The Genetics of Asymmetry: Whole Exome Sequencing in a Consanguineous Turkish Family with an Overrepresentation of Left-Handedness
Abstract
:1. Introduction
2. Materials and Methods
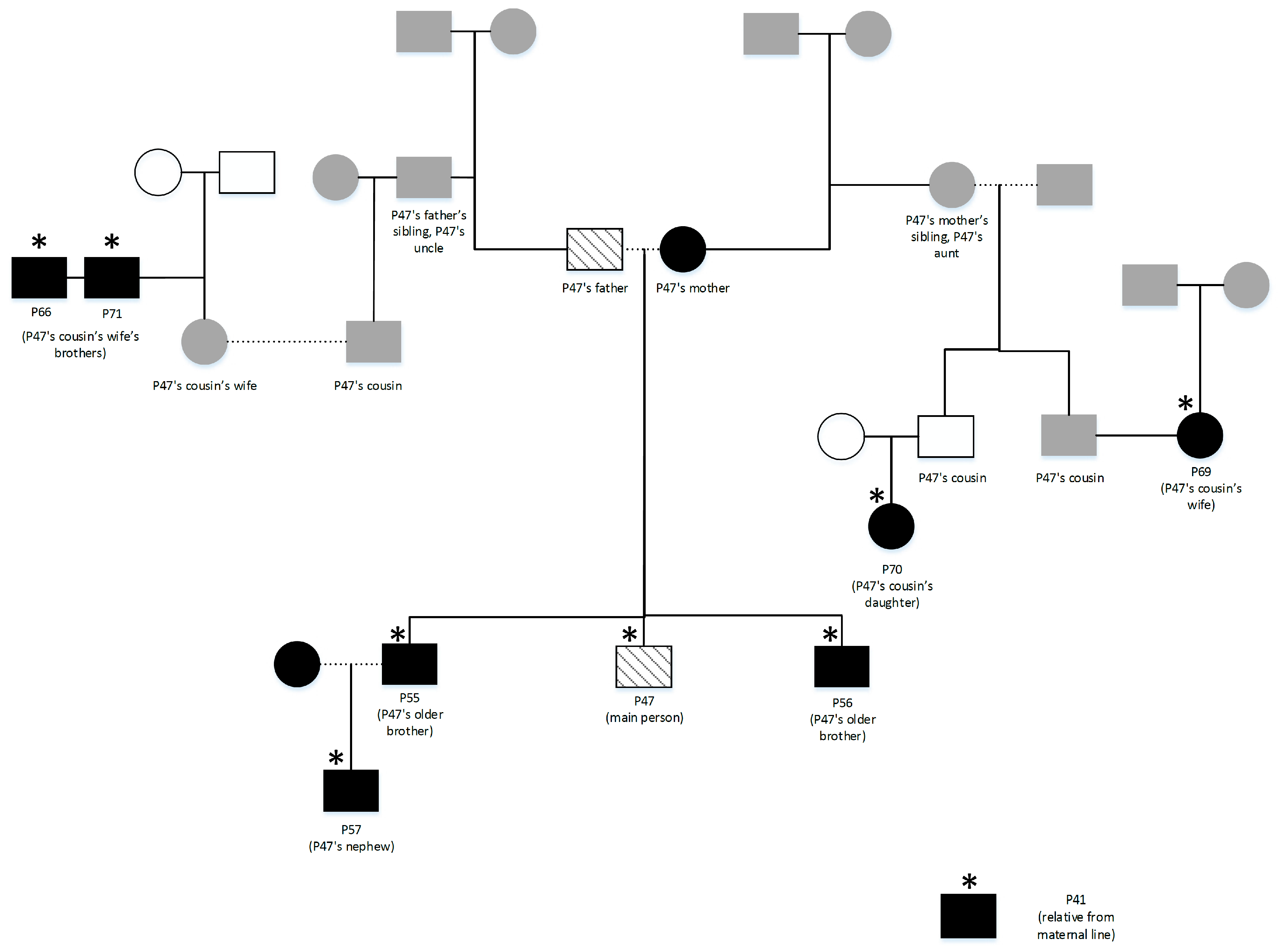
2.1. Participants
2.2. Phenotyping
2.2.1. Edinburgh Handedness Inventory
2.2.2. Pegboard Test
2.2.3. Dichotic Listening Task
2.3. Collection of DNA Samples
2.4. Whole Exome Sequencing
3. Results
3.1. Phenotyping
3.2. Sequencing Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lien, Y.J.; Chen, W.J.; Hsiao, P.C.; Tsuang, H.C. Estimation of heritability for varied indexes of handedness. Laterality 2015, 20, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Annett, M. Tests of the right shift genetic model for two new samples of family handedness and for the data of McKeever (2000). Laterality 2008, 13, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Armour, J.A.; Davison, A.; McManus, I.C. Genome-wide association study of handedness excludes simple genetic models. Heredity 2014, 112, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, N.; Macpherson, J.M.; Tung, J.Y.; Hon, L.S.; Naughton, B.; Saxonov, S.; Avey, L.; Wojcicki, A.; Pe’er, I.; Mountain, J. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet. 2010, 6, e1000993. [Google Scholar] [CrossRef] [PubMed]
- McManus, I.C.; Davison, A.; Armour, J.A. Multilocus genetic models of handedness closely resemble single-locus models in explaining family data and are compatible with genome-wide association studies. Ann. NY Acad. Sci. 2013, 1288, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Beste, C.; Güntürkün, O. Handedness: A neurogenetic shift of perspective. Neurosci. Biobehav. Rev. 2013, 37, 2788–2793. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Beste, C.; Arning, L.; Peterburs, J.; Güntürkün, O. The ontogenesis of language lateralization and its relation to handedness. Neurosci. Biobehav. Rev. 2014, 43, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Rentería, M.E. Cerebral asymmetry: A quantitative, multifactorial, and plastic brain phenotype. Twin Res. Hum. Genet. 2012, 15, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Arning, L.; Ocklenburg, S.; Schulz, S.; Ness, V.; Gerding, W.M.; Hengstler, J.G.; Falkenstein, M.; Epplen, J.T.; Güntürkün, O.; Beste, C. PCSK6 VNTR Polymorphism Is Associated with Degree of Handedness but Not Direction of Handedness. PLoS ONE 2013, 8, e67251. [Google Scholar] [CrossRef] [PubMed]
- Arning, L.; Ocklenburg, S.; Schulz, S.; Ness, V.; Gerding, W.M.; Hengstler, J.G.; Falkenstein, M.; Epplen, J.T.; Güntürkün, O.; Beste, C. Handedness and the X chromosome: The role of androgen receptor CAG-repeat length. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Brandler, W.M.; Morris, A.P.; Evans, D.M.; Scerri, T.S.; Kemp, J.P.; Timpson, N.J.; St. Pourcain, B.; Smith, G.D.; Ring, S.M.; Stein, J.; et al. Common variants in left/right asymmetry genes and pathways are associated with relative hand skill. PLoS Genet. 2013, 9, e1003751. [Google Scholar] [CrossRef] [PubMed]
- Francks, C.; Maegawa, S.; Laurén, J.; Abrahams, B.S.; Velayos-Baeza, A.; Medland, S.E.; Colella, S.; Groszer, M.; McAuley, E.Z.; Caffrey, T.M.; et al. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Mol. Psychiatr. 2007, 12, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Leach, E.L.; Prefontaine, G.; Hurd, P.L.; Crespi, B.J. The imprinted gene LRRTM1 mediates schizotypy and handedness in a nonclinical population. J. Hum. Genet. 2014, 59, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.J.; Hurd, P.L.; Read, S.; Crespi, B.J. The PCSK6 gene is associated with handedness, the autism spectrum, and magical ideation in a non-clinical population. Neuropsychologia 2016, 84, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Scerri, T.S.; Brandler, W.M.; Paracchini, S.; Morris, A.P.; Ring, S.M.; Richardson, A.J.; Talcott, J.B.; Stein, J.; Monaco, A.P. PCSK6 is associated with handedness in individuals with dyslexia. Hum. Mol. Genet. 2011, 20, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Somers, M.; Ophoff, R.A.; Aukes, M.F.; Cantor, R.M.; Boks, M.P.; Dauwan, M.; de Visser, K.L.; Kahn, R.S.; Sommer, I.E. Linkage analysis in a Dutch population isolate shows no major gene for left-handedness or atypical language lateralization. J. Neurosci. 2015, 35, 8730–8736. [Google Scholar] [CrossRef] [PubMed]
- Kavaklioglu, T.; Ajmal, M.; Hameed, A.; Francks, C. Whole exome sequencing for handedness in a large and highly consanguineous family. Neuropsychologia 2015, 93, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- McManus, I.C. Right- and left- hand skill: Failure of the right shift model. Br. J. Psychol. 1985, 76, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Bryden, M.P.; Tapley, S.M. A group test for the assessment of performance between the hands. Neuropsychologia 1985, 23, 215–221. [Google Scholar]
- Annett, M. Left, Right, Hand and Brain: The Right Shift Theory; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1985. [Google Scholar]
- Annett, M. Handedness and Brain Asymmetry: The Right Shift Theory; Psychology Press: Abingdon, UK, 2002. [Google Scholar]
- Hugdahl, K.; Eichele, T.; Rimol, L.M. The effect of voice-onset-time on dichotic listening with consonant–vowel syllables. Neuropsychologia 2006, 44, 191–196. [Google Scholar]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar]
- Somers, M.; Shields, L.S.; Boks, M.P.; Kahn, R.S.; Sommer, I.E. Cognitive benefits of right-handedness: A meta-analysis. Neurosci. Biobehav. Rev. 2015, 51, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Hirnstein, M.; Hugdahl, K. Excess of non-right-handedness in schizophrenia: Meta-analysis of gender effects and potential biases in handedness assessment. Br. J. Psychiatr. 2014, 205, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Foundas, A.L.; Corey, D.M.; Hurley, M.M.; Heilman, K.M. Verbal dichotic listening in right and left-handed adults: laterality effects of directed attention. Cortex 2006, 42, 79–86. [Google Scholar] [CrossRef]
- Kaibara, M.; Ishihara, K.; Doi, Y.; Hayashi, H.; Ehara, T.; Taniyama, K. Identification of human Kir2.2 (KCNJ12) gene encoding functional inward rectifier potassium channel in both mammalian cells and Xenopus oocytes. FEBS Lett. 2002, 531, 250–254. [Google Scholar] [CrossRef]
- Kiesecker, C.; Zitron, E.; Scherer, D.; Lueck, S.; Bloehs, R.; Scholz, E.P.; Pirot, M.; Kathöfer, S.; Thomas, D.; Kreye, V.A.; et al. Regulation of cardiac inwardly rectifying potassium current IK1 and Kir2.x channels by endothelin-1. J. Mol. Med. 2006, 84, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Karkanis, T.; Li, S.; Pickering, J.G.; Sims, S.M. Plasticity of KIR channels in human smooth muscle cells from internal thoracic artery. Am. J. Physiol. Heart. Circ. Physiol. 2003, 284, H2325–H2334. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Park, C.; Kang, W.K. Knockdown of inwardly rectifying potassium channel Kir2.2 suppresses tumorigenesis by inducing reactive oxygen species-mediated cellular senescence. Mol. Cancer Ther. 2010, 9, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.E.; Aryal, P.; Suen, K.F.; Slesinger, P.A. Evidence for association of GABA(B) receptors with Kir3 channels and regulators of G protein signalling (RGS4) proteins. J. Physiol. 2007, 580, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, A.H.; Grubb, B.D.; Pringle, J.H.; Norman, R.I.; Stanfield, P.R.; Brammar, W.J. Nuclear immunostaining in rat neuronal cells using two anti-Kir2.2 ion channel polyclonal antibodies. J. Mol. Neurosci. 2003, 20, 189–194. [Google Scholar] [CrossRef]
- Sauerhöfer, S.; Yuan, G.; Braun, G.S.; Deinzer, M.; Neumaier, M.; Gretz, N.; Floege, J.; Kriz, W.; van der Woude, F.; Moeller, M.J. L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 2007, 56, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Fiumara, A.; Jaeken, J. Congenital disorders of glycosylation with emphasis on cerebellar involvement. Semin. Neurol. 2014, 34, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Drake, R.R. Glycosylation and cancer: moving glycomics to the forefront. Adv. Cancer Res. 2015, 126, 1–10. [Google Scholar] [PubMed]
- Polašek, O.; Leutenegger, A.L.; Gornik, O.; Zgaga, L.; Kolcic, I.; McQuillan, R.; Wilson, J.F.; Hayward, C.; Wright, A.F.; Lauc, G.; et al. Does inbreeding affect N-glycosylation of human plasma proteins? Mol. Genet. Genom. 2011, 285, 427–432. [Google Scholar]
- Ertas, U.; Canakçi, E. Prevalence and handedness correlates of recurrent aphthous stomatitis in the Turkish population. J. Publ. Health Dent. 2004, 64, 151–156. [Google Scholar]
- Canakci, V.; Akgül, H.M.; Akgül, N.; Canakci, C.F. Prevalence and handedness correlates of traumatic injuries to the permanent incisors in 13–17-year-old adolescents in Erzurum, Turkey. Dent. Traumatol. 2003, 19, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Tan, U. The distribution of the Geschwind scores to familial left-handedness. Int. J. Neurosci. 1988, 42, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, B.J.; Yeni-Komshian, G.H. Incidence of left-handed writing in a college population with reference to family patterns of hand preference. Neuropsychologia 1983, 21, 651–659. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Schmitz, J.; Moinfar, Z.; Moser, D.; Klose, R.; Lor, S.; Kunz, G.; Tegenthoff, M.; Faustmann, P.; Francks, C.; et al. Epigenetic regulation of lateralized fetal spinal gene expression underlies hemispheric asymmetries. eLife 2017, 1, e22784. [Google Scholar] [CrossRef] [PubMed]
| Chr. | Gene | dbSNP ID | Likely Function |
|---|---|---|---|
| 2 | ANKRD36C | 202102082 | Ion channel inhibitor activity |
| 3 | MUC20 | 2688539 | Cellular protein metabolism |
| 3828408 | |||
| 4 | ZNF595 | - | Regulation of DNA transcription |
| 4 | FRG1 | 199978807 | Associated with facioscapulohumeral muscular dystrophy |
| 201142987 | |||
| 7 | MUC3A | 71540917 | Cellular protein metabolism |
| 775174499 | |||
| 747768677 | |||
| 759956700 | |||
| 796070497 | |||
| 796719496 | |||
| 796627084 | |||
| 796799995 | |||
| 796422604 | |||
| 796558082 | |||
| 796345426 | |||
| 796976589 | |||
| 62483696 | |||
| 10 | FRG2 | 200347477 | Protein coding in the nucleus |
| 11 | MUC6 | 770290437 | Cellular protein metabolism/ production of gastric mucin |
| 34490696 | |||
| 200644196 | |||
| 796934918 | |||
| 111641154 | |||
| 112301388 | |||
| 78265558 | |||
| 11 | MUC5AC | 74390930 | Cellular protein metabolism |
| 749291344 | |||
| 11 | TRIM49 | 74584169 | Protein-protein interactions, preferentially expressed in testis |
| 14 | HOMEZ | 148005528 | Regulation of DNA transcription |
| 15 | GOLGA6L2 | 76062343 | Protein binding |
| 16 | CBFA2T3 | 71395351 | Transcription corepressor activity |
| 71395352 | |||
| 17 | CCDC144NL | 73298040 | Affects blood copper, selenium and zinc |
| 17 | KCNJ12 | 77987694 | Encodes an inwardly rectifying K+ channel in neurons, heart and muscle cells. |
| 80335301 | |||
| 17 | RECQL5 | 142406301 | DNA helicase activity |
| 18 | CNDP1 | 10663835 | Encodes a member of the M20 metalloprotease family that is specifically expressed in the brain |
| 19 | MUC16 | 4992693 | Cellular protein metabolism |
| 19 | ZNF443 | 62114866 | Regulation of DNA transcription |
| 19 | SIGLEC11 | 9676436 | Anti-inflammatory and immunosuppressive signaling |
| 78673790 | |||
| 21 | BAGE2 | 9808647 | Melanoma antigen |
| 21 | BAGE5 | 113315187 | Melanoma antigen |
| X | RBMX | 76876438 | RNA binding |
| 74463481 | |||
| 74667874 | |||
| 35899675 | |||
| 77794331 |
| GO Group | Genes | Adjusted p-Value |
|---|---|---|
| O-glycan processing | 5 | 0.0000002 |
| Protein O-linked glycosylation | 5 | 0.0000005 |
| Post-translational protein modification | 5 | 0.00005 |
| Protein glycosylation | 5 | 0.0001 |
| Macromolecule glycosylation | 5 | 0.0001 |
| Glycosylation | 5 | 0.0001 |
| Glycoprotein biosynthetic process | 5 | 0.0002 |
| Glycoprotein metabolic process | 5 | 0.0005 |
| Golgi lumen | 5 | 0.0000007 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocklenburg, S.; Barutçuoğlu, C.; Özgören, A.Ö.; Özgören, M.; Erdal, E.; Moser, D.; Schmitz, J.; Kumsta, R.; Güntürkün, O. The Genetics of Asymmetry: Whole Exome Sequencing in a Consanguineous Turkish Family with an Overrepresentation of Left-Handedness. Symmetry 2017, 9, 66. https://doi.org/10.3390/sym9050066
Ocklenburg S, Barutçuoğlu C, Özgören AÖ, Özgören M, Erdal E, Moser D, Schmitz J, Kumsta R, Güntürkün O. The Genetics of Asymmetry: Whole Exome Sequencing in a Consanguineous Turkish Family with an Overrepresentation of Left-Handedness. Symmetry. 2017; 9(5):66. https://doi.org/10.3390/sym9050066
Chicago/Turabian StyleOcklenburg, Sebastian, Ceren Barutçuoğlu, Adile Öniz Özgören, Murat Özgören, Esra Erdal, Dirk Moser, Judith Schmitz, Robert Kumsta, and Onur Güntürkün. 2017. "The Genetics of Asymmetry: Whole Exome Sequencing in a Consanguineous Turkish Family with an Overrepresentation of Left-Handedness" Symmetry 9, no. 5: 66. https://doi.org/10.3390/sym9050066






