N2 and CO2 Adsorption by Soils with High Kaolinite Content from San Juan Amecac, Puebla, México
Abstract
:1. Introduction
2. Materials and Methods
2.1. X-Ray Diffraction (XRD) and Energy-Dispersive X-Ray Spectroscopy (EDS)
2.2. N2 Adsorption
2.3. CO2 Adsorption
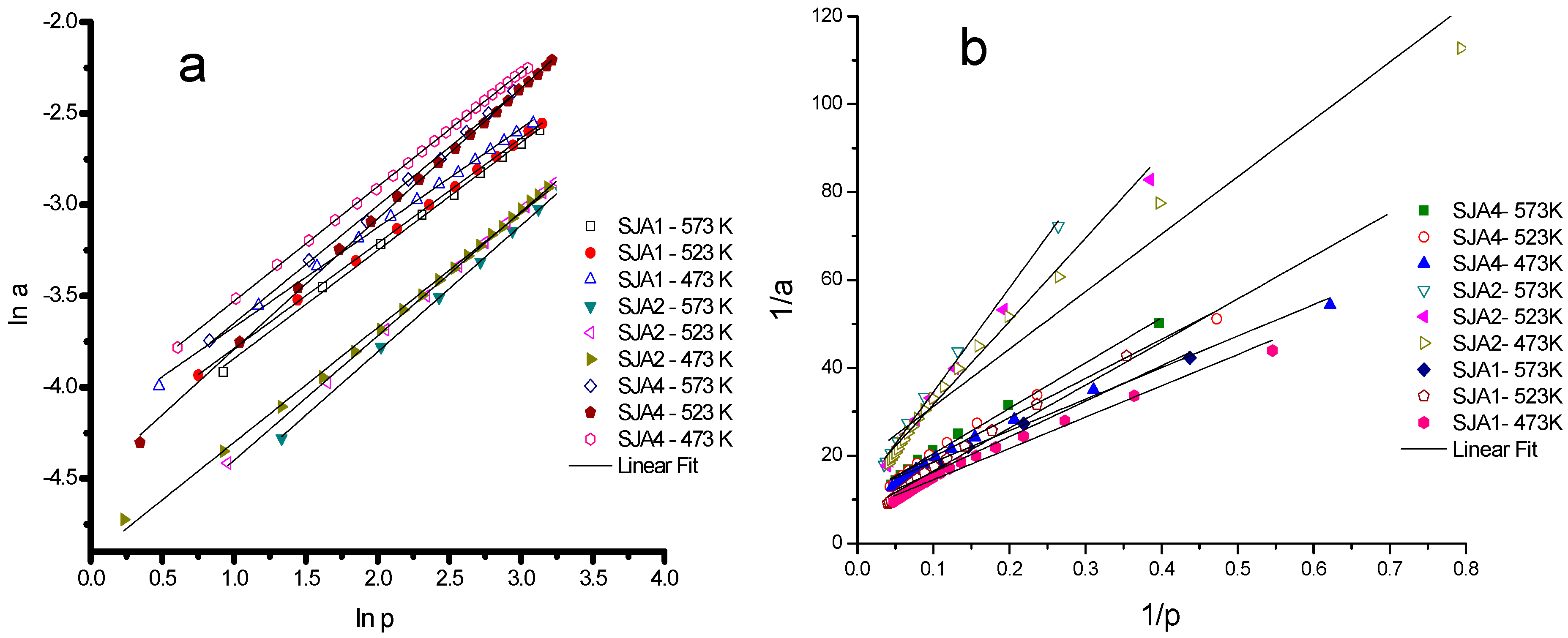
2.4. Data Analysis
3. Results and Discussion
3.1. Crystalline Phases and Chemical Composition
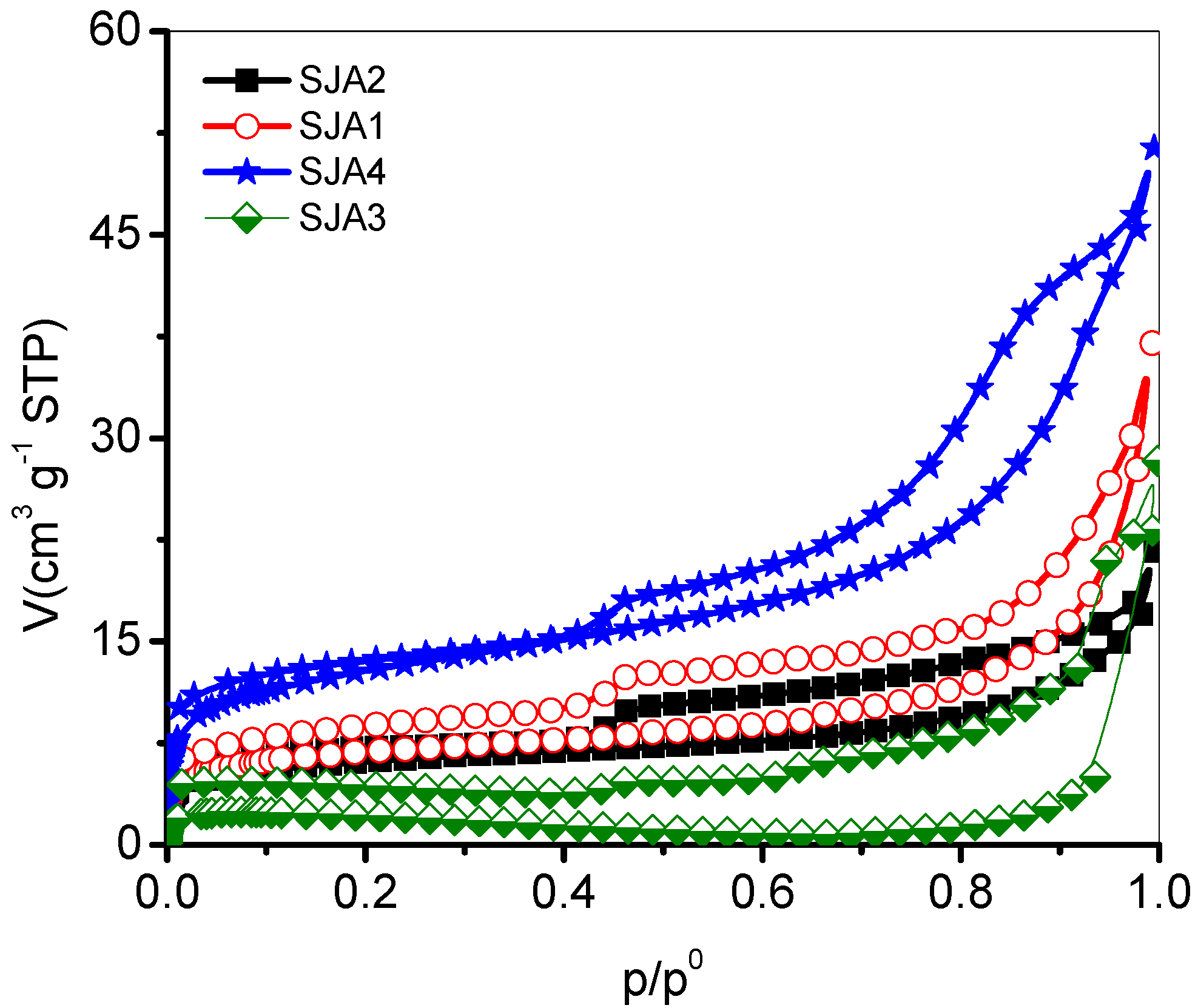
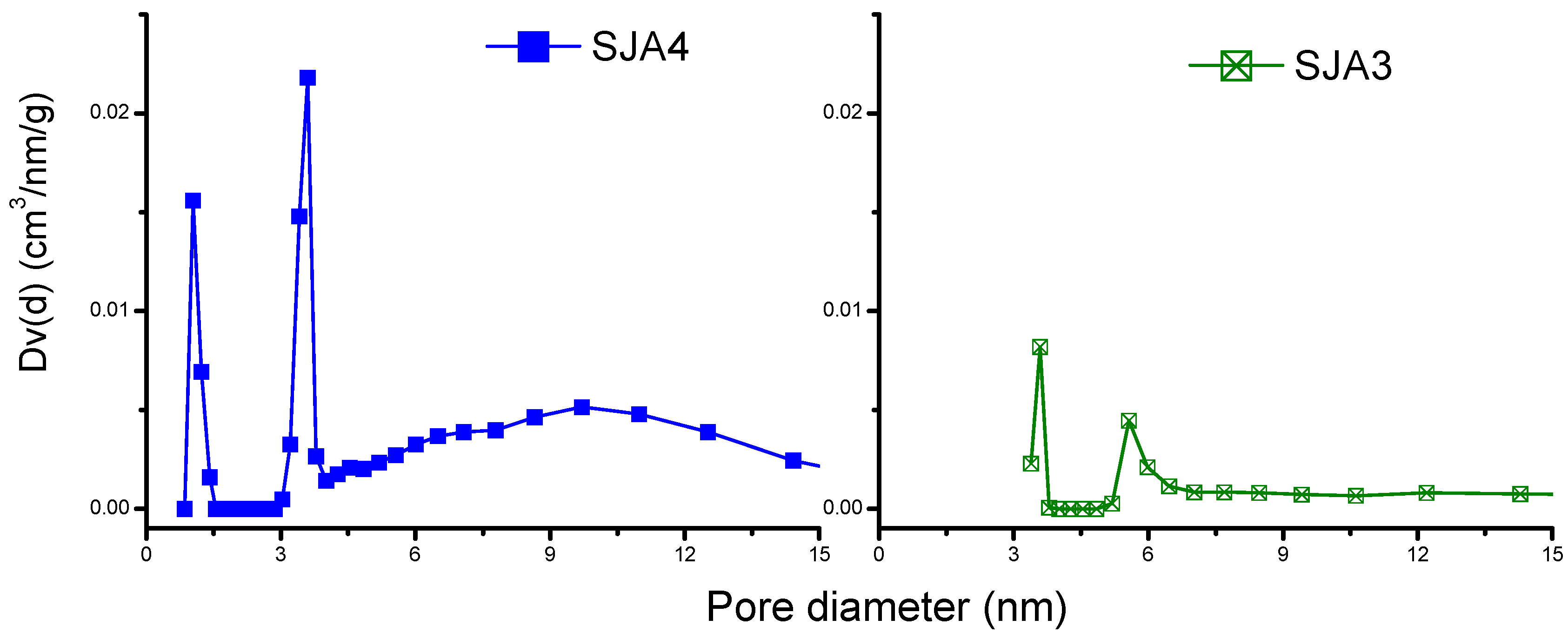
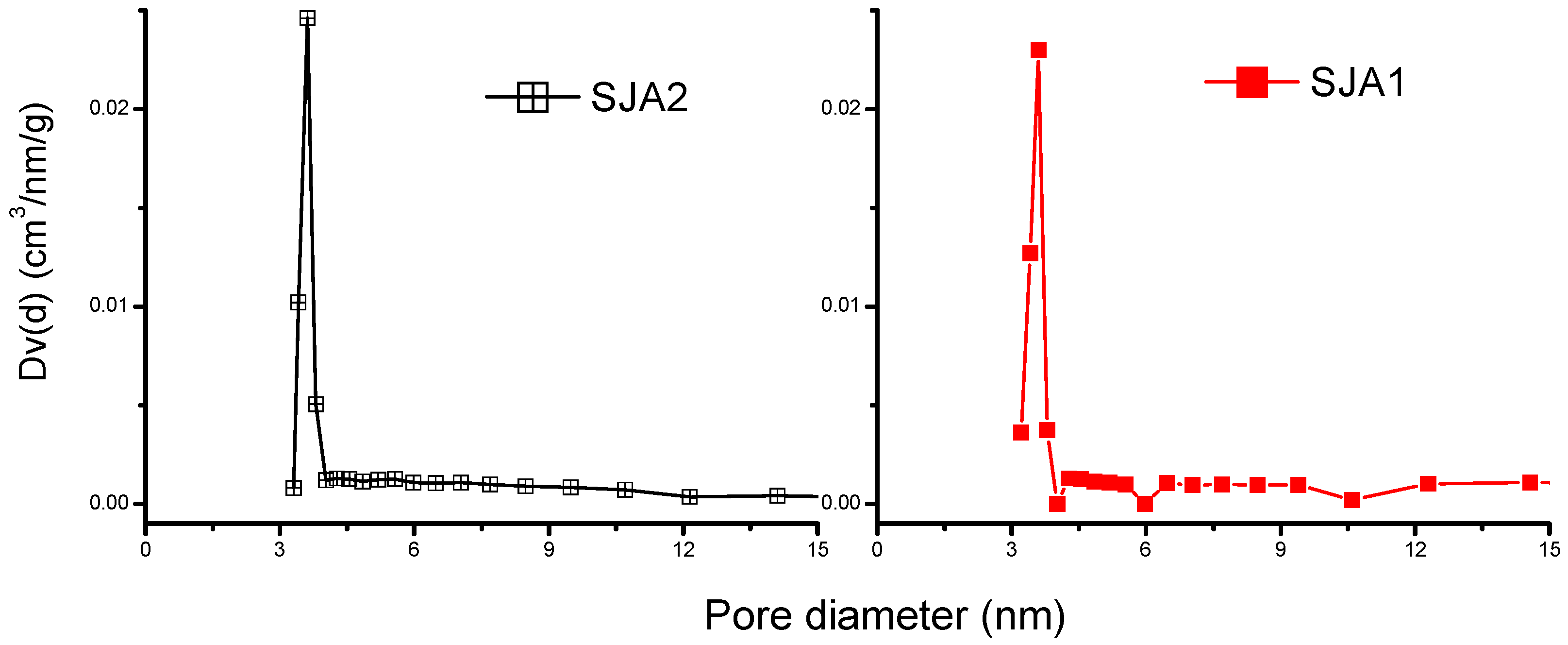
3.2. N2 Adsorption
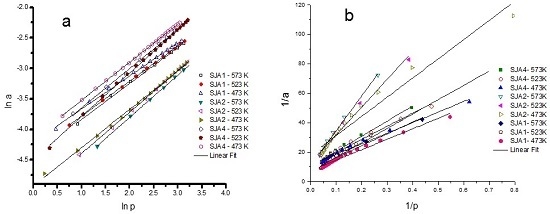
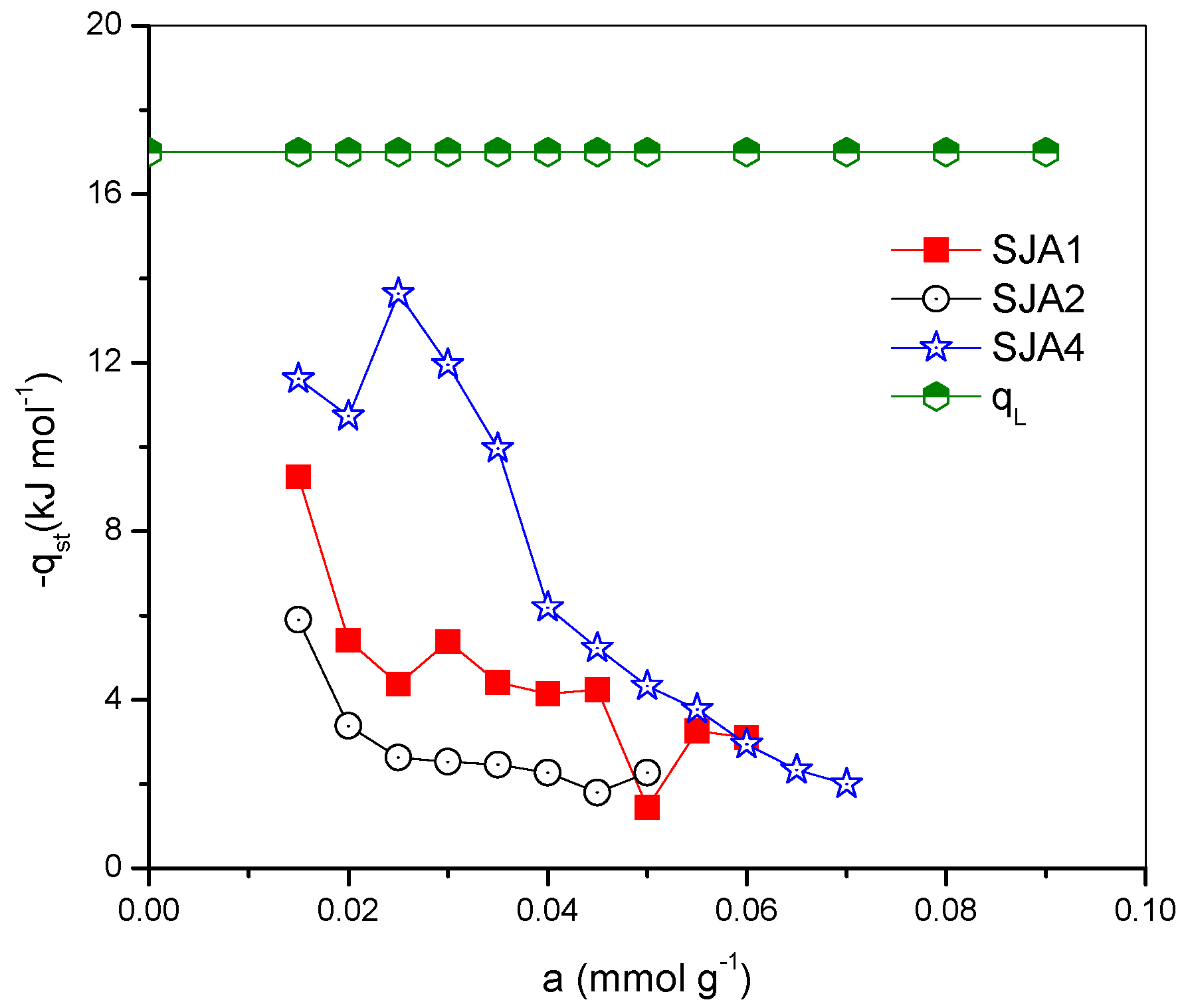
3.3. CO2 Adsorption
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Férey, G.; Serre, C.; Devic, T.; Maurin, G.; Jobic, H.; Llewellyn, P.; De Weireld, G.; Vimont, A.; Daturi, M.; Chang, J. Why hybrid porous solids capture greenhouse gases? Chem. Soc. Rev. 2011, 40, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Maurin, G.; Llewellyn, P.; Bell, R. Adsorption mechanism of carbon dioxide in Faujasites: Grand Canonical Monte Carlo simulations and microcalorimetry measurements. J. Phys. Chem. B 2005, 109, 16084–16091. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, W.; Zhang, Z.S.; Xing, W.; Zhuo, S. Carbon dioxide adsorption performance of N-doped zeolite Y templated carbons. RSC Adv. 2012, 2, 161–167. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L., Jr.; Chen, Z. Climate Change 2007: The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 4. [Google Scholar]
- Canadell, J.; Le Quére, C.; Raupach, M.; Field, C.; Buitenhuis, E.; Ciais, P.; Conway, T.; Gillett, N.; Houghton, R.; Marland, G. Contribution to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc. Natl. Acad. Sci. USA 2007, 104, 18866–18870. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; McGinley, P.M.; Katz, L.E. A distributed reactivity model for sorption by soils and sediments. Conceptual basis and equilibrium assessments. Environ. Sci. Technol. 1992, 26, 1955–1962. [Google Scholar] [CrossRef]
- Hernández, M.A.; González, A.I.; Corona, L.; Hernández, F.; Rojas, F.; Asomoza, M.; Solís, S.; Portillo, R.; Salgado, M.A. Chlorobenzene, chloroform, and carbon tetrachloride adsorption on undoped and metal-doped sol-gel substrates SiO2, Ag/SiO2, Cu/SiO2 and Fe/SiO2. J. Hazard. Mater. 2009, 162, 254–263. [Google Scholar] [CrossRef] [PubMed]
- López, L.; Hernández, M.A.; Ruiz, J.; Carcaño, M.; Medina, G.; Portillo, R.; Muñoz, J. Adsorción de ácidos carboxílicos de origen vegetal y bacteriano en un suelo agrícola. Terra Latinoam. 2012, 30, 261–270. (In Spanish) [Google Scholar]
- Carbon Dioxide Capture and Storage. Available online: https://www.ipcc.ch/report/srccs/ (accessed on 20 June 2016).
- Bereznitski, Y.; Jaroniec, M.; Maurice, P. Adsorption characterization of two clay minerals society standard kaolinites. J. Colloid Interface Sci. 1998, 205, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, D. CO2 capture by kaolinite and its adsorption mechanism. Appl. Clay Sci. 2015, 104, 221–228. [Google Scholar] [CrossRef]
- Ghosh, D.; Bhattacharyya, K. Adsorption of methylene blue on kaolinite. Appl. Clay Sci. 2002, 20, 295–300. [Google Scholar] [CrossRef]
- Hitch, M.; Li, J. Carbone dioxide sorption isotherm study on pristine and acid-treated olivine and its application in the vacuum swing adsorption process. Minerals 2015, 5, 259–275. [Google Scholar]
- Sen Guptaa, S.; Bhattacharyya, K. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef] [PubMed]
- Filimonova, S.V.; Knicker, H.; Kögel-Knabner, T.I. Soil micro- and mesopores studied by N2 adsorption and 129Xe NMR of adsorbed xenon. Geoderma 2006, 130, 218–228. [Google Scholar] [CrossRef]
- Hauchhum, L.; Mahanta, P. Carbon Dioxide adsorption on zeolites and activated carbon by pressure swing adsorption on a fixed bed. Int. J. Energy Environ. Eng. 2014, 5, 349–356. [Google Scholar] [CrossRef]
- Plaza, M.; Pevida, C.; Pis, J.; Rubiera, F. Evaluation of the cyclic capacity of low-cost carbon adsorbents for post- combustion CO2 capture. Energy Proced. 2011, 4, 1228–1234. [Google Scholar] [CrossRef]
- Pacala, S.; Socolow, R. Stabilization wedges: Solving the climate problem for the next 50 years with current technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.A.; Quiroz, K.; Rojas, F.; Portillo, R.; Salgado, M.; Hernández, F.; Rivera, A. Experiment and modeling of low coverage uptake of N2 and O2 on H-clinoptilolite zeolite from Tehuacán, Puebla, Mexico. J. Chem. Chem. Eng. 2014, 8, 1–10. [Google Scholar]
- Schaef, H.; Glezakou, V.; Owen, A.; Ramprasad, S.; Martin, P.; McGrail, B. Surface condensation of CO2 onto kaolinite. Environ. Sci. Technol. Lett. 2014, 1, 142–145. [Google Scholar] [CrossRef]
- Hernández, M.A.; Portillo, R.; Salgado, M.A.; Rojas, F.; Petranoskii, V.; Pérez, G.; Salas, R. Comparación de la capacidad de adsorción de CO2 en clinoptilolitas naturales y tratadas químicamente. Superf. Vacío 2010, 23, 67–72. (In Spanish) [Google Scholar]
- Choudary, V.R.; Mantri, K. Adsorption of aromatic hydrocarbons on highly siliceous MCM-41. Langmuir 2000, 16, 7031–7037. [Google Scholar] [CrossRef]
- Petacek, P.; fuvátová, D.; Havlica, J.; Brandstetr, J.; Soukal, F.; Opravil, T. Isothermal kinetic analysis of the thermal decomposition of kaolinite: The thermogravimetric study. Thermochim. Acta 2010, 501, 24–29. [Google Scholar] [CrossRef]
- Meroufel, B.; Benali, O.; Benyahia, M.; Benmoussa, Y.; Zenasni, M.A. Adsorptive removal of anionic dye from aqueous solutions by Algerian kaolin: Characteristics, isotherm, kinetic and thermodynamic studies. J. Mater. Environ. Sci. 2013, 4, 482–491. [Google Scholar]
- Besoain, E. Mineralogía de Arcillas de Suelo. Minerales secundarios del suelo: Silicatos cristalinos. In Mineralogia de Arcillas de Suelos; Escoto, J., Ed.; IICA: San José, Costa Rica, 1985; Volume 1, pp. 311–533. (In Spanish) [Google Scholar]
- Volzone, C.; Thompson, J.G.; Melnitchenko, A.; Ortiga, J.; Palothorpe, R.S. Selective gas adsorption by amorphous clay mineral derivatives. Clays Clay Miner. 1999, 47, 647–657. [Google Scholar] [CrossRef]
- Melnitchenko, A.; Thompsion, J.; Volzone, C.; Ortiga, J. Selective gas adsorption by metal exchanged amorphous kaolinite derivatives. Appl. Clay Sci. 2000, 17, 35–53. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, X.; Chen, Y.; Huang, J.; Luo, H.; Deng, S. Adsorption of CO2, CH4, and N2 on ordered mesoporous carbon: Approach for greenhouse capture and biogas upgrading. Environ. Sci. Technol. 2013, 47, 5474–5480. [Google Scholar] [CrossRef] [PubMed]
- Hutson, N.; Speakman, S.; Payzant, A. Structural effects on the high temperature adsorption of CO2 on a synthetic hydrotalcite. Chem. Mater. 2004, 16, 4135–4143. [Google Scholar] [CrossRef]
- Ng, C.; Losso, J.N.; Marshall, W.E.; Rao, R.M. Freundlich adsorption isotherms of agricultural by-product-based powdered activated carbons in a geosmin–water system. Bioresour. Technol. 2002, 85, 131–135. [Google Scholar] [CrossRef]
- Hernández, M.A.; Velasco, J.; Rojas, F.; Campos, E.; Lara, V.; Torres, J.; Salgado, M. Adsorción de compuestos orgánicos volátiles en arcillas del estado de Puebla, México. Rev. Int. Contam. Ambient. 2003, 19, 191–196. (In Spanish) [Google Scholar]
- Hernández, M.A.; Corona, L.; González, A.I. Quantitative study of the adsorption of aromatic hydrocarbons (Benzene, Toluene, and p-Xylene) on dealuminated clinoptilolites. Ind. Eng. Chem. Res. 2005, 44, 2908–2916. [Google Scholar] [CrossRef]
- Alzaydien, A.S.; Manasreh, W. Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto activated phosphate rock. Int. J. Phys. Sci. 2009, 4, 172–181. [Google Scholar]


| Compound | SJA1 | SJA2 | SJA3 | SJA4 |
|---|---|---|---|---|
| Na2O | 1.685 | 2.042 | 0.517 | 1.685 |
| MgO | 0.000 | 0.000 | 4.488 | 2.338 |
| Al2O3 | 14.191 | 14.455 | 10.575 | 15.211 |
| SiO2 | 32.173 | 44.346 | 30.148 | 30.227 |
| K2O | 0.258 | 0.295 | 0.000 | 0.000 |
| CaO | 1.994 | 4.869 | 0.541 | 1.758 |
| TiO2 | 0.000 | 1.043 | 0.323 | 0.167 |
| Fe2O3 | 4.046 | 3.710 | 7.058 | 3.408 |
| SiO2/AlO3 Ratio | 2.267 | 3.068 | 2.851 | 1.987 |
| Sample | AsBET m2·g−1 | R | CBET | BET p/p0 Plot | VΣ cm3·g−1 |
|---|---|---|---|---|---|
| SJA1 | 25 | 0.999 | 240 | 0.010–0.131 | 0.033 |
| SJA2 | 22.2 | 0.999 | 265 | 0.010–0.164 | 0.023 |
| SJA3 | 6.73 | 0.998 | 33.24 | 0.084–0.189 | 0.034 |
| SJA4 | 45.9 | 0.999 | 270 | 0.010–0.189 | 0.064 |
| Muestra | T (K) | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|---|
| R | n | KF | R | am | KL | ||
| SJA1 | 573 | 0.997 | 1.357 | 0.0107 | 0.981 | 0.152 | 0.072 |
| 523 | 0.999 | 1.398 | 0.011 | 0.994 | 0.149 | 0.077 | |
| 473 | 0.997 | 1.598 | 0.0157 | 0.972 | 0.11 | 0.2 | |
| SJA2 | 573 | 0.999 | 1.443 | 0.0056 | 0.994 | 0.092 | 0.046 |
| 523 | 0.999 | 1.47 | 0.0062 | 0.984 | 0.079 | 0.067 | |
| 473 | 0.999 | 1.497 | 0.0064 | 0.974 | 0.067 | 0.088 | |
| SJA4 | 573 | 0.998 | 1.691 | 0.0119 | 0.992 | 0.099 | 0.098 |
| 523 | 0.999 | 1.739 | 0.0128 | 0.979 | 0.092 | 0.121 | |
| 473 | 0.998 | 1.84 | 0.0148 | 0.985 | 0.088 | 0.158 | |
| Muestra | T (K) | −ΔH (kJ·mol−1) | −ΔG (kJ·mol−1) | −ΔU (kJ·mol−1) | ΔS (J·K−1·mol−1) |
|---|---|---|---|---|---|
| SJA1 | 573 | 4.505 | 71.524 | 11.491 | 116.962 |
| 483 | 55.797 | 106.193 | |||
| 453 | 51.148 | 102.964 | |||
| SJA2 | 573 | 2.904 | 69.190 | 6.648 | 115.682 |
| 483 | 55.237 | 108.352 | |||
| 453 | 50.597 | 105.283 | |||
| SJA4 | 573 | 8.037 | 70.621 | 10.394 | 109.223 |
| 483 | 55.754 | 96.413 | |||
| 453 | 50.911 | 102.798 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroz-Estrada, K.; Hernández-Espinosa, M.Á.; Rojas, F.; Portillo, R.; Rubio, E.; López, L. N2 and CO2 Adsorption by Soils with High Kaolinite Content from San Juan Amecac, Puebla, México. Minerals 2016, 6, 73. https://doi.org/10.3390/min6030073
Quiroz-Estrada K, Hernández-Espinosa MÁ, Rojas F, Portillo R, Rubio E, López L. N2 and CO2 Adsorption by Soils with High Kaolinite Content from San Juan Amecac, Puebla, México. Minerals. 2016; 6(3):73. https://doi.org/10.3390/min6030073
Chicago/Turabian StyleQuiroz-Estrada, Karla, Miguel Ángel Hernández-Espinosa, Fernando Rojas, Roberto Portillo, Efraín Rubio, and Lucía López. 2016. "N2 and CO2 Adsorption by Soils with High Kaolinite Content from San Juan Amecac, Puebla, México" Minerals 6, no. 3: 73. https://doi.org/10.3390/min6030073