Mineral Sequestration of Carbon Dioxide in Circulating Fluidized Bed Combustion Boiler Bottom Ash
Abstract
:1. Introduction
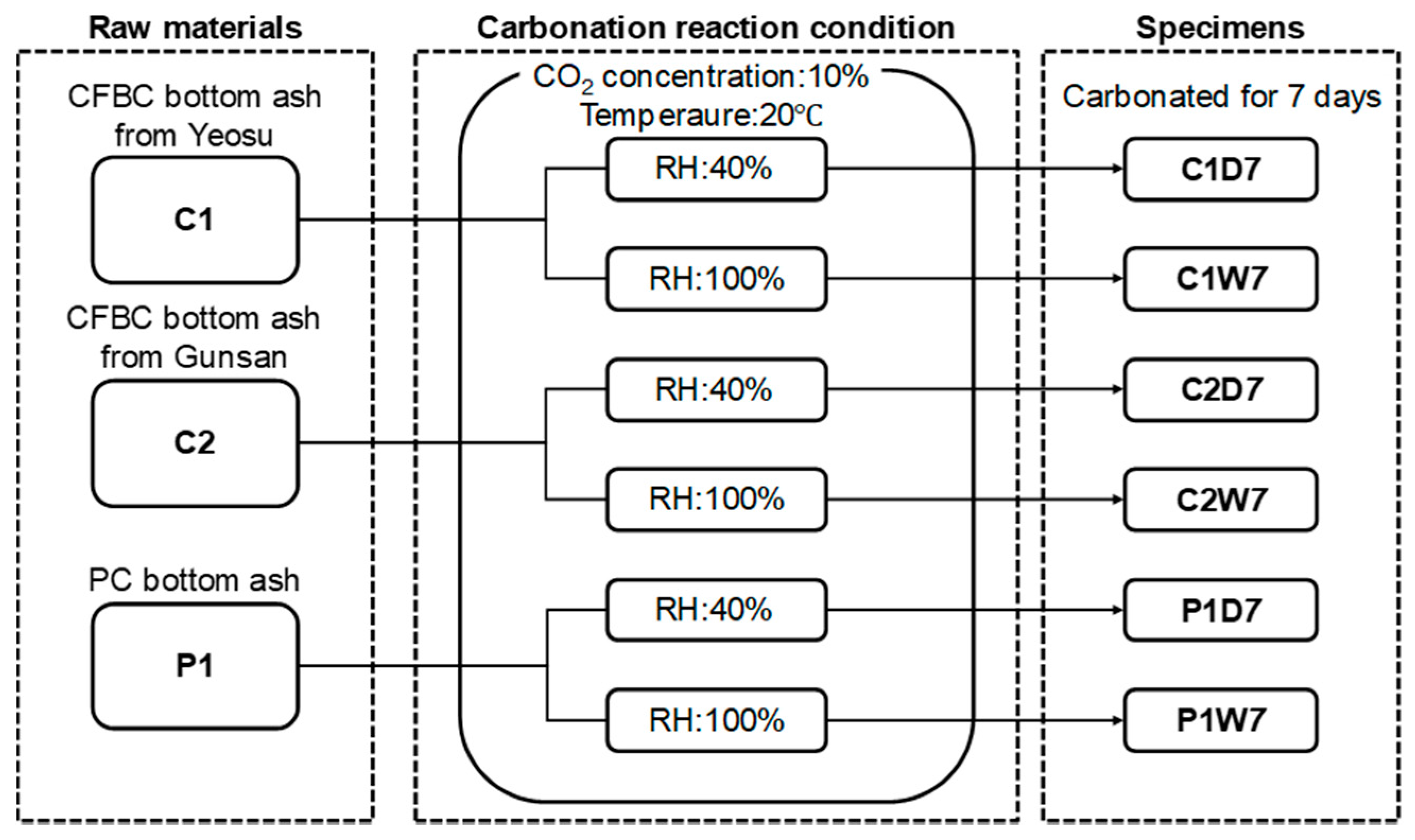
2. Materials and Methods
2.1. Materials Used and Sample Preparation
- CFBC bottom ash: Two samples were prepared using the bottom ash obtained from the Yeosu power plant in South Korea (‘hereinafter, C1’), and the other from the Gunsan plant (‘hereinafter, C2’). Both were bottom ash generated from a CFBC boiler.
- PC bottom ash: One sample was prepared using PC boiler bottom ash obtained from the Seocheon power plant in South Korea (hereinafter, P1).
2.2. Experimental Details
3. Results
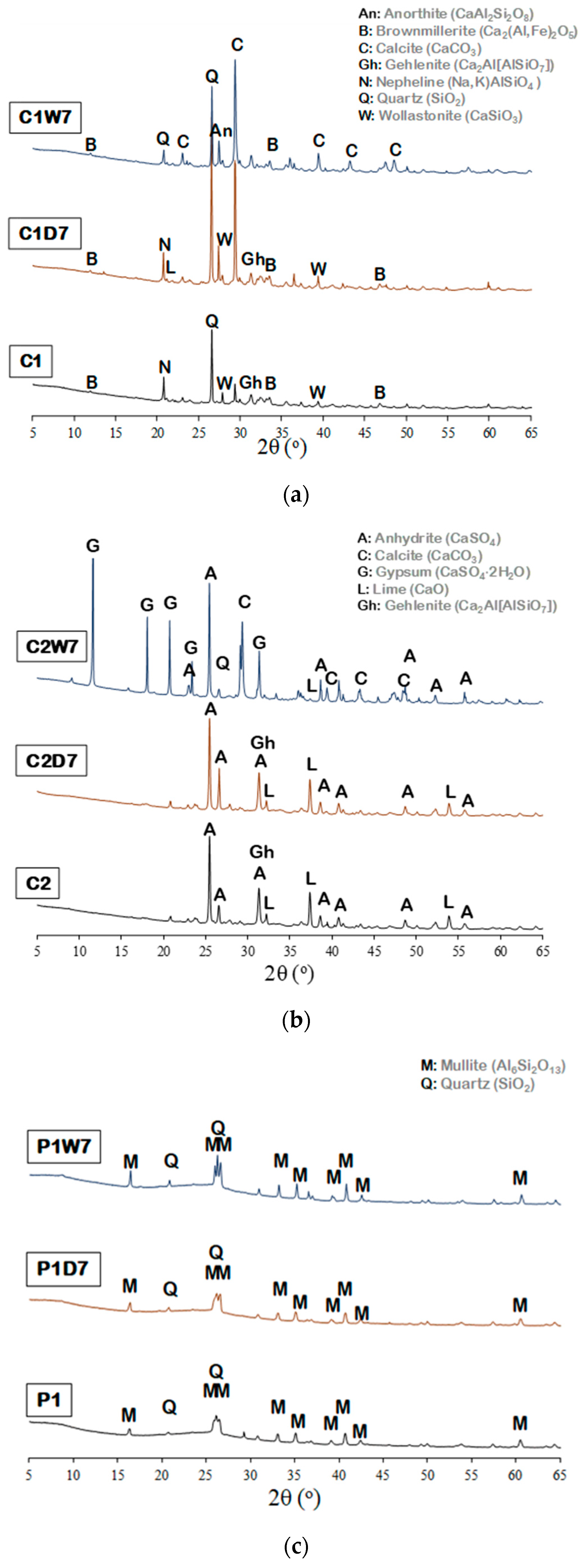
3.1. Characterization of Raw Bottom Ash
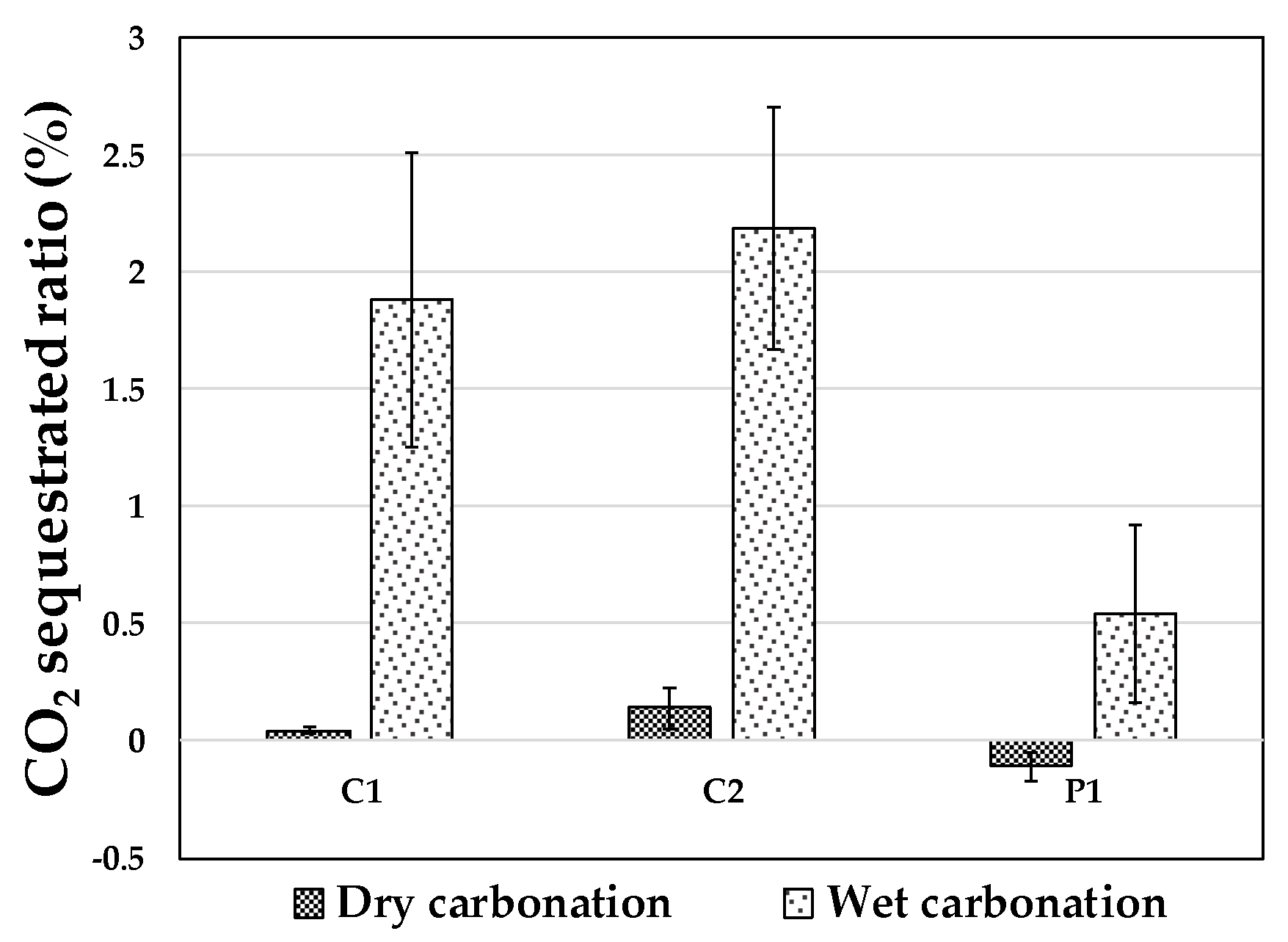
3.2. CO2 Sequestration in Bottom Ash
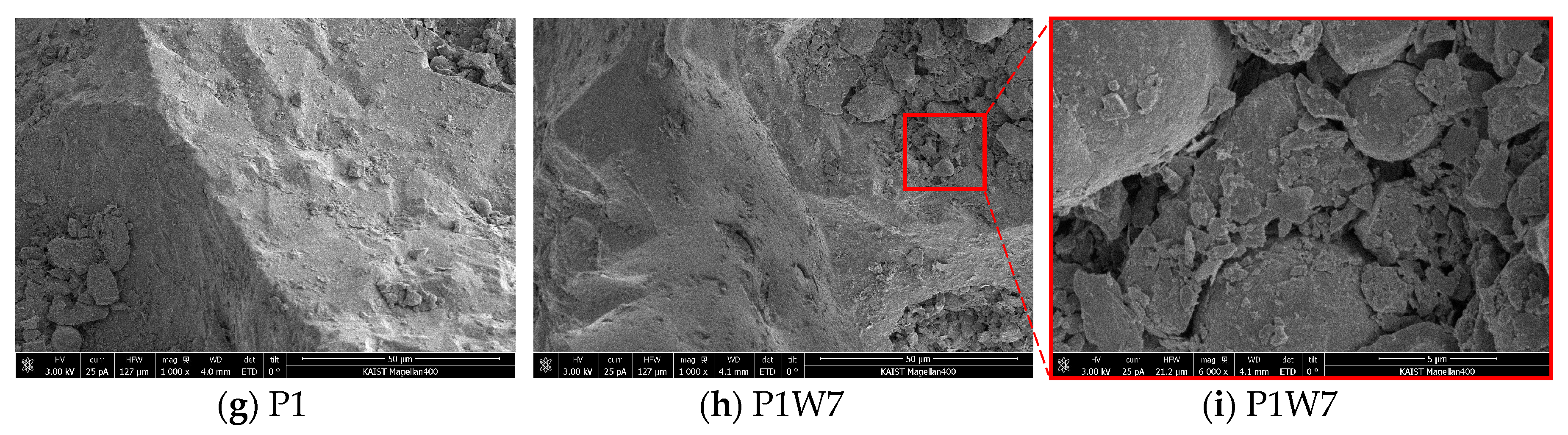
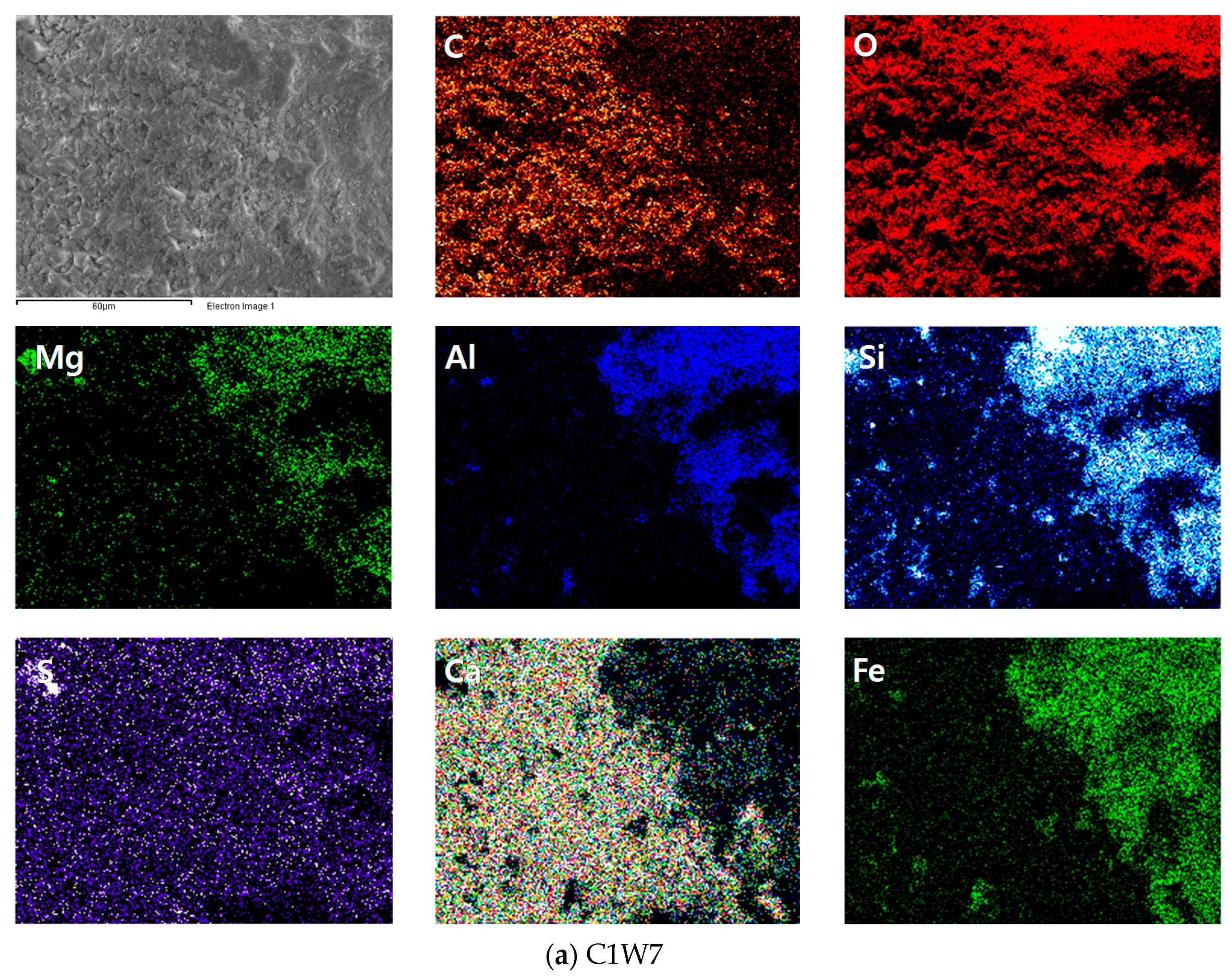
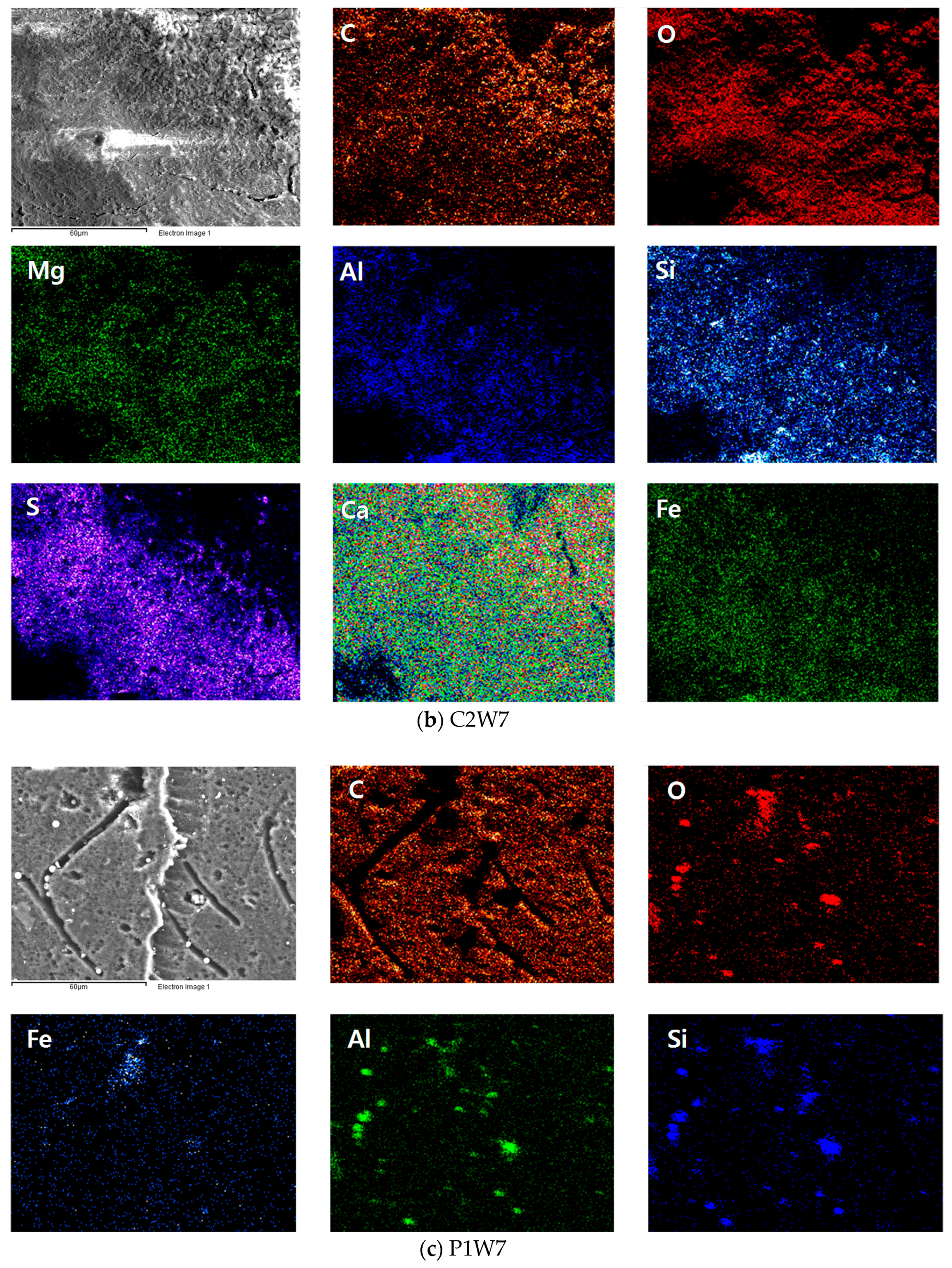
3.3. Morphology of Carbonated Bottom Ash
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bruhn, T.; Naims, H.; Olfe-Kräutlein, B. Separating the debate on CO2 utilisation from carbon capture and storage. Environ. Sci. Policy 2016, 60, 38–43. [Google Scholar] [CrossRef]
- Cox, P.M.; Betts, R.A.; Jones, C.D.; Spall, S.A.; Totterdell, I.J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 2000, 408, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Energy Information Administration. Monthly Energy Review; Energy Information Administration: Washington, DC, USA, 2014.
- Haynes, R. Reclamation and revegetation of fly ash disposal sites—Challenges and research needs. J. Environ. Manag. 2009, 90, 43–53. [Google Scholar] [CrossRef] [PubMed]
- U.S. Energy Infromation Administration. International Energy Outlook 2016; U.S. Energy Infromation Administration: Washington, DC, USA, 2016.
- Anthony, E.; Bulewicz, E.M.; Dudek, K.; Kozak, A. The long term behaviour of cfbc ash—Water systems. Waste Manag. 2002, 22, 99–111. [Google Scholar] [CrossRef]
- Selcuk, N.; Ozkan, M. Simulation of circulating fluidized bed combustors firing indigenous lignite. Int. J. Therm. Sci. 2011, 50, 1109–1115. [Google Scholar] [CrossRef]
- Selvakumaran, P.; Lawerence, A.; Lakshminarasimhan, M.; Bakthavatsalam, A. Mineralogical influence of mining intrusions in CFB combustion of indian lignite. Int. J. Energy Environ. Eng. 2013, 4, 34. [Google Scholar]
- Innovation for Cool Earth Forum, Global Roadmap for Implementing CO2 Utilization. 2016. Available online: www.globalco2initiative.org/webinar (accessed on 18 April 2017).
- Chi, M.; Huang, R. Effect of circulating fluidized bed combustion ash on the properties of roller compacted concrete. Cem. Concr. Compos. 2014, 45, 148–156. [Google Scholar] [CrossRef]
- Havlica, J.; Brandstetr, J.; Odler, I. Possibilities of utilizing solid residues from pressured fluidized bed coal combustion (PSBC) for the production of blended cements. Cem. Concr. Res. 1998, 28, 299–307. [Google Scholar] [CrossRef]
- Zhang, W.; Choi, H.; Sagawa, T.; Hama, Y. Compressive strength development and durability of an environmental load-reduction material manufactured using circulating fluidized bed ash and blast-furnace slag. Constr. Build. Mater. 2017, 146, 102–113. [Google Scholar] [CrossRef]
- Bertos, M.F.; Simons, S.; Hills, C.; Carey, P. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar]
- Renforth, P.; Washbourne, C.-L.; Taylder, J.; Manning, D. Silicate Production and Availability for Mineral Carbonation; ACS Publications: Washington, DC, USA, 2011. [Google Scholar]
- Ukwattage, N.; Ranjith, P.; Yellishetty, M.; Bui, H.; Xu, T. A laboratory-scale study of the aqueous mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2015, 103, 665–674. [Google Scholar] [CrossRef]
- Wang, C.; Jia, L.; Tan, Y.; Anthony, E.J. Carbonation of fly ash in oxy-fuel CFB combustion. Fuel 2008, 87, 1108–1114. [Google Scholar] [CrossRef]
- Wee, J.-H. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Perez-Lopez, R.; Renard, F.; Nieto, J.; Charlet, L. Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J. Hazard. Mater. 2009, 161, 1347–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soong, Y.; Fauth, D.; Howard, B.; Jones, J.; Harrison, D.; Goodman, A.; Gray, M.; Frommell, E. CO2 sequestration with brine solution and fly ashes. Energy Convers. Manag. 2006, 47, 1676–1685. [Google Scholar] [CrossRef]
- Salvador, C.; Lu, D.; Anthony, E.; Abanades, J. Enhancement of CaO for CO2 capture in an FBC environment. Chem. Eng. J. 2003, 96, 187–195. [Google Scholar] [CrossRef]
- Cebrucean, D.; Cebrucean, V.; Ionel, I. CO2 capture and storage from fossil fuel power plants. Energy Procedia 2014, 63, 18–26. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Abanades, J.C.; Alvarez, D. Conversion limits in the reaction of CO2 with lime. Energy Fuels 2003, 17, 308–315. [Google Scholar] [CrossRef]
- Ozcan, D.C.; Macchi, A.; Lu, D.Y.; Kierzkowska, A.M.; Ahn, H.; Müller, C.R.; Brandani, S. Ca–Cu looping process for CO2 capture from a power plant and its comparison with ca-looping, oxy-combustion and amine-based CO2 capture processes. Int. J. Greenh. Gas Control 2015, 43, 198–212. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; Luis, F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef] [Green Version]
- Ridha, F.N.; Manovic, V.; Macchi, A.; Anthony, E.J. CO2 capture at ambient temperature in a fixed bed with CaO-based sorbents. Appl. Energy 2015, 140, 297–303. [Google Scholar] [CrossRef]
- Burnard, K.; Bhattacharya, S. Power Generation from Coal; IEA Information Paper; IEA: Paris, France, 2011; pp. 1–49. [Google Scholar]
- World Energy Council. World Energy Resources; World Energy Council: London, UK, 2016; pp. 1–73. [Google Scholar]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Loo, L.; Maaten, B.; Konist, A.; Siirde, A.; Neshumayev, D.; Pihu, T. Carbon dioxide emission factors for oxy-fuel CFBC and aqueous carbonation of the Ca-rich oil shale ash. Energy Procedia 2017, 128, 144–149. [Google Scholar] [CrossRef]
- Rao, A.; Anthony, E.; Jia, L.; Macchi, A. Carbonation of FBC ash by sonochemical treatment. Fuel 2007, 86, 2603–2615. [Google Scholar] [CrossRef]
- Uibu, M.; Uus, M.; Kuusik, R. CO2 mineral sequestration in oil-shale wastes from estonian power production. J. Environ. Manag. 2009, 90, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Chindaprasirt, P.; Rattanasak, U. Utilization of blended fluidized bed combustion (FBC) ash and pulverized coal combustion (PCC) fly ash in geopolymer. Waste Manag. 2010, 30, 667–672. [Google Scholar] [CrossRef] [PubMed]
- ASTM International Voluntary Organization C114–13. Standard Test Methods for Chemical Analysis of Hydraulic Cement; ASTM International Voluntary Organization: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Huijgen, W.J.; Witkamp, G.-J.; Comans, R.N. Mineral CO2 sequestration by steel slag carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Jens, G.; Nasdala, L.; Kleeberg, R.; Wenzel, M. Occurrence and distribution of “moganite” in agate/chalcedony: A combined micro Raman, Rietveld, and cathodoluminescence study. Contrib. Mineral. Petrol. 1998, 133, 96–105. [Google Scholar]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Hoschek, G. Gehlenite stability in the system CaO-Al2O3-SiO2-H2O-CO2. Contrib. Mineral. Petrol. 1974, 47, 245–254. [Google Scholar] [CrossRef]
- Singh, M.; Garg, M. Activation of gypsum anhydrite-slag mixtures. Cem. Concr. Res. 1995, 25, 332–338. [Google Scholar] [CrossRef]

| Oxide Contents (wt %) | C1 | C2 | P1 |
|---|---|---|---|
| CaO | 47.6 | 52.7 | 2.1 |
| Free CaO contents (%) | 0.61 | 4.94 | 0.04 |
| SiO2 | 7.4 | 11.3 | 27.0 |
| Al2O3 | 3.5 | 7.0 | 16.0 |
| Fe2O3 | 19.6 | 4.7 | 21.7 |
| MgO | 3.0 | 1.3 | 4.0 |
| Na2O | 16.0 | - | 17.0 |
| K2O | 0.5 | 0.4 | 6.1 |
| SO3 | 0.9 | 20.5 | 0.7 |
| TiO2 | 0.6 | 0.5 | 4.6 |
| MnO | 0.1 | 0.1 | 0.2 |
| P2O5 | - | 0.9 | 0.1 |
| Sample | Pre-Carbonation | Dry Carbonation | Wet Carbonation |
|---|---|---|---|
| C1 | 0.187% (0.05) | 0.231% (0.01) | 2.067% (0.16) |
| C2 | 0.197% (0.04) | 2.385% (0.14) | 2.385% (0.12) |
| P1 | 9.899% (4.34) | 9.792% (1.18) | 10.439% (2.67) |
| Mineral | C1 | C1D7 | C1W7 | C2 | C2D7 | C2W7 | P1 | P1D7 | P1W7 |
|---|---|---|---|---|---|---|---|---|---|
| Calcite (CaCO3) | 8.7 | 25.4 | 53.0 | 0.5 | 2.1 | 33.7 | 0.3 | 0.2 | |
| Quartz (SiO2) | 31.6 | 35.4 | 18.9 | 10.3 | 19.6 | 2.2 | 16.3 | 15.2 | 19.8 |
| Lime (CaO) | 0.7 | 1.0 | 0.4 | 14.6 | 17.0 | 0.1 | - | - | - |
| Brownmillerite (Ca2(Al,Fe)2O5) | 13.7 | 16.8 | 7.3 | - | - | - | - | - | - |
| Gehlenite (Ca2Al(AlSiO7)) | 18.3 | 13.4 | - | 24.8 | - | - | - | - | - |
| Mullite (Al6Si2O13) | - | - | - | - | - | - | 81.3 | 79.5 | 80.0 |
| Anhydrite (CaSO4) | - | - | - | 47.9 | 59.3 | 32.6 | - | - | - |
| Corundum (Al2O3) | - | - | - | - | - | - | 2.1 | 5.3 | - |
| Nepheline ((Na,K)AlSiO4) | 4.9 | 3.2 | - | - | - | - | - | - | - |
| Wollastonite (CaSiO3) | 4.6 | 4.7 | - | - | - | - | - | - | - |
| Magnesium Oxide (MgO) | - | - | - | 1.2 | 0.8 | - | - | - | - |
| Magnesite (MgCO3) | 17.3 | - | - | - | - | - | - | - | - |
| Cristobalite (SiO2) | 0.1 | - | - | - | - | - | - | - | - |
| Calcium Titanate (CaTiO3) | - | - | 0.8 | - | - | - | - | - | - |
| Anorthite sodian (CaAl2Si2O8) | - | - | 19.6 | - | - | - | - | - | - |
| Gypsum (CaSO4∙2H2O) | - | - | - | - | - | 29.4 | - | - | - |
| Portlandite Ca(OH)2 | - | - | - | - | 1.0 | - | - | - | - |
| Rutile (TiO2) | - | - | - | 0.7 | - | - | - | - | - |
| Ettringite (CaO)6(Al2O3)(SO3)3·32H2O | - | - | - | - | - | 2.1 | - | - | - |
| Sample | Dry Carbonation | Wet Carbonation |
|---|---|---|
| C1 | 7.34 | 19.48 |
| C2 | 0.70 | 14.60 |
| P1 | - | - |
| Sample | Pre-Carbonation | Post-Carbonation |
|---|---|---|
| C1 | 0.8887 | 3.2826 |
| C2 | 0.3208 | 18.6235 |
| P1 | 1.5973 | 1.5511 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Lee, H.-K. Mineral Sequestration of Carbon Dioxide in Circulating Fluidized Bed Combustion Boiler Bottom Ash. Minerals 2017, 7, 237. https://doi.org/10.3390/min7120237
Kim H-J, Lee H-K. Mineral Sequestration of Carbon Dioxide in Circulating Fluidized Bed Combustion Boiler Bottom Ash. Minerals. 2017; 7(12):237. https://doi.org/10.3390/min7120237
Chicago/Turabian StyleKim, Hee-Jeong, and Haeng-Ki Lee. 2017. "Mineral Sequestration of Carbon Dioxide in Circulating Fluidized Bed Combustion Boiler Bottom Ash" Minerals 7, no. 12: 237. https://doi.org/10.3390/min7120237




