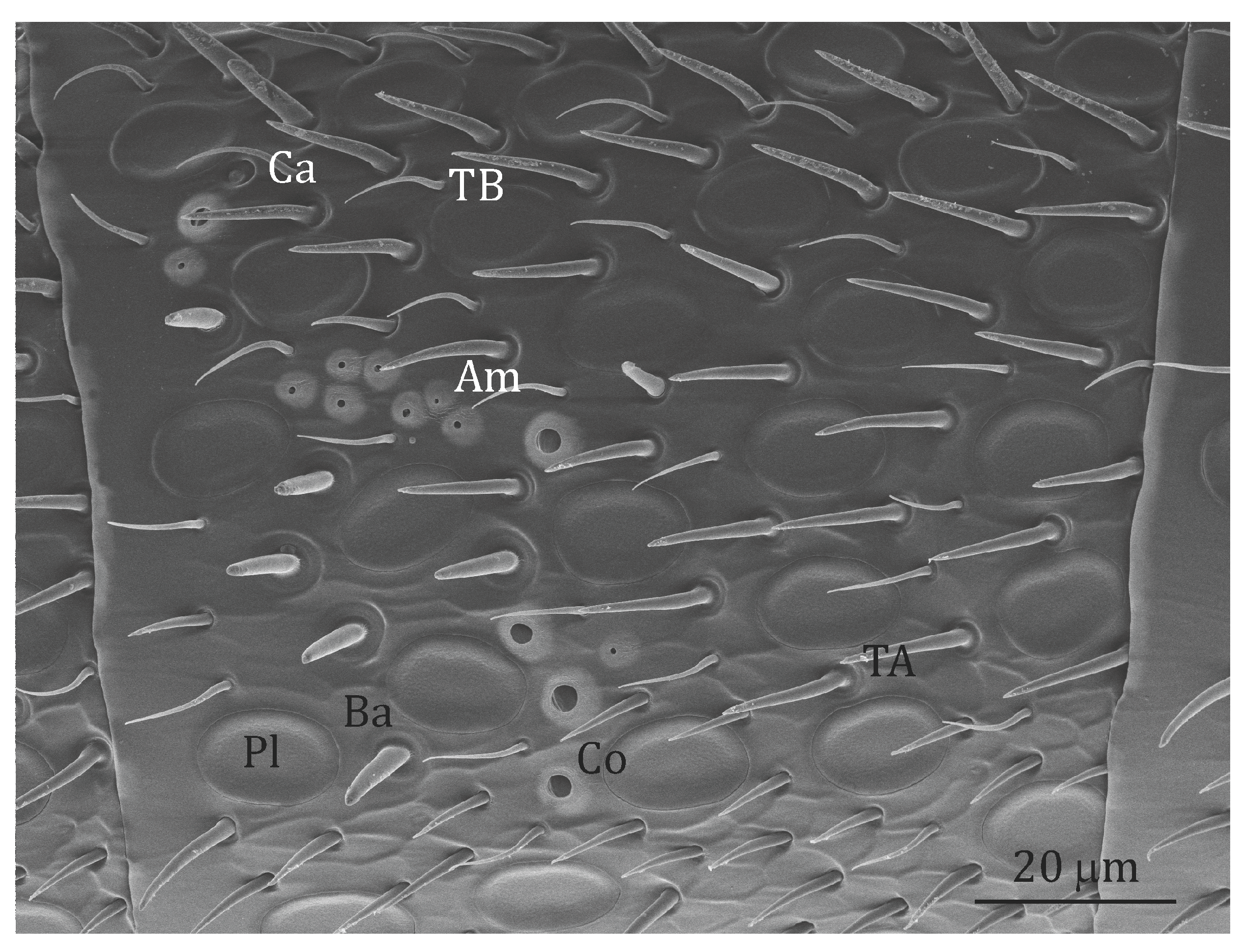
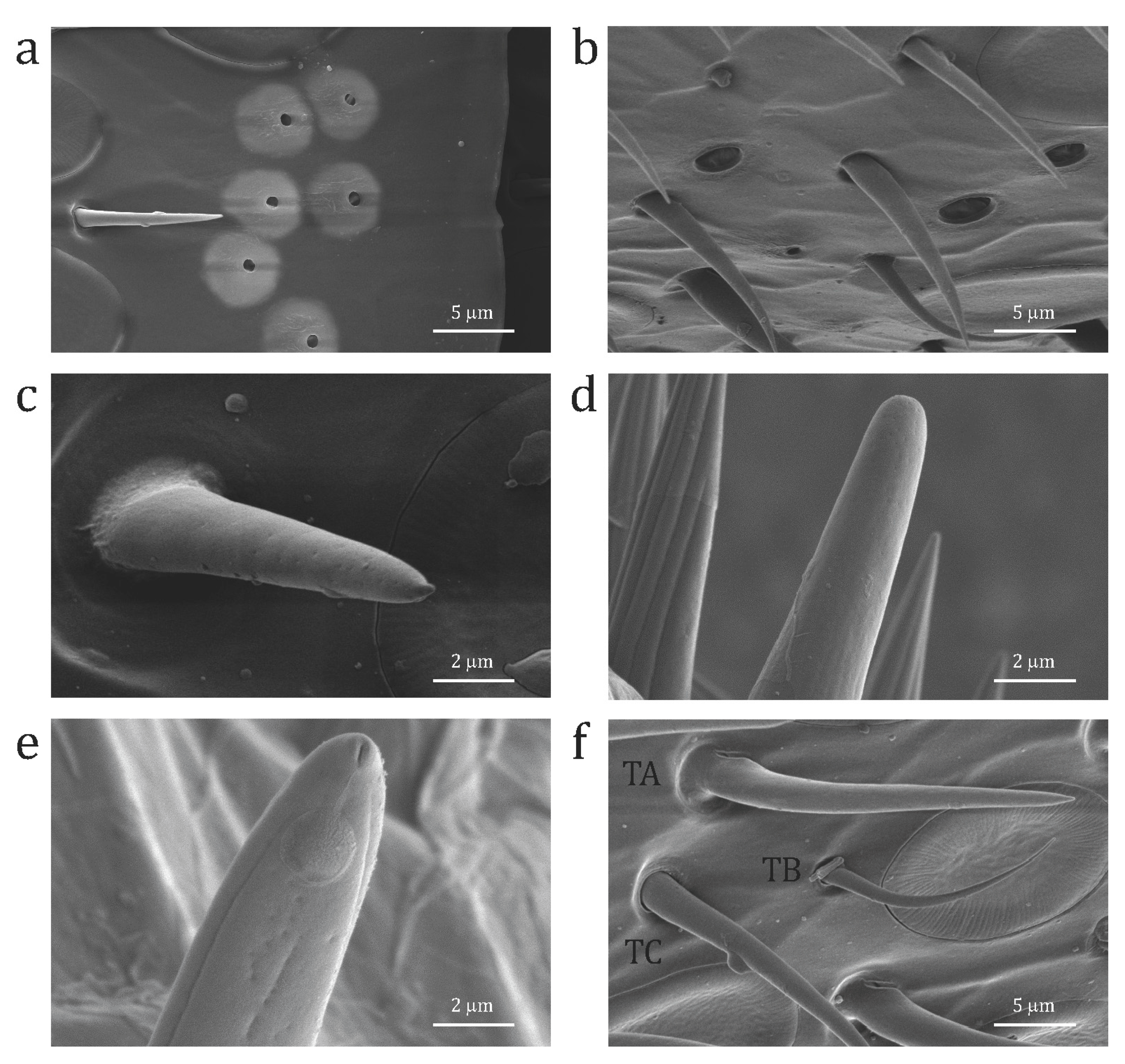
1. Introduction
The different functional specialization of the right and left sides of the nervous system (lateralization) is a feature shared by many vertebrates and also invertebrate species (see [
1,
2]). Lateralization manifests itself in a substantial range of behaviors and cognitive tasks, and mediates distinct sensory, motor and cognitive processes. In several species, behavioral asymmetries such as, for example, a side-bias in turning in one direction or a preferential use of one eye, ear or nostril to respond to specific stimuli, have been associated with corresponding asymmetries in the anatomical substrates of the nervous system (see [
1]). These anatomical differences can be present at different levels: (i) in macroscopic anatomy, such as, for example, the Sylvian fissure of the lateral sulcus in humans that, in most people, is longer in the left hemisphere [
3]; (ii) in the different size of the fibers that connect sensory reception and motor afference, such as the Mauthner cells responsible for the lateralization in the C-start bending reaction to danger in fishes [
4]; or (iii) at the cellular level, such as in the different arrangement of synapses for specific neurotransmitters between the right and the left side of specific cerebral structures (e.g., the glutamate
N-methyl-D-aspartate (NMDA) receptor, implied in learning and memory, in the left and right hippocampus of rodents [
5]).
Several species of social bees exhibit population-level lateralization in learning odors and recalling olfactory memories. The first evidence comes from a study by Letzkus and colleagues [
6], showing that honeybees
Apis mellifera trained with only one antenna in use to associate an odor with a sugar reward in the proboscis extension reflex (PER) paradigm performed better in a recall test 5–6 h after training when they used their right antenna. Letzkus et al. [
6] also looked at the distribution of one type of olfactory sensilla (the
sensilla placodea) on the antennae and found that the right antenna had more
sensilla placodea compared to the left antenna, and they linked this result with the better performance of the bees in the PER. Since the bees were both trained and tested with only one antenna in use, it is very difficult to establish whether the behavioral asymmetry observed related to the learning phase or to the recall of the olfactory memory.
Access to unilaterally acquired memories for odors is transferred to the other side of the brain in honeybees [
7] and this transfer seems to occur from the right to the left side of the brain. Specifically, Rogers and Vallortigara [
8] showed that there is a time-dependence in the behavioral asymmetry in the PER. Specifically, when honeybees are trained in a PER paradigm with both antennae in use, they are better at recalling the olfactory memory 1–2 h after training using their right antenna, and 8–12 h after training using their left antenna [
8,
9]. The same pattern of lateralization in the recall of short- and long-term olfactory memories has been found in the Australian social stingless bees
Trigona carbonaria,
Trigona hockingsi and
Austroplebeia australis—the three species are better able to recall short- and long-term memories through the right and left antenna respectively [
10]. It is important to underline that the results of the study conducted by Rogers and Vallortigara [
8] and those of the following studies [
9,
10,
11,
12,
13] investigated asymmetry in the recall of olfactory memories and not in the learning phase as the bees were trained with both antennae in use. Recent evidence has confirmed that in honeybees trained with only one antenna in use during olfactory learning, the left hemisphere is more responsible for long-term memory and the right hemisphere is more responsible for the learning and short-term memory [
14]. Moreover, the gene expression in the brain of these honeybees was also asymmetric, with more genes having higher expression in the right hemisphere than the left hemisphere [
14].
Interestingly, the non-social mason bees
Osmia rufa are not lateralized in this way for the recall of short-term memory since they can retrieve it both through the circuits of the right and left antenna [
11]. However, when tested for electroantennographic (EAG) responsivity to different odors, most mason bees showed individual lateralization (seven and eight individuals out of 21 showed significantly stronger responses respectively with the right and the left antenna), whereas honeybees show population-level lateralization with higher EAG responses on the right than on the left antenna [
11].
Bumble bees
Bombus terrestris trained on the PER paradigm with only one antenna in use and tested one hour after training show the same asymmetrical performance favoring the right antenna as do honeybees and the three species of Australian stingless bees. However, in bumble bees EAG responsivity is not lateralized at the population level, as it is in honeybees. In fact, as with mason bees, most bumble bees show individual lateralization (nine and three individuals out of 20 showed significantly stronger responses respectively with the right and the left antenna) [
12].
In honeybees, the population-level asymmetry in the recall of olfactory memories may be partially explained by a morphological asymmetry at the peripheral level—the right antenna has about 5% more olfactory sensilla than the left antenna [
6,
13]. However, this does not exclude that the right antenna may also have a more important role than the left antenna in learning the association between an odor and a sugar reward in the PER paradigm [
6]. As a consequence, it is possible that the morphological asymmetry observed in the number of olfactory sensilla influences the learning process and not the recall of the olfactory memory.
Moreover, honeybees with only the right antenna in use are better at discriminating a target from a background odorant in a cross-adaptation experiment (i.e., when a target odor is superimposed on the same or a different background odor), and this behavioral performance is not due to different discrimination of changes in odor concentration, nor to different learning abilities during odor discrimination [
15]. Indeed, Rigosi et al. [
15] showed that odor representations in the projection neurons of the right and left antennal lobes (ALs) are different with higher Euclidian distances between activity patterns in the right AL compared to the left. Interestingly, it is the odor representation in the right and the left ALs that is different. In fact, the functional activity patterns elicited by stimulation with different odors (both pheromones and environmental odors) in the right and the left AL of the same honeybee are bilaterally symmetrical [
16]. In addition, at 14 days post-emergence the levels of neuroligin-1 expression, a protein involved in learning and memory, are higher in honeybees with only their right antenna compared to honeybees with only the left or both antenna [
17].
In bumble bees
Bombus terrestris, morphological counting of the olfactory and non-olfactory sensilla show a predominance in the number of only one type of olfactory sensilla, the
s. trichodea type A, in the right antenna [
12]. In the Australian stingless bee
T. carbonaria, the right and the left antenna present the same number of olfactory and non-olfactory sensilla [
18].
The antennae of female wasps
Anastatus japonicus Ashmead (Hymenoptera: Eupelmidae), a non-social parasitoid, present more
s. placodea on the right antenna than on the left antenna. Interestingly, in this species the distribution of
s. trichodea and
s. basiconica is asymmetrical between the antennae, but depends on the segment. In fact, these sensilla are more abundant on the third flagellum antennomere of the right antenna than on the corresponding flagellum of the left antenna—the reverse results were observed for
s. trichodea on the scape, pedicle, and fourth to fifth flagellum antennomeres, and for
s. basiconica on the seventh flagellum antennomere and the third clava antennomere—suggesting that the asymmetry between the antennae can vary depending on the segment [
19].
Here we looked at the possible correlation between the distribution of antennal sensilla in those species mentioned above that have been previously studied for behavioral asymmetry in the recall of olfactory memories, specifically the social Australian stingless bee Austroplebeia australis and the non-social mason bee Osmia rufa.
3. Results
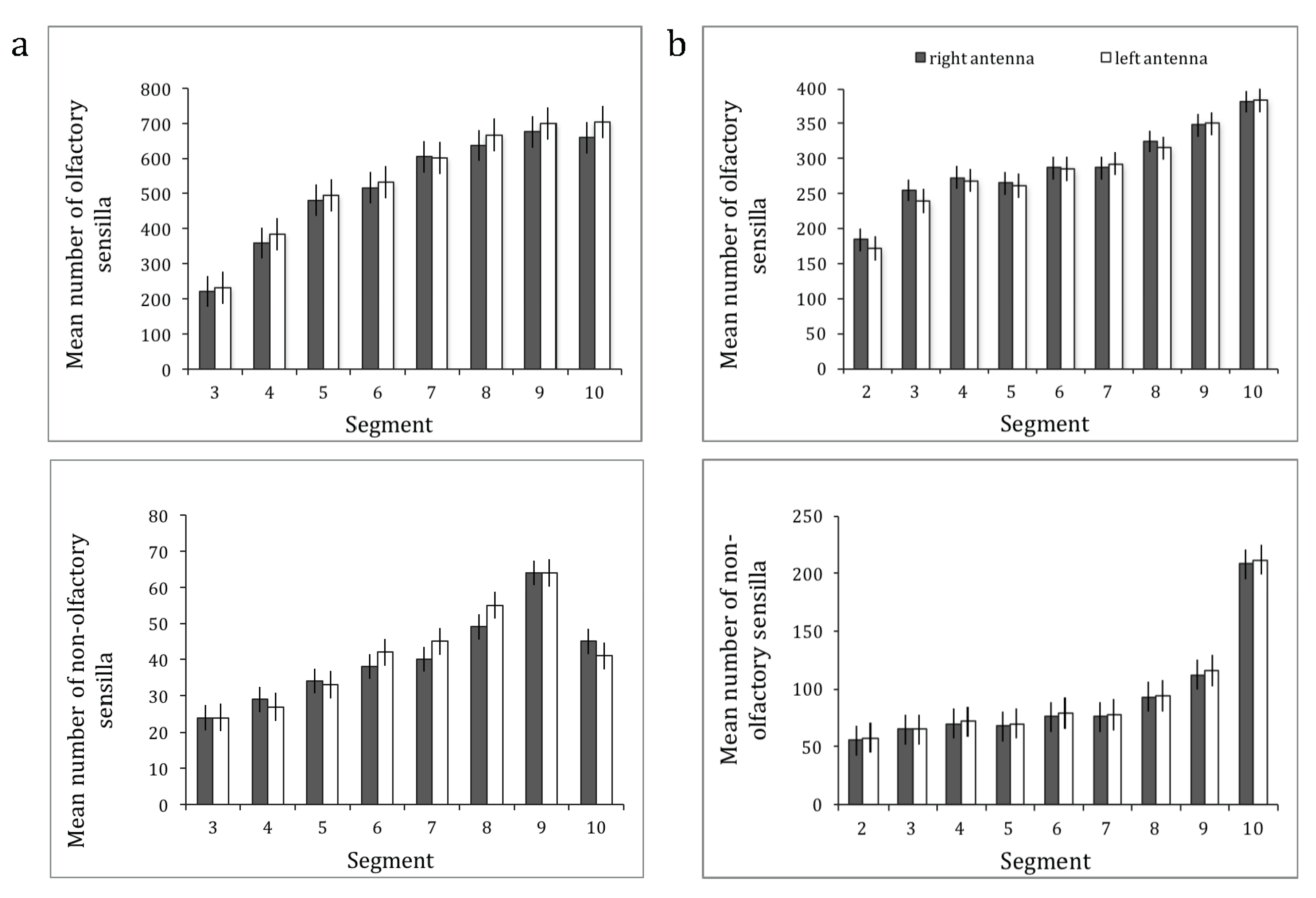
The results for both species are shown in
Figure 3. For
O. rufa, the overall ANOVA revealed significant main effects of segment (Greenhouse–Geisser, F
2.841,36.930 = 734.905,
p < 0.0001) and sensilla type (Greenhouse–Geisser, F
1.416,18.410 = 976.911,
p < 0.0001), but no effect of the antenna (left versus right) (sphericity assumed, F
1,13 = 4.217,
p = 0.061), although there was a tendency towards more sensilla on the left antenna. There was a significant interaction between segment × sensilla type (Greenhouse–Geisser, F
3.877,50.399 = 261.403,
p < 0.0001) but no significant interaction with antenna (antenna × type, Greenhouse–Geisser, F
2.154,28.006 = 2.031,
p = 0.147; antenna × segment, Greenhouse–Geisser, F
2.005,26.066 = 0.602,
p = 0.555; antenna × type × segment, Greenhouse–Geisser, F
3.734,48.538 = 1.269,
p = 0.296).
Similarly, for A. australis, the overall ANOVA revealed significant main effects of segment (Greenhouse–Geisser, F2.002,26.027 = 458.141, p < 0.0001) and sensilla type (Greenhouse–Geisser, F1.545,20.091 = 1982.535, p < 0.0001), but no effect of the antenna (left versus right) (sphericity assumed, F1,13 = 0.045, p = 0.835). There was a significant interaction between segment × sensilla type (Greenhouse–Geisser, F4.941,64.230 = 244.914, p < 0.0001) but no significant interactions with antenna (antenna × type, Greenhouse–Geisser, F1.324,17.206 = 1.380, p = 0.267; antenna × segment, Greenhouse–Geisser, F3.480,45.243 = 1.405, p = 0.251; antenna × type × segment, Greenhouse–Geisser, F5.609,72.911 = 0.917, p = 0.483).
We then summed up all the olfactory sensilla and all the non-olfactory sensilla, and conducted separate ANOVAs with sensilla type (olfactory vs. non-olfactory—two levels) as within-subjects factors to see whether there was any significant antenna × sensilla type interaction. For
O. rufa (
Figure 3a) we found a significant main effect of segment (Greenhouse–Geisser, F
2.841,36.930 = 734.905,
p < 0.0001) and sensilla type (sphericity assumed, F
1,13 = 6102.170,
p < 0.0001), but no effect although a tendency of the antenna (sphericity assumed, F
1,13 = 4.217,
p = 0.061). Again, there was a significant interaction between segment × sensilla type (Greenhouse–Geisser, F
2.835,36.855 = 533.080,
p < 0.0001) but no significant interaction with antenna (antenna × type, sphericity assumed, F
1,13 = 3.337,
p = 0.091; antenna × segment, Greenhouse–Geisser, F
2.005,26.066 = 0.602,
p = 0.555; antenna × type × segment, Greenhouse–Geisser, F
2.499,32.488 = 1.040,
p = 0.378). Likewise, ANOVA of the data for olfactory vs. non-olfactory sensilla for
A. australis (
Figure 3b) revealed significant main effects of segment (Greenhouse–Geisser, F
2.002,26.027 = 458.141,
p < 0.0001) and sensilla type (sphericity assumed, F
1,13 = 2269.510,
p < 0.0001), but no effect of the antenna (sphericity assumed, F
1,13 = 0.045,
p = 0.835). There was a significant interaction between segment × sensilla type (Greenhouse–Geisser, F
3.160,41.079 = 98.505,
p < 0.0001) but no significant interactions with antenna (antenna × type, sphericity assumed, F
1,13 = 1.893,
p = 0.192; antenna × segment, Greenhouse–Geisser, F
3.480,45.243 = 1.405,
p = 0.251; antenna × type × segment, Greenhouse–Geisser, F
3.086,40.112 = 1.029,
p = 0.391).
We also looked at possible differences between the right and the left antenna when the segment was not considered as a factor. For O. rufa, there was no significant effect of antenna (sphericity assumed, F1,13 = 4.217, p = 0.061) nor the antenna × type interaction (Greenhouse–Geisser, F2.154,28.006 = 2.031, p = 0.147), but only the effect of the sensilla type was significant (Greenhouse–Geisser, F1.416,18.410 = 976.911, p < 0.0001). For A. australis, the differences between the left and right antennae were not significant (sphericity assumed, F1,13 = 0.045, p = 0.835) nor the antenna × type interaction (Greenhouse–Geisser, F1.324,17.206 = 1.380, p = 0.267), but only the effect of the sensilla type was significant (Greenhouse–Geisser, F1.545,20.091 = 1982.535, p < 0.0001).
Interestingly, when we looked at possible individual differences between the number of olfactory and non-olfactory sensilla on the two antennae, we found that most individuals of both species showed individual-level asymmetry (
Table 1 and
Table 2). Ten out of 14 individual
O. rufa showed a significantly higher number of olfactory sensilla (estimated by the two-tailed binomial test,
p < 0.05) either on the right (two individuals) or the left (eight individuals) antenna (
Table 1). Nine of 14 mason bees, six of which were the same individuals that showed individual asymmetry for the olfactory sensilla, had more non-olfactory sensilla either on the right (four individuals) or the left (five individuals) antenna (
Table 1).
Six out of 14 individual
A. australis showed a significantly higher number of olfactory sensilla (estimated by two-tailed binomial test,
p < 0.05) either on the right (four individuals) or the left (two individuals) antenna (
Table 2). The same two individuals that had more olfactory sensilla on the left antenna, also had significantly more non-olfactory sensilla on the left antenna (
Table 2).
4. Discussion
We showed that both O. rufa and A. australis do not show differences at the population level in the number of olfactory and non-olfactory sensilla on the right and the left antennae. However, about half of the individuals of both species presented individual-level asymmetry—some bees had more olfactory (and/or non-olfactory) sensilla on the right antenna and others had more sensilla on the left antenna.
Our results seem to be partially in line with previous findings. In fact, although previous studies found that the right antenna presents on average (i.e., at the population level) a higher number of at least one type of olfactory sensilla compared to the left antenna in the species
A. mellifera [
13],
B. terrestris [
12] and
A. japonicas Ashmead [
19], no significant differences in the number of either olfactory or non-olfactory sensilla between the left and the right antennae were found in
T. carbonaria [
16].
As both
T. carbonaria and
A. australis show population level lateralized behavior in the recall of olfactory memories [
10], as do honeybees [
8], we are tempted to conclude that this behavioral asymmetry cannot be explained by a different number of sensilla on the right compared to the left antenna. Indeed, for honeybees the difference between the number of olfactory receptors between the right and the left antenna is only 5% [
13]. It is likely that behavioral asymmetry in the learning and recall of olfactory memories through the circuits of the right and left antenna is due to other functional asymmetries in the way these memories are represented and processed in the brain, as shown in the antennal lobe of
A. mellifera [
15]. Moreover, lateralized behavior may be due to asymmetries in the expression of specific neurotransmitters between the two sides on the nervous system, as suggested by a study by Biswas and colleagues [
17], showing that levels of neuroligin-1 expression are higher in honeybees with only their right antenna compared to honeybees with only the left or both antennae [
17].
It is also important to consider that the asymmetrical processing of odors may be advantageous to the single individual as this would allow, for example, the learning of new odors with one hemisphere and the keeping of memories of old odors in the other hemisphere [
9]; this does not necessarily imply that all the individuals should be aligned in the same direction. Indeed, in our study we showed that about half of the individuals in the population (depending on the species considered) are lateralized at the individual level. The individual asymmetry that we observed in the current study of
O. rufa matches well with previous findings of individual-level lateralization in the EAG responses [
11]. In fact, 15 individuals out of 21 (71%) were found to have significantly stronger responses with either the right (seven individuals) or the left antenna (eight individuals) [
11]. Here, 10 out of 14 (71%—exactly the same proportion) of mason bees showed significant asymmetry in the number of olfactory sensilla, and nine out of 14 individuals in the non-olfactory sensilla, regardless of the direction.
An alignment of lateralization within the population has been suggested to be a consequence of social pressure [
20] and to arise as an evolutionarily stable strategy when individually asymmetrical organisms must coordinate their behavior with that of other individually asymmetrical organisms within the same species [
21,
22]. Recently, evidence has started to emerge suggesting that also so-called “non-social” species of insects are lateralized at the population level when biases in social interactions are considered. This is the case for
O. rufa, a species that does not show behavioral asymmetry in the recall of short-term olfactory memory, but shows population-level lateralization in aggressive displays [
23], similarly to eusocial honeybees [
24] and social stingless bees
T. carbonaria [
18]. Thus, it is plausible that
A. australis would also exhibit population-level biases in competitive and/or cooperative interactions with other individuals.
Clearly, it is the possibility of being engaged in interactions with other individuals rather than the way in which the species nests (socially or not) that may affect lateralization. Nonetheless the reason why some species show individual-level or population-level lateralization at the peripheral level, i.e., in the antenna, and what, if any, is the functional significance of this remains at present unexplained. It is possible that morphological asymmetry in the number of olfactory sensilla on the antennae influences the learning process of odors rather than the recall of the memories associated with these odors. Likewise, an asymmetrical distribution of non-olfactory sensilla may influence other sensory processes and allow parallel processing of different kinds of information in the two sides of the brain.