.3390/land5040036
Chicago/Turabian StyleLiu, Zhanyu, Jiquan Chen, Jiaguo Qi, Ranjeet John, Jiaan Cheng, and Zengrong Zhu. 2016. "The Effect of Landscape Composition on the Abundance of Laodelphax striatellus Fallén in Fragmented Agricultural Landscapes" Land 5, no. 4: 36. https://doi.org/10.3390/land5040036







