Technological Proposals for Recycling Industrial Wastes for Environmental Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Original Samples
| Sample name | Sample characteristics |
|---|---|
| PG | Original phosphogypsum sample |
| PGTS | Solid phase from phosphogypsum + caustic soda for domestic use |
| PGOS | Solid phase from phosphogypsum + olive industry waste |
| PGOL | Liquid phase from phosphogypsum + olive industry waste |
| PGAS | Solid phase from phosphogypsum + aluminum industry waste |
| PGAS_CO2w | Weathered sample PGAS |
| PGAS_CO2b | Sample PGAS carbonated by bubbling CO2 in aqueous media |
| PGT_mortar | Mortar obtained from PGTS |
2.1.1. Phosphogypsum Waste
| Technique | Posphogypsum waste [9] | Olive industry waste | Aluminum-rich waste |
|---|---|---|---|
| XRF (wt %) | [SO3] = 50.2% | - | [Na2O] = 80.24% |
| [CaO] = 44.7% | [Al2O3] = 17.81% | ||
| [Na2O] = 1.2% | [Cl] = 1.23% | ||
| [F] = 1.6% | [SiO2] = 0.52% | ||
| Chemical and elemental analyses | - | [Na] = 45.7 ± 0.1 mg/L | [Na] = 148 ± 2 g/L [Al] = 87.3 ± 1.4 g/L |
| [Ca] = 17 ± 1 mg/L | |||
| [C] = 1.5 ± 0.1% | |||
| [N] ≤ 0.01% |
2.1.2. Low-Cost Soda Samples
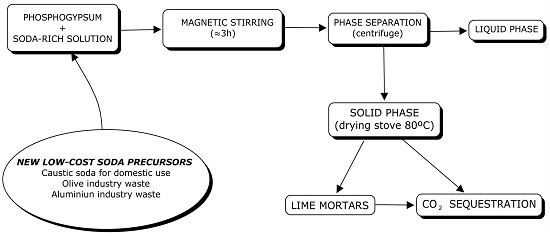
2.2. Sample Preparation
2.3. CO2 Sequestration Experiments
2.4. Characterization Techniques

3. Results and Discussion
3.1. Phase Separation
3.1.1. Phosphogypsum and Caustic Soda

3.1.2. Phosphogypsum and Olive Industry Waste
3.1.3. Phosphogypsum and Aluminum-Rich Waste


3.2. CO2 Sequestration by Reusing Phosphogypsum and Aluminum-Rich Wastes

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Keeling Curve. A Daily Record of Atmospheric Carbon Dioxide from Scripps Institution of Oceanography at UC San Diego. Available online: http://keelingcurve.ucsd.edu/ (accessed on 31 June 2014).
- Bahor, B.; van Brunt, M.; Stovall, J.; Blue, K. Integrated waste management as a climate change stabilization wedge. Waste Manag. Res. 2009, 27, 839–849. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M. Carbon Mineralization: From Natural Analogues to Engineered Systems. Rev. Mineral. Geochem. 2013, 77, 305–360. [Google Scholar] [CrossRef]
- Kirchofer, A.; Becker, A.; Brandt, A.; Wilcox, J. CO2 Mitigation Potential of Mineral Carbonation with Industrial Alkalinity Sources in the United States. Environ. Sci. Technol. 2013, 47, 7548–7554. [Google Scholar]
- Bobicki, E.R.; Liu, Q.X.; Xu, Z.H.; Zeng, H.B. Carbon capture and storage using alkaline industrial waste. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- International Energy Agency. Tracking Industrial Energy Efficiency and CO2 Emissions; International Energy Agency: Paris, France, 2007. Available online: https://www.iea.org/publications/freepublications/publication/tracking_emissions.pdf (accessed on 16 March 2014).
- Best Available Techniques (BAT) Reference Document for theProduction of Cement, Lime and Magnesium Oxide. European IPPC Bureau, Institute for Prospective Technological Studies, European Commission: Seville, Spain, 2013. Available online: http://eippcb.jrc.ec.europa.eu/reference/BREF/CLM_30042013_DEF.pdf (accessed on 6 August 2014).
- Cárdenas-Escudero, C.; Morales-Flórez, V.; Pérez-López, R.; Santos, A.; Esquivias, L. Procedure to use phosphogypsum industrial waste for mineral CO2 sequestration. J. Hazard. Mater. 2011, 196, 431–435. [Google Scholar] [CrossRef]
- Habashi, F. Bayer’s process for alumina production: A historical perspective. Bull. Hist. Chem. 1995, 17–18, 15–19. [Google Scholar]
- Morales-Flórez, V.; Santos, A.; Lemus, A.; Esquivias, L. Artificial weathering pools of calcium-rich industrial waste for CO2 sequestration. Chem. Eng. J. 2011, 166, 132–137. [Google Scholar] [CrossRef]
- Herrero, J.; Artieda, O.; Hudnall, W.H. Gypsum, a Tricky Material. Soil Sci. Soc. Am. J. 2009, 73, 1757–1763. [Google Scholar] [CrossRef]
- Aïtcin, P.C. High Performance Concrete, 1st ed.; E & FN Spon Ltd.: London, UK, 1998. [Google Scholar]
- UNE-EN 13139/AC:2004. Available online: https://www.aenor.es/AENOR/normas/normas/fichanorma.asp?tipo=N&codigo=N0032204&PDF=Si-.U9p9sFZSHIs (accessed on 5 August 2014).
- Pérez-López, R.; Nieto, J.M.; López-Coto, I.; Aguado, J.L.; Bolívar, J.P.; Santisteban, M. Dynamics of contaminants in phosphogysump of fertilizer industry of Huelva (SW Spain): From phosphate rock ore to the environment. Appl. Geochem. 2010, 25, 705–715. [Google Scholar] [CrossRef]
- Santos, A.; Ajbary, M.; Morales-Flórez, V.; Kherbeche, A.; Piñero, M.; Esquivias, L. Larnite powders and larnite/silica aerogel composites as effective agents for CO2 sequestration by carbonation. J. Hazard. Mater. 2009, 168, 1397–1403. [Google Scholar] [CrossRef]
- Morales-Flórez, V.; Santos, A.; López, A.; Moriña, I.; Esquivias, L. Calcium Silicates synthesized from industrial residues with ability for CO2 sequestration. Waste Manag. Res. 2014. accepted for publication. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997; pp. 19–24. [Google Scholar]
- Puertas, F.; Blanco-Varela, M.T.; Vazquez, T. Behaviour of cement mortars containing an industrial waste from aluminiun refining: Stability in Ca(OH)2 solutions. Cem. Concr. Res. 1999, 29, 1673–1680. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Romero-Hermida, I.; Morales-Flórez, V.; Santos, A.; Villena, A.; Esquivias, L. Technological Proposals for Recycling Industrial Wastes for Environmental Applications. Minerals 2014, 4, 746-757. https://doi.org/10.3390/min4030746
Romero-Hermida I, Morales-Flórez V, Santos A, Villena A, Esquivias L. Technological Proposals for Recycling Industrial Wastes for Environmental Applications. Minerals. 2014; 4(3):746-757. https://doi.org/10.3390/min4030746
Chicago/Turabian StyleRomero-Hermida, Isabel, Víctor Morales-Flórez, Alberto Santos, Antonio Villena, and Luis Esquivias. 2014. "Technological Proposals for Recycling Industrial Wastes for Environmental Applications" Minerals 4, no. 3: 746-757. https://doi.org/10.3390/min4030746