2.1. Research Site
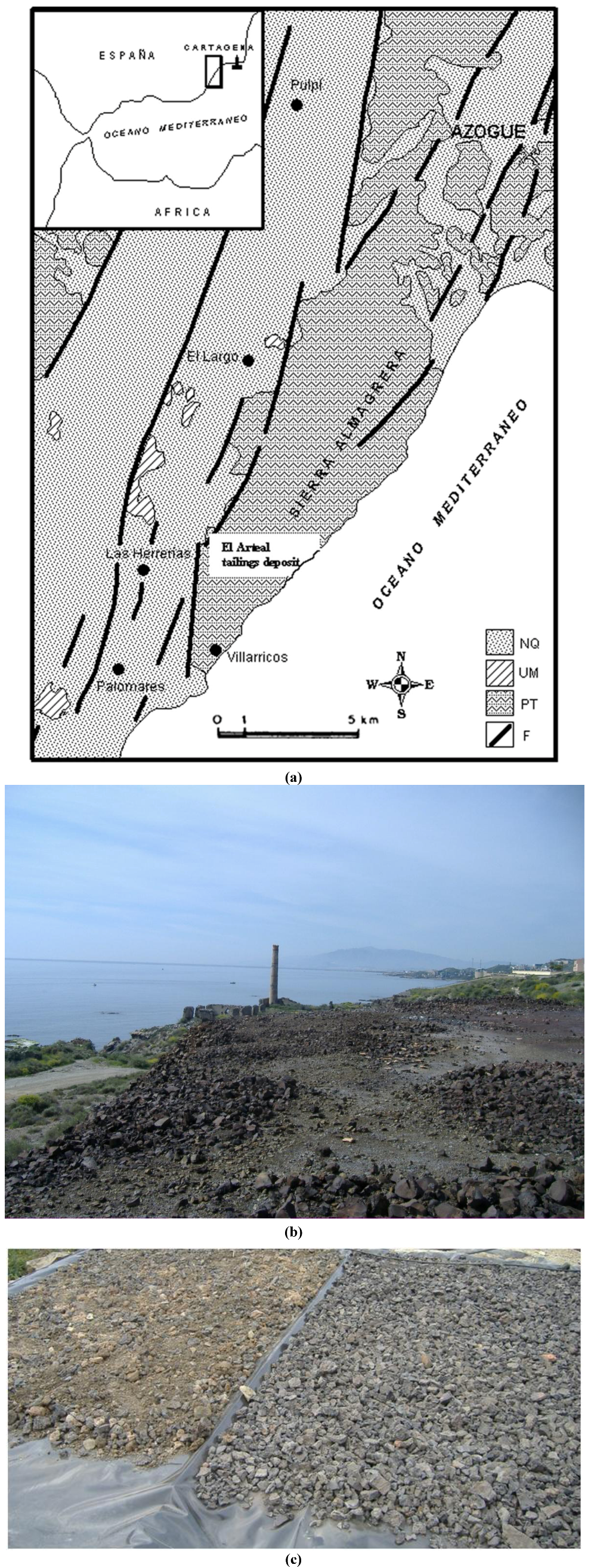
The area comprising the Sierra Almagrera (
Figure 1), located 90 km NE of the city of Almería (SE Spain), together with the Iberian Pyrite Belt and the Cartagena mining district, is the oldest metallurgical and mining area in the Iberian Peninsula [
33,
34,
35]. During the nineteenth century and the early twentieth century, the smelting plants located along the Mediterranean coastline of the Sierra Almagrera district produced approximately 550,000 t of Pb and 3000 t of Ag. The Sierra Almagrera area is located in a semi-arid region that is characterized by a highly irregular hydrological regime with long dry periods and intermittent periods of rainfall, in which the volume of precipitation in a few hours can exceed the annual average. The study area is close to the shoreline in the coastal part of the Sierra Almagrera, where the main slag dumps were generated between 1850 and 1885 (
Figure 1). The waste deposits are now covered by anthropogenic soils and industrial facilities built over part of the metallurgical slags, which were partially used in the road construction. The site covers an area of approximately 120,000 m
2 and has a simple geological structure that comprises a metamorphic basement (phyllites and schists), a level of weathered phyllites, layers of smelter slags, and topsoil mixed with other waste. The study area, which is now used for industrial activities, contains a 4–5 m deep unconfined aquifer. The 2 m deep saturated zone is related to levels established by old smelter slags and/or colluvial deposits.
An important environmental concern related with these contaminated areas is associated with the damage of
Posidonia Oceanica prairies in the coastal area [
36], and the presence of significative contents of metals in the superficial marine sediments. Since
Posidonia Oceanica is a seagrass endemic to Mediterranean Sea and is protected by the European laws, the conservation of this seagrass and, therefore, the control of metal contamination in the terrestrial source-areas may be of environmental concern in the old mining areas of SE Spain. The detailed characterization of the slags dumped in this area was described by Navarro
et al. [
9].
Figure 1.
(a) Location of the study area and regional Geology. NQ: Neogene and Quaternay; UM: Volcanic-shoshonitic rocks of Miocene age; PT: Metamorphic basement; F: Main fractures. (b) Detail of smelting slags dumps in Sierra Almagrera (Almería, Spain). (c) Detail of the experimental device.
Figure 1.
(a) Location of the study area and regional Geology. NQ: Neogene and Quaternay; UM: Volcanic-shoshonitic rocks of Miocene age; PT: Metamorphic basement; F: Main fractures. (b) Detail of smelting slags dumps in Sierra Almagrera (Almería, Spain). (c) Detail of the experimental device.
2.2. Organic Amendments
The sewage sludge used is a stabilized biosolid from a depuration process in which seawater was used to eliminate organic matter from pharmaceutical waste waters. The sewage sludge showed a grain size distribution that was dominated by fine particles (94%–98%), an elevated content of organic matter (6.2%–13%) and a high content of soluble salts and gypsum (
Table 1). Other physical parameters of the biosolid were a low dry density and a high degree of saturation.
In addition, the mechanical properties of sewage sludge were determined from soil laboratory tests. Thus, Atterberg limits were calculated showing a liquid limit of 60.8–65.4, a plastic limit of 59.1–60.0 and a plasticity index of 0.8–6.2 (
Table 1). Besides, the air-dried compacted sludge was tested in vane shear assays, showing an effective angle of shearing resistance of 29.2–30.3, below the values obtained in similar experiences [
37]. The sewage sludge had a high content of Ca (17.7%), S (5.28%), Mg (0.74%), P (5.71%) and particularly Na (1.38%), possibly due to the use of seawater in the treatment process that created the sludge. The metal content in the sludge was very low (
Table 2), except for V (142 mg/kg). The amounts of As (9.9 mg/kg), Cd (0.5 mg/kg), Cu (12 mg/kg), Fe (7900 mg/kg), Pb (7 mg/kg) and Zn (78 mg/kg) were close to the concentrations detected in non-contaminated soils in the nearby region (
Table 2). However, concentrations of Ba and Ni are above the values detected in the non-contaminated soils. The metal content of the sludge did not exceed the limits imposed by Spanish environmental regulations (RD 13-10/1990) for the total metal content in sewage sludge used on agricultural land and are above the Netherlands Soil Contamination Guidelines (intervention values).
2.3. Contaminated Soils and Smelting Slags
The total amount of metals and trace elements was analyzed by Instrumental Neutron Activation Analysis (INAA) and Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). Au, Ag, As, Ba, Br, Ca, Ce, Co, Cr, Cs, Eu, Fe, Hf, Hg, Ir, La, Lu, Na, Ni, Nd, Rb, Sb, Sc, Se, Sm, Sn, Sr, Ta, Th, Tb, U, W, Y and Yb were found by INAA, and Mo, Cu, Pb, Zn, Ag, Ni, Mn, Sr, Cd, Bi, V, Ca, P, Mg, Tl, Al, K, Y and Be were found by ICP-OES.
Previously, the mineralogy of soils and some slags was examined under a binocular microscope and analyzed using X-ray powder diffraction (XRD) at Actlabs (Ontario, Canada) and the Autonomous University of Barcelona (UAB).
Contaminated soils and smelting slags used in the leaching experiments showed high concentrations of Ag (46.1 mg/kg), As (51.6 mg/kg), Ba (101,000 mg/kg), Cu (227 mg/kg), Fe (20.4%), Mn (10,600 mg/kg), Pb (4900 mg/kg), Sb (243 mg/kg) and Zn (5070 mg/kg) (
Table 2).
The slags were composed mainly of quartz, fayalite, barite, orthoclase and Zn-Pb-Fe alloys. In addition, the following weathering-related secondary phases were detected: jarosite-natrojarosite, corrensite, ferrihydrite, kastningite and wuestite. No significant glass phases were detected in previous analyzed slags [
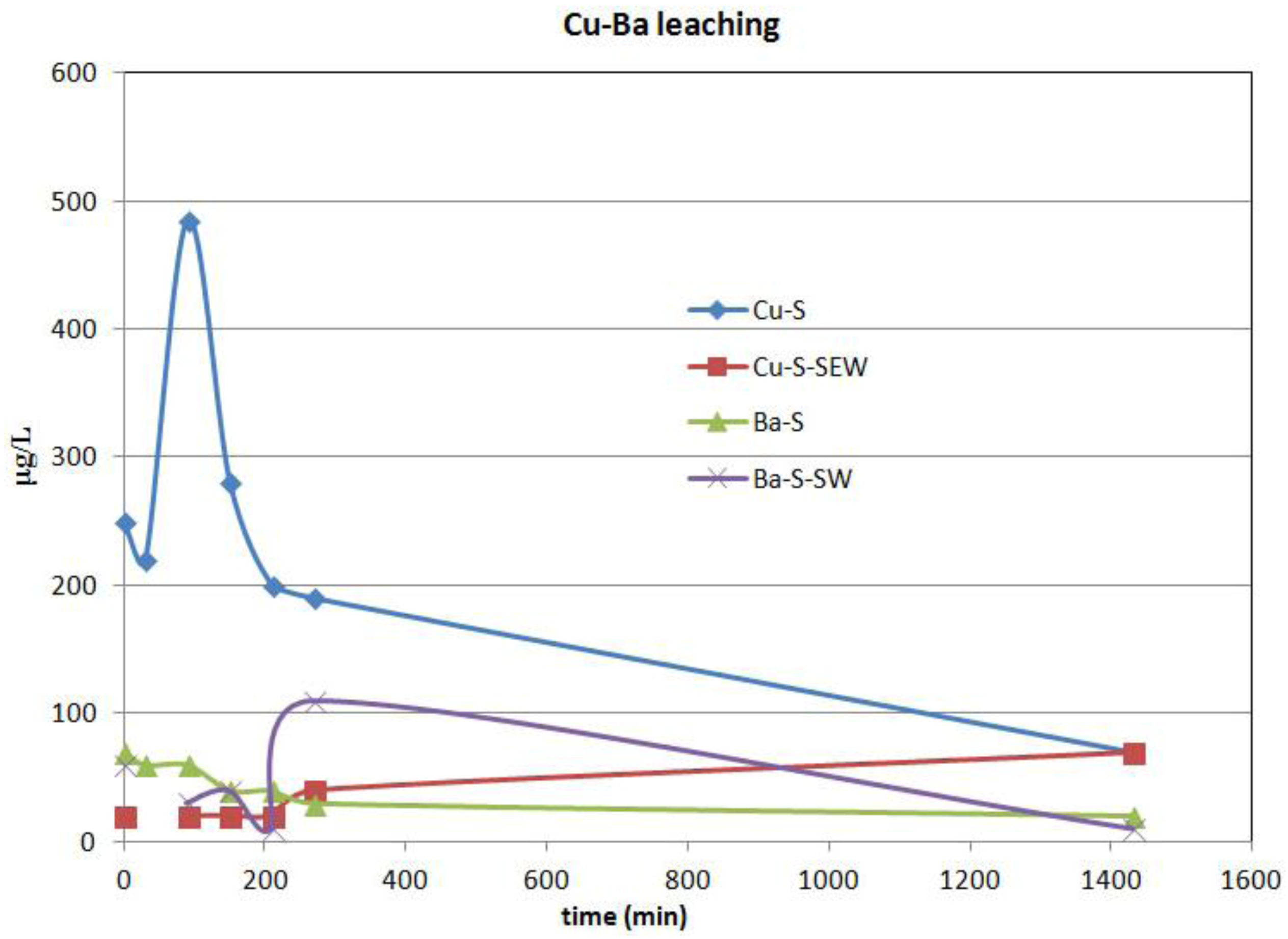
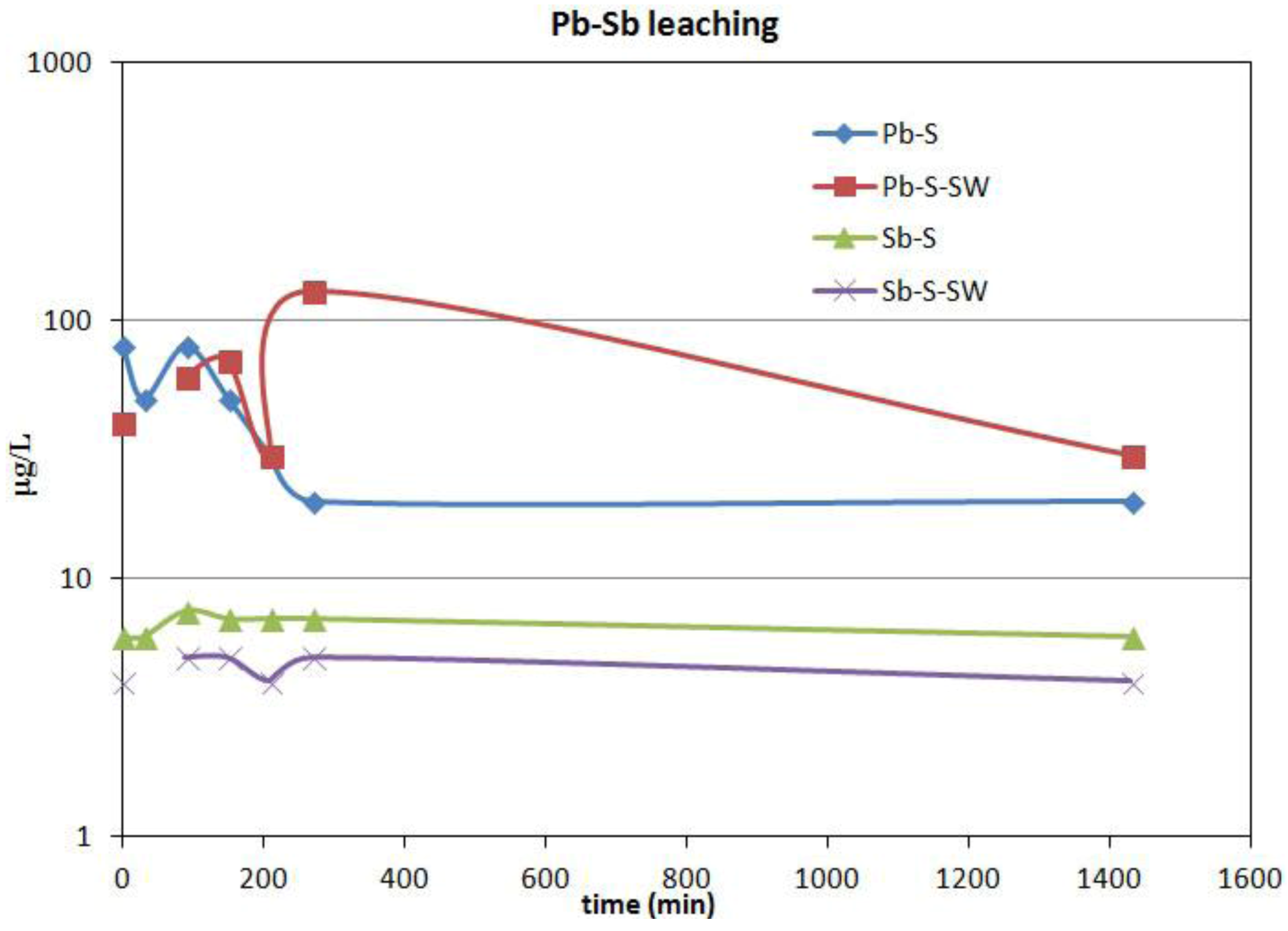
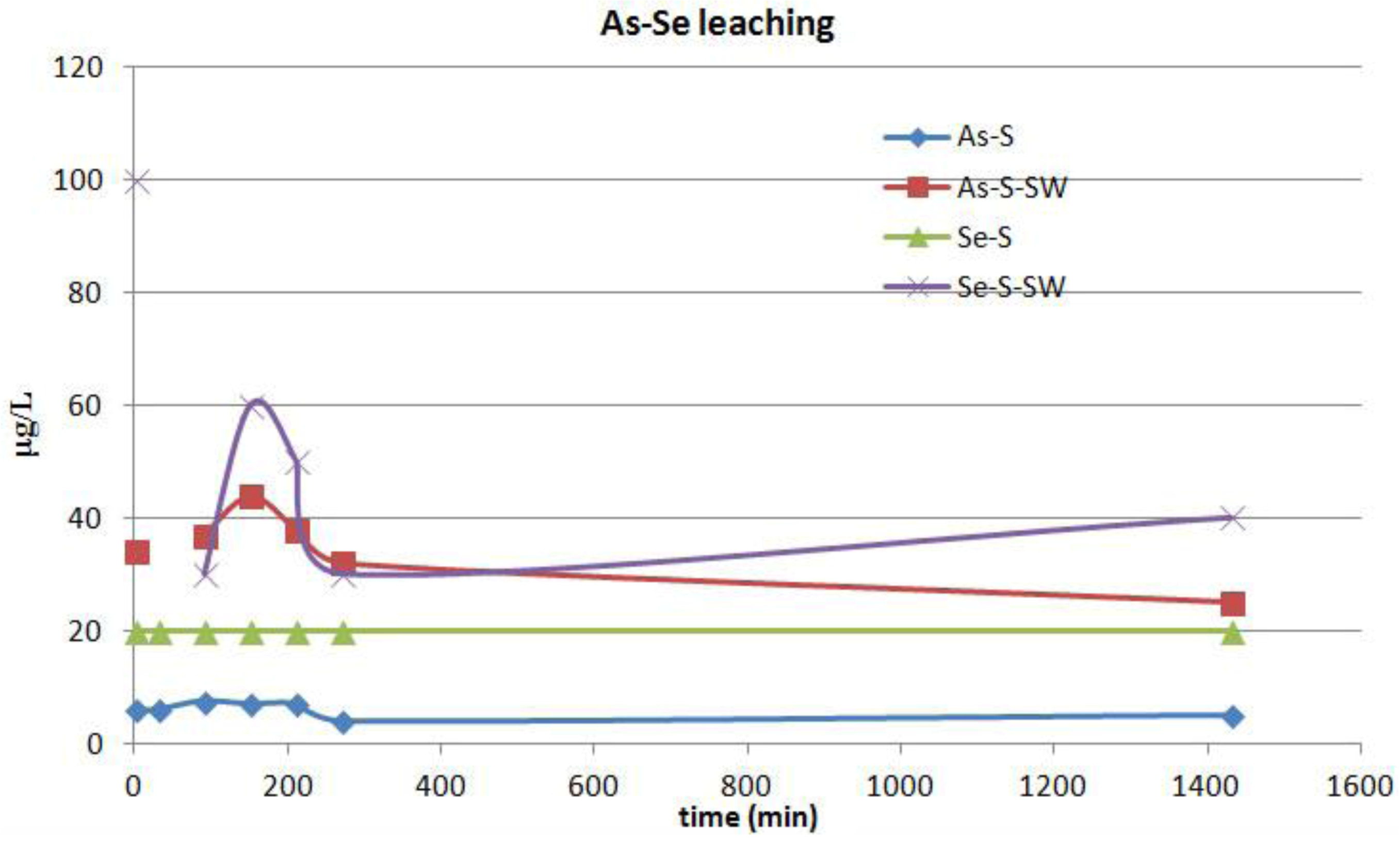
9]. In addition, previous hydrogeochemical data showed that groundwater in the area had been contaminated by Cu, Fe, Mn, Pb, Zn and As to maximum concentrations of 0.64 mg/L Cu, 40 mg/L Fe, 0.6 mg/L Mn, 7.6 mg/L Zn, 5.1 mg/L Pb and 0.019 mg/L As [
9]. Differences in the geochemical composition between the historically analyzed slags [
9] and the slags used in the experiments may reflect the variation in furnace charges and smelting conditions over time [
6].
Table 1.
Main characteristics of biosolid (sewage sludge) used in the experiments.
Table 1.
Main characteristics of biosolid (sewage sludge) used in the experiments.
| Sample | Gravel (%) | Sand (%) | Fine Sand (%) | Fines (%) | Organic Matter (%) | Soluble Salts (%) | Gypsum (%) | W (%) | ρs | ρp | e0 | S (%) | WL | WP | IP |
|---|
| M01 | 0 | 0 | 2 | 98 | 13 | 29.55 | 32.78 | 112.5 | 0.61 | 2.3 | 2.78 | 93.0 | 60.8 | 60.0 | 0.8 |
| M02 | 0 | 0 | 6 | 94 | 6.2 | 24.7* | - | 41.67 | 1.09 | 2.41 | - | - | 65.4 | 59.1 | 6.2 |
Table 2.
Geochemical composition of biosolid, and contaminated materials (contaminated soils P1 to P4 and smelting slags) used in the leaching experiments. Values in mg/kg, except Au (ppb) and Fe (%).
Table 2.
Geochemical composition of biosolid, and contaminated materials (contaminated soils P1 to P4 and smelting slags) used in the leaching experiments. Values in mg/kg, except Au (ppb) and Fe (%).
| Material | Au | Ag | Cu | Cd | Mo | Pb | Ni | Zn | As | Ba | Co | Cr | Fe | Hg | Mn | Sb | Se | V |
|---|
| Biosolid | <2 | 0.3 | 12 | 0.5 | 2 | 7 | 39 | 78 | 9.9 | 540 | 3 | 40 | 0.79 | <1 | 62 | 2.8 | <3 | 142 |
| P1-A | <2 | <0.3 | 20 | 1.9 | <1 | 97 | 57 | 227 | 34.9 | 900 | 18 | 139 | 6.29 | <1 | 489 | 4.7 | <3 | 142 |
| P1-B | <2 | <0.3 | 20 | 1.8 | <1 | 508 | 43 | 122 | 87.1 | 660 | 19 | 102 | 4.97 | <1 | 417 | 16.5 | <3 | 64 |
| P2-A | <2 | 0.4 | 22 | 12 | 1 | 2450 | 33 | 157 | 278 | 850 | 10 | 100 | 3.93 | <1 | 358 | 40.8 | <3 | 110 |
| P2-B | <2 | 0.6 | 23 | 8.8 | <1 | 2390 | 28 | 110 | 324 | 580 | 10 | 88 | 3.63 | <1 | 358 | 47.3 | <3 | 102 |
| P3-A | <2 | 0.6 | 27 | 7.6 | 2 | 4900 | 39 | 192 | 337 | 1030 | 15 | 105 | 4.39 | <1 | 469 | 63.4 | <3 | 129 |
| 3-B | <2 | <0.3 | 33 | 8.5 | <1 | 2440 | 49 | 229 | 367 | 690 | 18 | 126 | 5.19 | <1 | 457 | 34.2 | <3 | 162 |
| P4-A | <2 | <0.3 | 30 | 0.6 | <1 | 73 | 41 | 112 | 31.3 | 690 | 13 | 82 | 3.45 | <1 | 571 | 4.9 | <3 | 107 |
| P4-B | <2 | <0.3 | 34 | 1.4 | <1 | 67 | 52 | 125 | 60.5 | 530 | 16 | 117 | 5.18 | <1 | 513 | 7.8 | <3 | 142 |
| Smelting slags | <2 | 46.1 | 227 | 0.6 | 18 | 4460 | 17 | 5070 | 51.6 | 10.1* | 10 | 40 | 20.4 | <1 | 1.0* | 243 | <3 | 87 |
| NCS | 3 | <0.4 | 15 | <0.5 | <1 | 17 | 26 | 57 | 16 | 310 | 8 | 55 | 2.69 | <1 | 136 | 1.8 | <3 | 96 |
| NSGS | -- | -- | 190 | 12 | 200 | 530 | 210 | 720 | 55 | 625 | 240 | 380 | -- | 10 | -- | 15 | -- | -- |
2.4. Column Leaching Experiments
A leaching experiment was conducted in order to characterize the behavior of biosolid and the mobility of their components. The experiment comprised a column test consisted of a water reservoir, a peristaltic pump, a methacrylate column, and a series of instruments for determining some parameters of the effluents
in situ, such as pH, Eh, EC (electrical conductivity), temperature and dissolved O
2. The column was made in a methacrylate cylinder, thus allowing for visual examination of the progress of the wetting front and detection of preferential flow channels along the column walls. The column was 750 mm long, with an outer diameter of 150 mm and a thickness of 5 mm [
15,
38].
Low mineralized water (LMW) entered the column through an injection system connected to a metering pump, which emitted a maximum flow of 10 L·h
−1. The chemical characteristics of LMW are shown in
Table 3. A constant-head reservoir was used to deliver influent water at a flow rate of 2.4 L·h
−1. The inflow fluid was low mineralization water, which approximates the behavior of rainwater when it comes into contact with the waste in environmental conditions (
Table 3). The samples were collected at the bottom of the column as a function of time. The first sample, which corresponds to time 0, was taken when water started to flow from the lower part of the column. The flow, pH and EC (electrical conductivity) were measured immediately after the sample was collected. The number of pore volumes reached in the column experiments is given by the following expression:
where
Ta is the number of pore volumes,
V the pore velocity,
L the column length and
t the leaching time. In the column experiment, the final
Ta was 4.7 pore volumes, which indicates a time period that can be considered as representative.
Table 3.
Chemical composition of fluids from column leaching experiment with biosolid. Values in μg/L.
Table 3.
Chemical composition of fluids from column leaching experiment with biosolid. Values in μg/L.
| Samples | PV | Cu | Pb | Sb | As | Se | Mn | Ni | EC (μS/cm) | Cl (mg/L) | SO4 (mg/L) |
|---|
| SL-1 | 0 | 178 | <0.1 | 0.59 | 28.1 | <0.2 | 274 | 161 | 5860 | 570 | 980 |
| SL-2 | 0.45 | 13 | 134 | 0.25 | 5.59 | 1.3 | 9.8 | 14 | - | - | - |
| SL-3 | 0.9 | 9.3 | <0.1 | 0.21 | 5.31 | 0.5 | 5.4 | 5.5 | - | - | - |
| SL-4 | 1.35 | 4.8 | <0.1 | 0.23 | 4.09 | <0.2 | 7.9 | 2.2 | - | - | - |
| SL-5 | 1.8 | 9.6 | <0.1 | 0.19 | 4.21 | <0.2 | 3.3 | 1.8 | - | - | - |
| SL-6 | 2.2 | 2.7 | <0.1 | 0.16 | 3.17 | <0.2 | 2.4 | 1.9 | - | - | - |
| SL-7 | 2.7 | 1.6 | <0.1 | 0.16 | 2.5 | <0.2 | 1.4 | 0.5 | - | - | - |
| SL-8 | 3.4 | 2.4 | <0.1 | 0.13 | 2.2 | <0.2 | 2.8 | 0.7 | - | - | - |
| SL-9 | 4.0 | 1.5 | <0.1 | 0.11 | 2.02 | <0.2 | 1.1 | 0.4 | - | - | - |
| SL-10 | 4.7 | 6.5 | <0.1 | 0.12 | 1.89 | <0.2 | 1.5 | 0.4 | - | - | - |
| LMW | - | <0.2 | 0.1 | 0.02 | 0.34 | <0.2 | <0.1 | <0.3 | 250 | 5.6 | 11.1 |
2.5. Pilot-Scale Experiments
The experimental device covered a surface of 30 m
2 approximately, divided into two sub-areas of 15 m
2: an area of not-amended materials leaching, and an area of leaching of contaminated materials and organic amendments. These amendments comprised about 10% by weight of total soil and slags used in the experiment. The experimental system was fed by low mineralized water (LMW) (
Table 4 and
Table 5) for 24 h with a constant flow rate of 5 L·h
−1. The materials were laid over a low hydraulic conductivity liner (HDEP) with a slope of approximately 5%, to facilitate the leachate movement.
The LMW was introduced in the top of the experimental cells and flowed by means of a trickle irrigation system, which divided the total flow rate into several points of water recharge. The leachate sampling device comprised a narrow channel located in the lower area of each experimental sub-cell leaching, where the leachates were sampled with the aid of a syringe. We measured the pH, redox potential (Eh; mV), temperature, and EC (μS/cm) in the leachates in situ using portable devices, and corrected the values using standard solutions (HACH sensION TM378). The samples were filtered using a cellulose nitrate membrane with a pore size of 0.45 μm. The samples for cation analysis were later acidified to pH<2.0 by adding ultra-pure HNO3. The samples were collected in 110 mL high-density polypropylene bottles, which were sealed with a double cap and stored in a refrigerator until analysis.
The cation concentrations were measured using inductively coupled plasma mass spectroscopy (ICP-MS) at ACTLABS (Ontario, Canada). The concentrations of chloride, nitrate and sulfate (in a second, untreated sample) were analyzed by ion chromatography. The alkalinity of the waters was analyzed by titration. The standard reference material NIST 1640 (ICP-MS) was used to evaluate accuracy.
To assess the potential role of organic matter in the mobility of metals, we used the Visual Minteq numerical code [
39]. Visual Minteq includes a submodel for estimations of the complexation of metals with dissolved organic matter, which assumes that the composite ligand consists of a population of discrete binding sites in which the probability of occurrence of a binding site is normally distributed (Gaussian model) with respect to its log K value for proton or metal binding [
40].