Three-Dimensional Facial Asymmetry in Attractive and Normal People from Childhood to Young Adulthood
Abstract
:1. Introduction
1.1. Facial Symmetry and Attractiveness
1.2. Selection of Attractive Persons
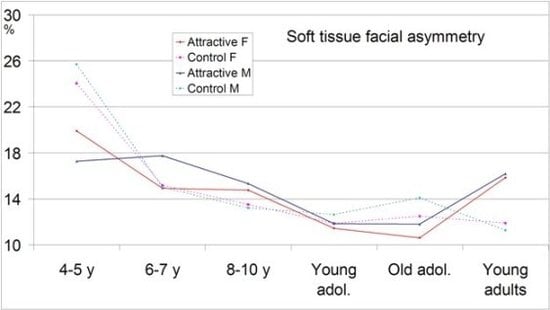
2. Results and Discussion
2.1. Total, Midline and Lateral Facial Asymmetry
2.2. Landmark Asymmetry
2.3. General Discussion
2.4. Study Limitations
3. Experimental Section
3.1. Analyzed Subjects
3.2. Collection of Facial Landmarks
3.3. Method Error
3.4. Calculation of Facial Asymmetry
3.5. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Ercan, I.; Ozdemir, S.T.; Etoz, A.; Sigirli, D.; Tubbs, R.S.; Loukas, M.; Guney, I. Facial asymmetry in young healthy subjects evaluated by statistical shape analysis. J. Anat. 2008, 213, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, V.F.; Sforza, C.; Schmitz, J.H.; Santoro, F. Three-dimensional facial morphometric assessment of soft tissue changes after orthognathic surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 549–556. [Google Scholar] [CrossRef]
- Ferrario, V.F.; Sforza, C.; Ciusa, V.; Dellavia, C.; Tartaglia, G.M. The effect of sex and age on facial asymmetry in healthy subjects: a cross-sectional study from adolescence to mid-adulthood. J. Oral Maxillofac. Surg. 2001, 59, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Leamy, L.J.; Klingenberg, C.P. The genetics and evolution of fluctuating asymmetry. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 1–21. [Google Scholar] [CrossRef]
- Peck, S.; Peck, L.; Kataja, M. Skeletal asymmetry in esthetically pleasing faces. Angle Orthod. 1991, 61, 43–48. [Google Scholar]
- Ramieri, G.A.; Nasi, A.; Dell’acqua, A.; Verzé, L. Facial soft tissue changes after transverse palatal distraction in adult patients. Int. J. Oral Maxillofac. Surg. 2008, 37, 810–818. [Google Scholar] [CrossRef]
- Rossi, M.; Ribeiro, E.; Smith, R. Craniofacial asymmetry in development: an anatomical study. Angle Orthod. 2003, 73, 381–385. [Google Scholar]
- Sforza, C.; Peretta, R.; Grandi, G.; Ferronato, G.; Ferrario, V.F. Three-dimensional facial morphometry in skeletal Class III patients. A non-invasive study of soft-tissue changes before and after orthognathic surgery. Br. J. Oral Maxillofac. Surg. 2007, 45, 138–144. [Google Scholar] [CrossRef]
- Stauber, I.; Vairaktaris, E.; Holst, A.; Schuster, M.; Hirschfelder, U.; Neukam, F.W.; Nkenke, E. Three-dimensional analysis of facial symmetry in cleft lip and palate patients using optical surface data. J. Orofac. Orthop. 2008, 69, 268–282. [Google Scholar] [CrossRef]
- Borod, J.C.; Koff, E.; Yecker, S.; Santschi, C.; Schmidt, J.M. Facial asymmetry during emotional expression: gender, valence, and measurement technique. Neuropsychologia 1998, 36, 1209–1215. [Google Scholar] [CrossRef]
- Okamoto, H.; Haraguchi, S.; Takada, K. Laterality of asymmetry in movements of the corners of the mouth during voluntary smile. Angle Orthod. 2010, 80, 223–239. [Google Scholar] [CrossRef]
- Zaidel, D.W. Neuropsychology of Art; Psychology Press: Howe, East Sussex, UK, 2005; Chapter 9; pp. 168–169. [Google Scholar]
- Brown, W.M.; Price, M.E.; Kang, J.; Pound, N.; Zhao, Y.; Yu, H. Fluctuating asymmetry and preferences for sex-typical bodily characteristics. Proc. Natl. Acad. Sci. USA 2008, 105, 12938–12943. [Google Scholar] [CrossRef] [PubMed]
- Shaner, D.J.; Peterson, A.E.; Beattie, O.B.; Bamforth, J.S. Assessment of soft tissue facial asymmetry in medically normal and syndrome-affected individuals by analysis of landmarks and measurements. Am. J. Med. Genet. 2000, 93, 143–154. [Google Scholar] [CrossRef]
- Lu, K.H. Harmonic analysis of the human face. Biometrics. 1965, 21, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Farkas, L.G. Anthropometry of the Head and Face, 2nd ed.; Raven Press: New York, NY, USA, 1994. [Google Scholar]
- Haraguchi, S.; Iguchi, Y.; Takada, K. Asymmetry of the face in orthodontic patients. Angle Orthod. 2008, 78, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ing, E.; Safarpour, A.; Ing, T.; Ing, S. Ocular adnexal asymmetry in models: a magazine photograph analysis. Can. J. Ophthalmol. 2006, 41, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Penton-Voak, I.S.; Jones, B.C.; Little, A.C.; Baker, S.; Tiddeman, B.; Burt, D.M.; Perrett, D.I. Symmetry, sexual dimorphism in facial proportions and male facial attractiveness. Proc. Biol. Sci. 2001, 268, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Bashour, M. History and current concepts in the analysis of facial attractiveness. Plast. Reconstr. Surg. 2006, 118, 741–756. [Google Scholar] [CrossRef]
- Baudouin, J.Y.; Tiberghien, G. Symmetry, averageness, and feature size in the facial attractiveness of women. Acta Psychol. (Amst). 2004, 117, 313–332. [Google Scholar] [CrossRef]
- Jones, B.C.; DeBruine, L.M.; Little, A.C. The role of symmetry in attraction to average faces. Percept. Psychophys. 2007, 69, 1273–1277. [Google Scholar] [CrossRef]
- Little, A.C.; Burt, D.M.; Penton-Voak, I.S.; Perrett, D.I. Self-perceived attractiveness influences human female preferences for sexual dimorphism and symmetry in male faces. Proc. Biol. Sci. 2001, 268, 39–44. [Google Scholar] [CrossRef]
- Little, A.C.; Jones, B.C.; Waitt, C.; Tiddeman, B.P.; Feinberg, D.R.; Perrett, D.I.; Apicella, C.L.; Marlowe, F.W. Symmetry is related to sexual dimorphism in faces: data across culture and species. PLoS One 2008, 3, e2106. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, G. The evolutionary psychology of facial beauty. Annu. Rev. Psychol. 2006, 57, 199–226. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, G.; Yoshikawa, S.; Palermo, R.; Simmons, L.W.; Peters, M.; Lee, K.; Halberstadt, J.; Crawford, J.R. Perceived health contributes to the attractiveness of facial symmetry, averageness, and sexual dimorphism. Perception 2007, 36, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Springer, I.N.; Wannicke, B.; Warnke, P.H.; Zernial, O.; Wiltfang, J.; Russo, P.A.; Terheyden, H.; Reinhardt, A.; Wolfart, S. Facial attractiveness: visual impact of symmetry increases significantly towards the midline. Ann. Plast. Surg. 2007, 59, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Matoula, S.; Pancherz, H. Skeletofacial morphology of attractive and nonattractive faces. Angle Orthod. 2006, 76, 204–210. [Google Scholar] [PubMed]
- Orsini, M.G.; Huang, G.J.; Kiyak, H.A.; Ramsay, D.S.; Bollen, A.M.; Anderson, N.K.; Giddon, D.B. Methods to evaluate profile preferences for the anteroposterior position of the mandible. Am. J. Orthod. Dentofacial Orthop. 2006, 130, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sforza, C.; Laino, A.; D’Alessio, R.; Dellavia, C.; Grandi, G.; Ferrario, V.F. Three-dimensional facial morphometry of attractive children and normal children in the deciduous and early mixed dentition. Angle Orthod. 2007, 77, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Sforza, C.; Laino, A.; D’Alessio, R.; Grandi, G.; Tartaglia, G.M.; Ferrario, V.F. Soft-tissue facial characteristics of attractive and normal adolescent boys and girls. Angle Orthod. 2008, 78, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Sforza, C.; Laino, A.; D’Alessio, R.; Grandi, G.; Binelli, M.; Ferrario, V.F. Soft-tissue facial characteristics of attractive Italian women as compared to normal women. Angle Orthod. 2009, 79, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.; Neave, N.; Seydel, H. Male facial appearance signals physical strength to women. Am. J. Hum. Biol. 2007, 19, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.M.; Naidoo, S.D.; Bardi, K.M.; Brandon, C.A.; Neiswanger, K.; Resick, J.M.; Martin, R.A.; Marazita, M.L. Face shape of unaffected parents with cleft affected offspring: combining three-dimensional surface imaging and geometric morphometrics. Orthod. Craniofac. Res. 2009, 12, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Albert, A.M.; Greene, D.L. Bilateral asymmetry in skeletal growth and maturation as an indicator of environmental stress. Am. J. Phys. Anthropol. 1999, 110, 341–349. [Google Scholar] [CrossRef]
- Zaidel, D.W.; Hessamian, M. Asymmetry and symmetry in the beauty of human faces. Symmetry 2010, 2, 136–149. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Zaidel, D.W.; Aarde, S.M.; Baig, K. Appearance of symmetry, beauty, and health in human faces. Brain Cogn. 2005, 57, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, V.F.; Sforza, C.; Dellavia, C.; Tartaglia, G.M.; Colombo, A.; Carù, A. A quantitative three-dimensional assessment of soft-tissues facial asymmetry of cleft lip and palate adult patients. J. Craniofac. Surg. 2003, 14, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Bock, M.T.; Bowman, A.W. On the measurement and analysis of asymmetry with applications to facial modelling. J. R. Stat. Soc., Ser. C, Appl. Stat. 2006, 55, 77–91. [Google Scholar] [CrossRef]
- Kokich, V.O.; Kokich, V.G.; Kiyak, H.A. Perceptions of dental professionals and laypersons to altered dental esthetics: asymmetric and symmetric situations. Am. J. Orthod. Dentofacial Orthop. 2006, 130, 141–151. [Google Scholar] [CrossRef]
- Kiekens, R.M.; van ’t Hof, M.A.; Straatman, H.; Kuijpers-Jagtman, A.M.; Maltha, J.C. Influence of panel composition on aesthetic evaluation of adolescent faces. Eur. J. Orthod. 2007, 29, 95–99. [Google Scholar] [CrossRef]
- Scott, C.R.; Goonewardene, M.S.; Murray, K. Influence of lips on the perception of malocclusion. Am. J. Orthod. Dentofacial Orthop. 2006, 130, 152–162. [Google Scholar] [CrossRef]
- Sforza, C.; Ferrario, V.F. Soft-tissue facial anthropometry in three dimensions: from anatomical landmarks to digital morphology in research, clinics and forensic anthropology. J. Anthropol. Sci. 2006, 84, 97–124. [Google Scholar]
- De Menezes, M.; Rosati, R.; Ferrario, V.F.; Sforza, C. Accuracy and reproducibility of a 3D stereophotogrammetric imaging system. J. Oral Maxillofac. Surg. 2010, 68, 2129–2135. [Google Scholar]
- Hajeer, M.Y.; Ayoub, A.F.; Millett, D.T. Three-dimensional assessment of facial soft-tissue asymmetry before and after orthognathic surgery. Br. J. Oral Maxillofac. Surg. 2004, 42, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Nkenke, E.; Vairaktaris, E.; Kramer, M.; Schlegel, A.; Holst, A.; Hirschfelder, U.; Wiltfang, J.; Neukam, F.W.; Stamminger, M. Three-dimensional analysis of changes of the malar-midfacial region after LeFort I osteotomy and maxillary advancement. Oral Maxillofac. Surg. 2008, 12, 5–12. [Google Scholar] [CrossRef]
- Shi, J.; Samal, A.; Marx, D. How effective are landmarks and their geometry for face recognition? Comput. Vis. Image Underst. 2006, 102, 117–133. [Google Scholar] [CrossRef]
- Ozsoy, U.; Demirel, B.M.; Yildirim, F.B.; Tosun, O.; Sarikcioglu, L. Method selection in craniofacial measurements: Advantages and disadvantages of 3D digitization method. J. Craniomaxillofac. Surg. 2009, 37, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Bruner, E.; Bastir, M. Digital Morphology: modelling anatomy and evolution. J. Anthropol. Sci. 2008, 86, 3–5. [Google Scholar] [PubMed]
- Ferrario, V.F.; Sforza, C.; Poggio, C.E.; Cova, M.; Tartaglia, G. Preliminary evaluation of an electromagnetic three-dimensional digitizer in facial anthropom etry. Cleft Palate Craniofac J. 1998, 35, 9–15. [Google Scholar] [CrossRef]








| DFSl | DFSm | DFSt | % | Ft-Ft | Os-Os | En-En | Zy-Zy | T-T | Ala-Ala | Ch-Ch | Go-Go | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | ||||||||||||
| Age | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Attractiveness | 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | NS | 0.0025 | 0.0001 | 0.0040 | NS | < 0.0001 | 0.0026 | NS |
| Interaction | 0.0299 | < 0.0001 | NS | NS | < 0.0001 | < 0.0001 | NS | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0008 |
| Males | ||||||||||||
| Age | < 0.0001 | 0.0003 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0003 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Attractiveness | 0.0002 | < 0.0001 | 0.0006 | 0.0009 | 0.0450 | NS | < 0.0001 | NS | 0.0006 | 0.0004 | 0.0298 | < 0.0001 |
| Interaction | < 0.0001 | 0.0438 | < 0.0001 | < 0.0001 | < 0.0001 | NS | < 0.0001 | < 0.0001 | < 0.0001 | NS | NS | 0.0012 |
| Children | Adolescents | Adults | ||||
|---|---|---|---|---|---|---|
| 4-5 y | 6-7 y | 8-10 y | “Young”* | “Old”§ | 18-30 y | |
| Females | ||||||
| Attractive | 14 | 18 | 14 | 24 | 23 | 139 |
| Control | 11 | 42 | 42 | 48 | 60 | 69 |
| Males | ||||||
| Attractive | 8 | 21 | 13 | 21 | 24 | 60 |
| Control | 19 | 41 | 55 | 99 | 55 | 128 |
© 2010 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sforza, C.; Laino, A.; Grandi, G.; Pisoni, L.; Ferrario, V.F. Three-Dimensional Facial Asymmetry in Attractive and Normal People from Childhood to Young Adulthood. Symmetry 2010, 2, 1925-1944. https://doi.org/10.3390/sym2041925
Sforza C, Laino A, Grandi G, Pisoni L, Ferrario VF. Three-Dimensional Facial Asymmetry in Attractive and Normal People from Childhood to Young Adulthood. Symmetry. 2010; 2(4):1925-1944. https://doi.org/10.3390/sym2041925
Chicago/Turabian StyleSforza, Chiarella, Alberto Laino, Gaia Grandi, Luca Pisoni, and Virgilio Ferruccio Ferrario. 2010. "Three-Dimensional Facial Asymmetry in Attractive and Normal People from Childhood to Young Adulthood" Symmetry 2, no. 4: 1925-1944. https://doi.org/10.3390/sym2041925