Discovery of a Novel Cationic Surfactant: Tributyltetradecyl-Phosphonium Chloride for Iron Ore Flotation: From Prediction to Experimental Verification
Abstract
:1. Introduction
2. Methodology
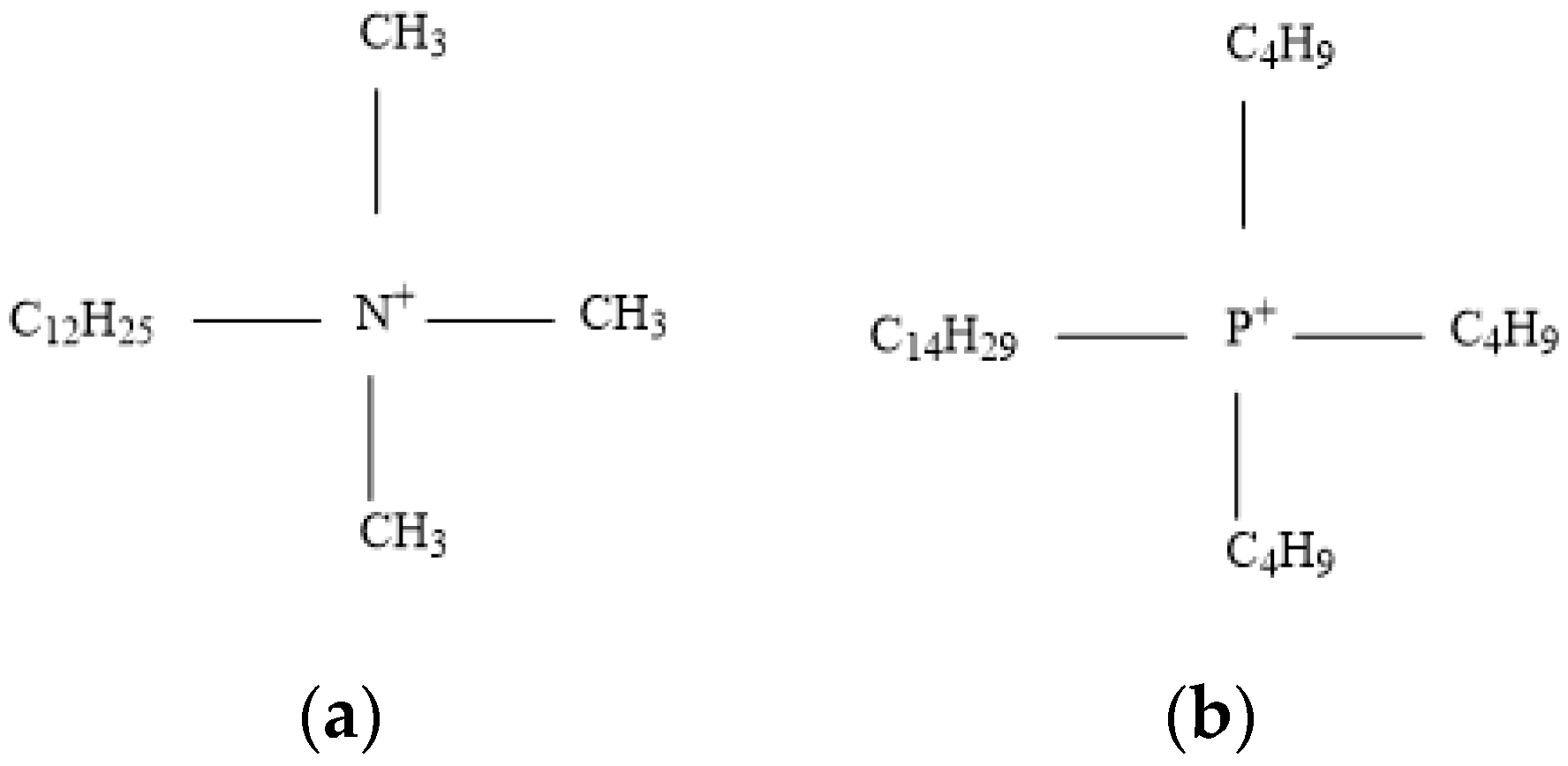
2.1. Materials and Reagents
2.2. The DFT Calculations
2.3. Zeta Potential Measurements
2.4. Adsorption Tests
2.5. Micro-Flotation Tests
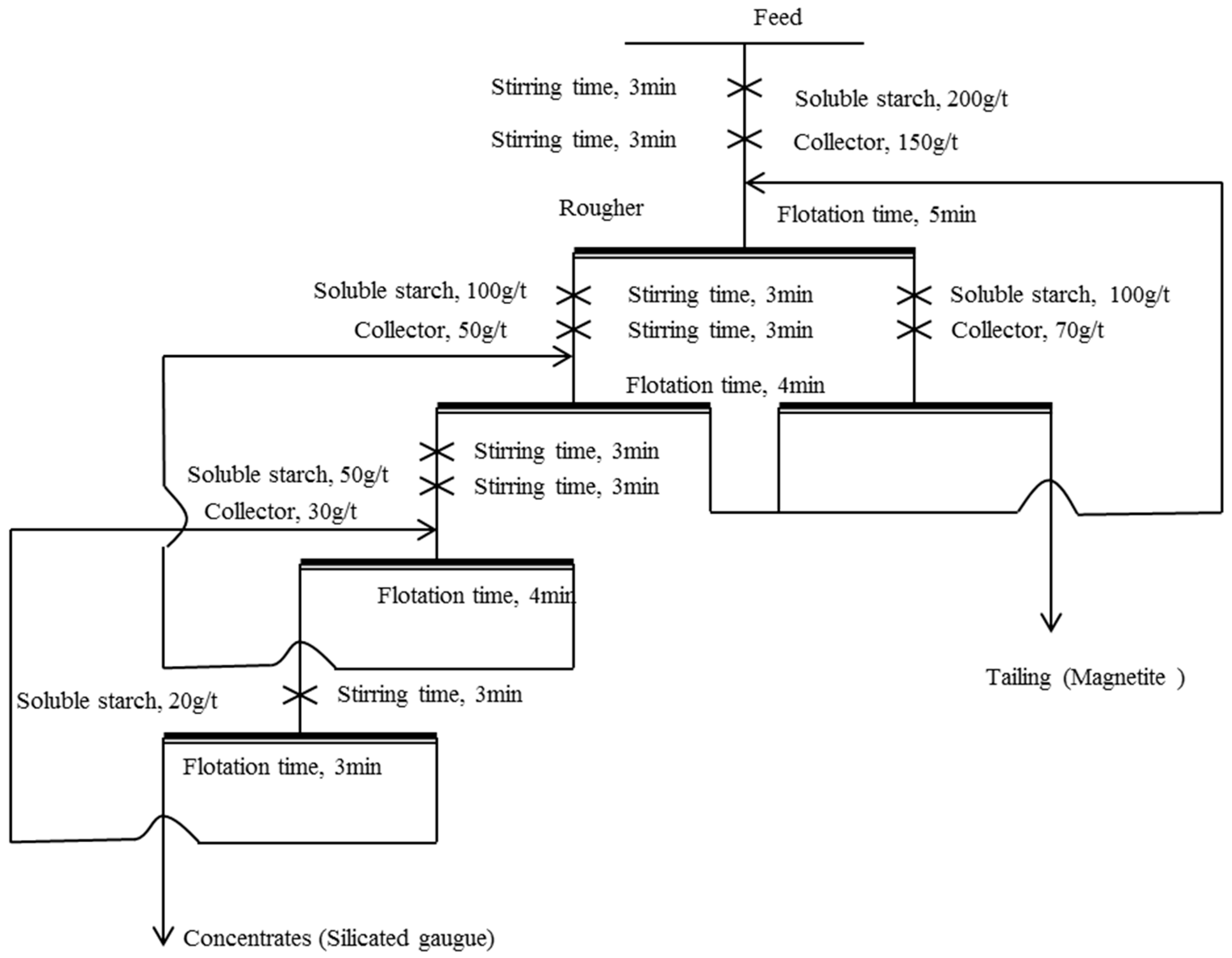
2.6. Bench-Scale Flotation
3. Results and Discussions
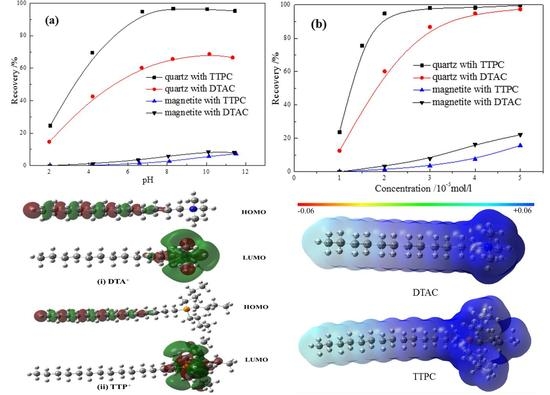
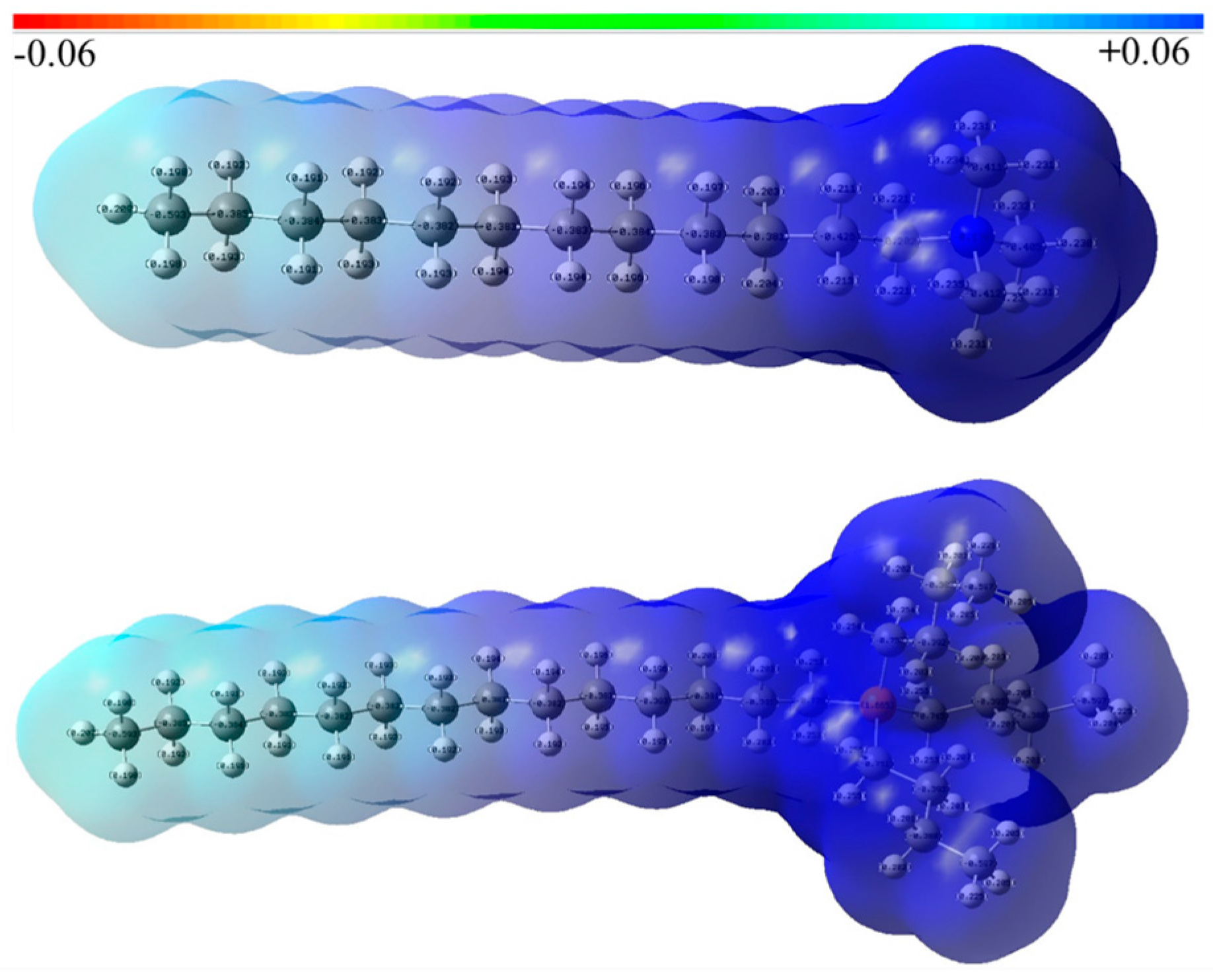
3.1. The DFT Calculations
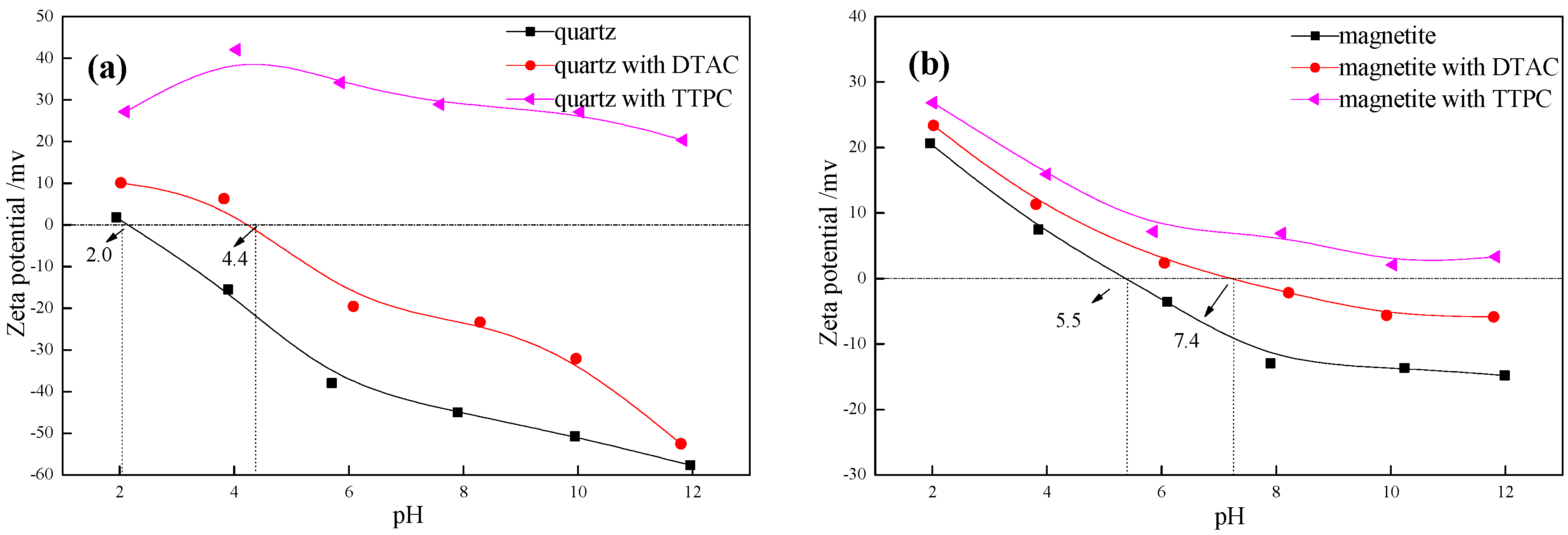
3.2. Zeta Potential Measurements
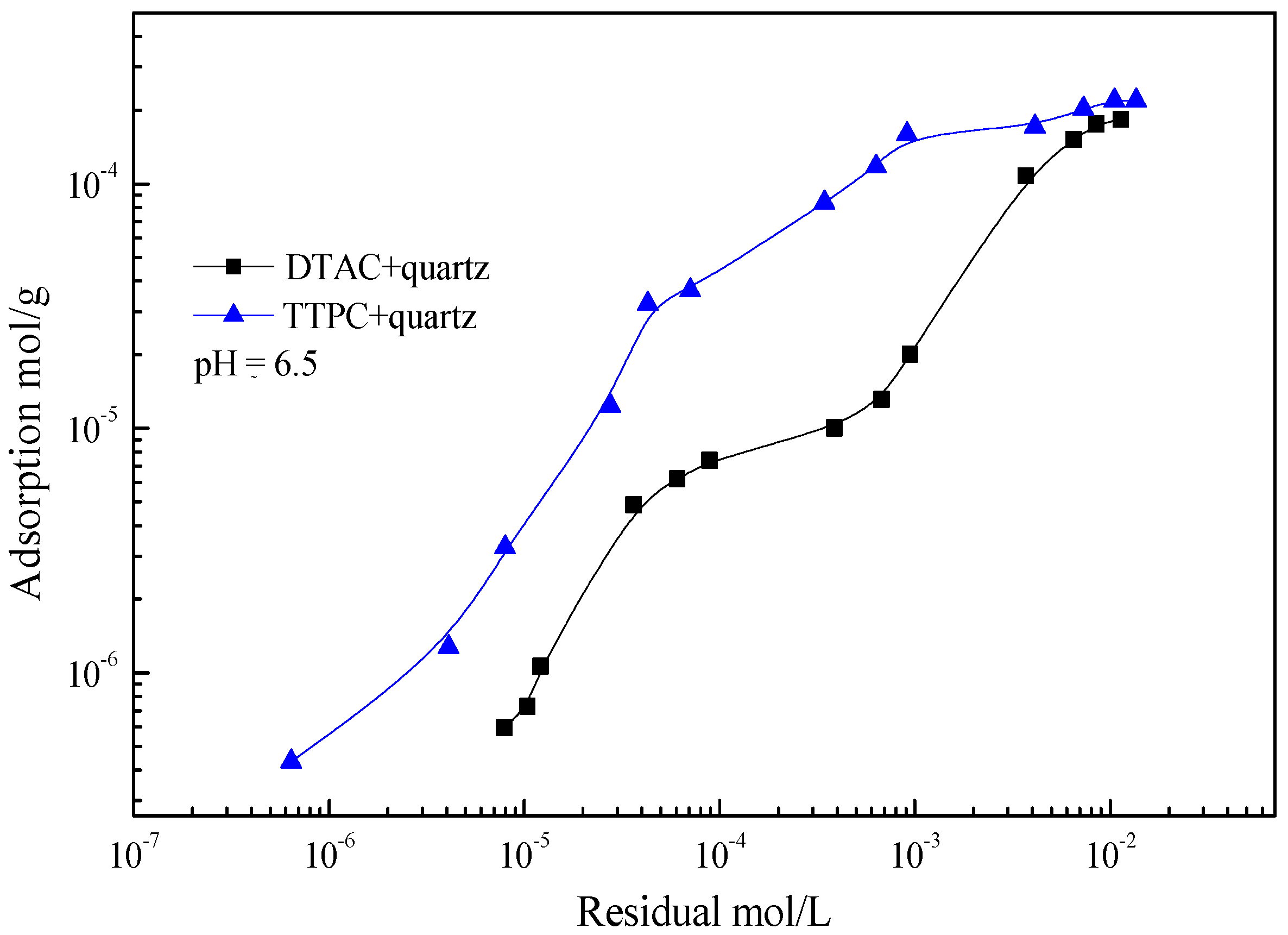
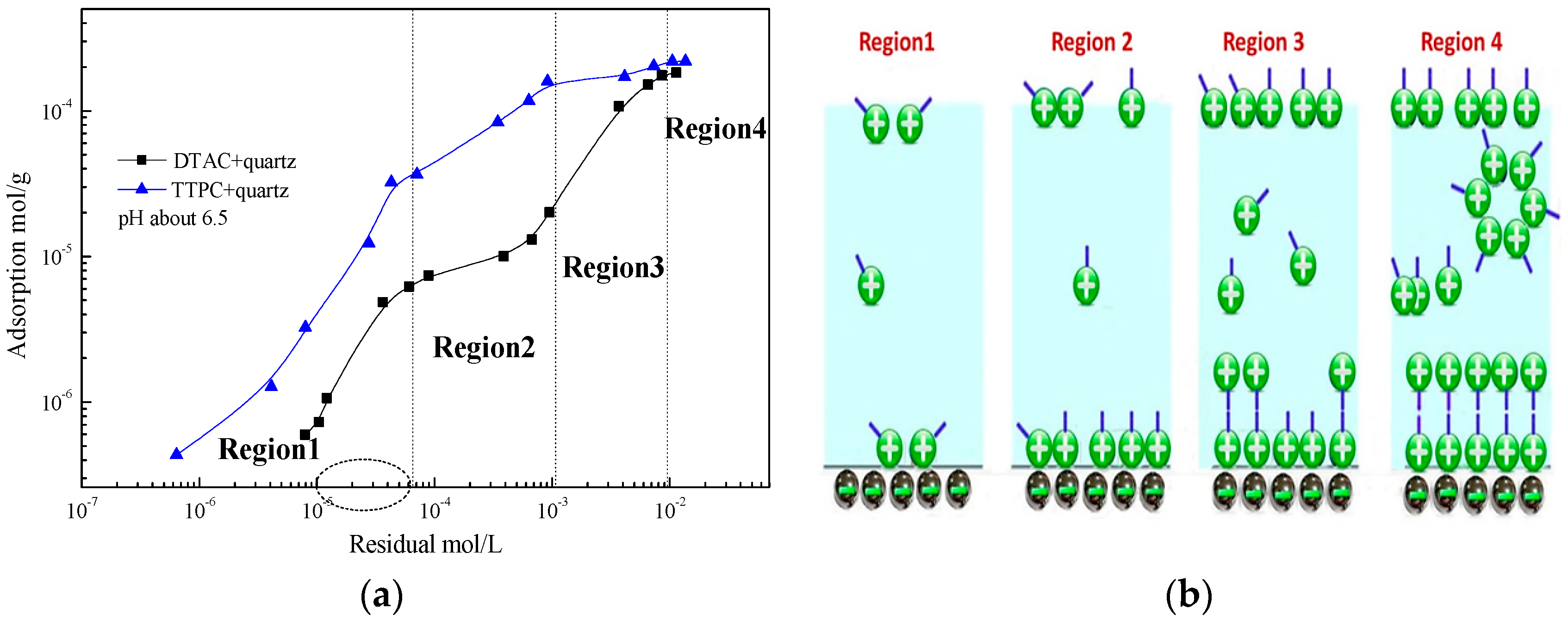
3.3. Adsorption Isotherm Measurements
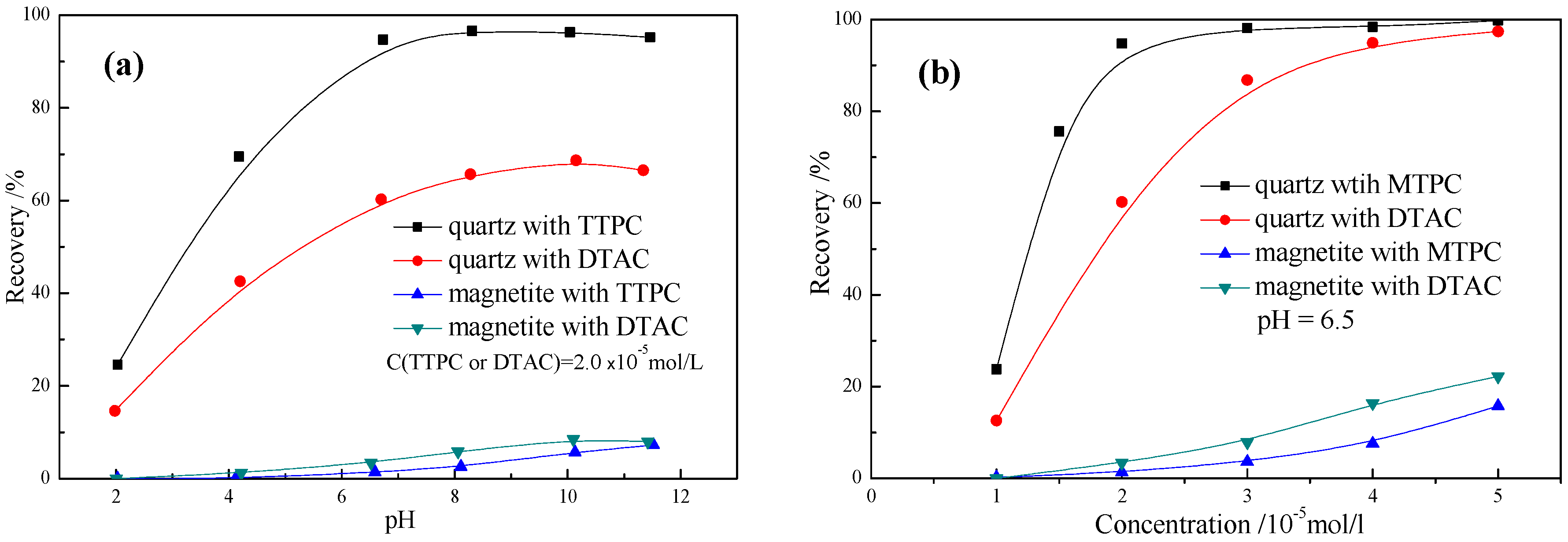
3.4. Micro-Flotation Tests
3.5. Bench-Scale Flotation
3.6. Adsorption Mechanism
4. Conclusions
- (1)
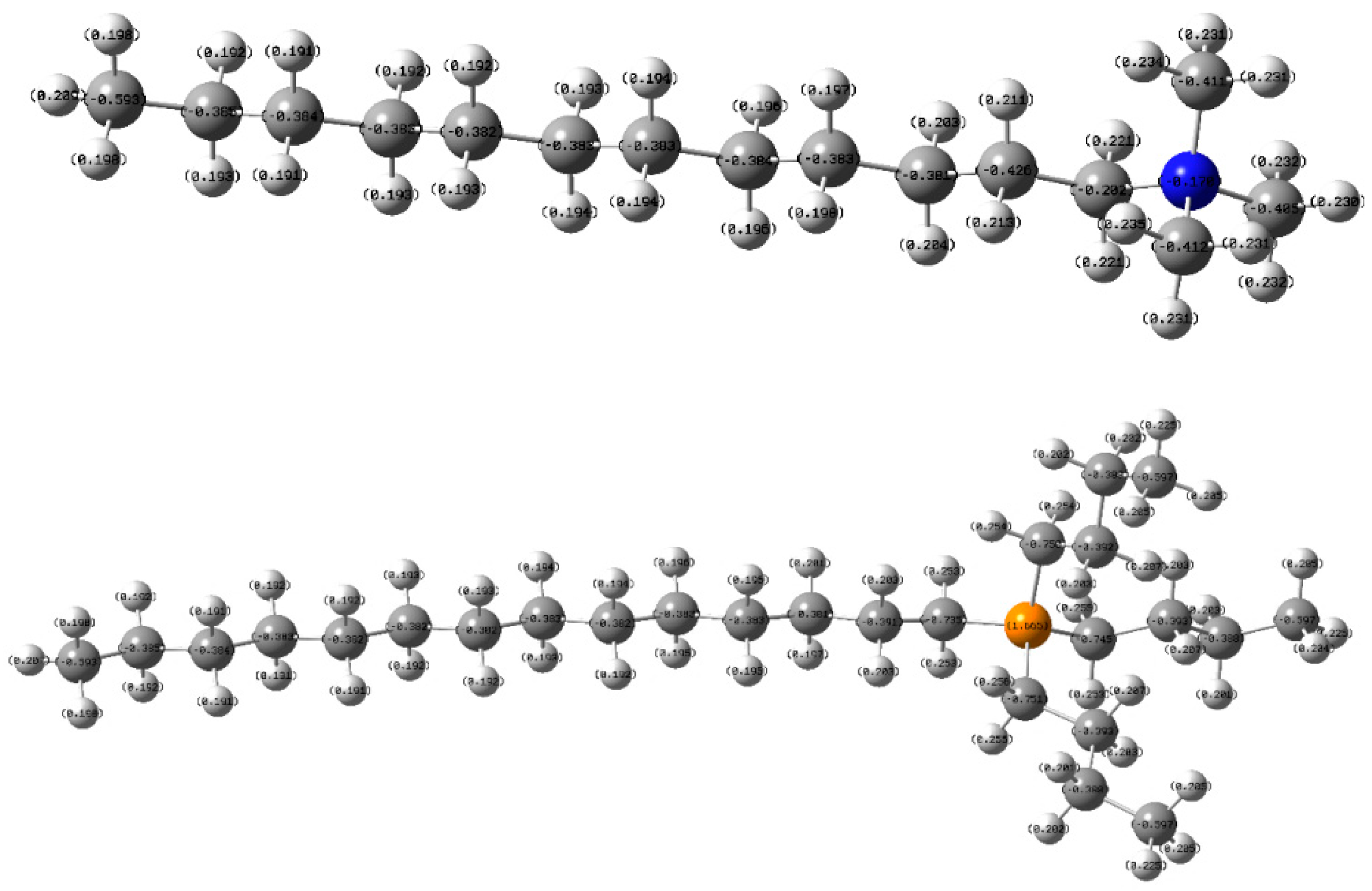
- TTPC prefers to physically adsorb on the objective mineral surface by electrostatic force, like the DTAC. Furthermore, the calculation of NPA charge indicates that active part (P+(C4H9)) of TTP+ takes much more positive charges than that (N+(CH3)3) of DTA+, meaning that TTPC may be a promising collector like or even better than DTAC;
- (2)
- Zeta potential measurements show that the potential of TTPC or DTAC adsorbed quartz surface is much higher than the untreated quartz surface. Particularly, the potential of the TTPC adsorbed quartz surface is significantly more positive than that of the DTAC adsorbed quartz surface, which is in good agreement with the computational results;
- (3)
- Adsorption tests illustrate that the adsorption amount of TTPC is much more than that of DTAC at low concentration, and the flotation experiments confirm that the adsorption amount of TTPC or DTAC is positively correlated to the flotation recovery, suggesting that TTPC is a potential candidate collector for iron ore flotation;
- (4)
- Micro-flotation and Bench-scale flotation results further verify that TTPC presents stronger collecting power and much better selectivity in the separation of quartz from magnetite in comparison with the conventional collector DTA.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Filippov, L.O.; Severov, V.V.; Filippova, I.V. An overview of the beneficiation of iron ores via reverse cationic flotation. Int. J. Miner. Process. 2014, 127, 62–69. [Google Scholar] [CrossRef]
- Clemmer, J.B. Flotation of iron ores. In Proceedings of the 8th Annual Mining Symposium, Duluth, MN, USA, January 1947. [Google Scholar]
- Araujo, A.C.; Viana, P.R.M.; Peres, A.E.C. Reagents in iron ores flotation. Miner. Eng. 2005, 18, 219–224. [Google Scholar] [CrossRef]
- Weng, X.; Mei, G.; Zhao, T.; Zhu, Y. Utilization of novel ester-containing quaternary ammonium surfactant as cationic collector for iron ore flotation. Sep. Purif. Technol. 2013, 103, 187–194. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V.; Severov, V.V. The use of collectors mixture in the reverse cationic flotation of magnetite ore: The role of Fe-bearing silicates. Miner. Eng. 2010, 23, 91–98. [Google Scholar] [CrossRef]
- Batisteli, G.M.B.; Peres, A.E.C. Residual amine in iron ore flotation. Miner. Eng. 2008, 21, 873–876. [Google Scholar] [CrossRef]
- Uwadiale, G.G.O.O. Flotation of iron oxides and quartz—A review. Miner. Process. Extr. Metall. Rev. 1992, 11, 129–161. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J. The flotation of quartz from iron minerals with a combined quaternary ammonium salt. Int. J. Miner. Process. 2005, 77, 116–122. [Google Scholar]
- Vieira, A.M.; Peres, A.E.C. The effect of amine type, pH, and size range in the flotation of quartz. Miner. Eng. 2007, 20, 1008–1013. [Google Scholar] [CrossRef]
- Huang, Z.; Hong, Z.; Shuai, W.; Xia, L.; Zou, W.; Liu, G. Investigations on reverse cationic flotation of iron ore by using a Gemini surfactant: Ethane-1,2-bis(dimethyl-dodecyl-ammonium bromide). Chem. Eng. J. 2014, 257, 218–228. [Google Scholar] [CrossRef]
- Kar, B.; Sahoo, H.; Rath, S.S.; Das, B. Investigations on different starches as depressants for iron ore flotation. Miner. Eng. 2013, 49, 1–6. [Google Scholar] [CrossRef]
- Turrer, H.D.G.; Peres, A.E.C. Investigation on alternative depressants for iron ore flotation. Miner. Eng. 2010, 23, 1066–1069. [Google Scholar] [CrossRef]
- Lima, N.P.; Valadão, G.E.S.; Peres, A.E.C. Effect of amine and starch dosages on the reverse cationic flotation of an iron ore. Miner. Eng. 2013, 45, 180–184. [Google Scholar] [CrossRef]
- Pavlovic, S.; Brandao, P.R.G. Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and quartz. Miner. Eng. 2003, 16, 1117–1122. [Google Scholar] [CrossRef]
- Montes-Sotomayor, S.; Houot, R.; Kongolo, M. Flotation of silicated gangue iron ores: Mechanism and effect of starch. Miner. Eng. 1998, 11, 71–76. [Google Scholar] [CrossRef]
- Ge, H.; Wang, Y.; Li, C.; Chen, N.; Xie, Y.; Xu, M.; He, Y.; Gu, X.; Wu, R.; Gu, Q. Molecular dynamics-based virtual screening: Accelerating the drug discovery process by high-performance computing. J. Chem. Inf. Model. 2013, 53, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Brédas, J.L.; Persson, K.; Seshadri, R. Computational design of functional materials. Chem. Mater. 2017, 29, 2399–2401. [Google Scholar] [CrossRef]
- Zhang, Y.; Miller, G.J.; Fokwa, B.P.T. Computational design of rare-earth-free magnets with the Ti3Co5B2-type structure. Chem. Mater. 2015, 29, 2535–2541. [Google Scholar] [CrossRef]
- Curtarolo, S.; Hart, G.L.; Nardelli, M.B.; Mingo, N.; Sanvito, S.; Levy, O. The high-throughput highway to computational materials design. Nat. Mater. 2013, 12, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H.; He, J.Y.; Zhang, C.H.; Zhang, C.Y.; Sun, W.; Zhao, D.B.; Chen, P.; Han, H.S.; Gao, Z.Y.; Liu, R.Q.; et al. Insights into the activation mechanism of calcium ions on the sericite surface: A combined experimental and computational study. Appl. Surf. Sci. 2018, 427, 162–168. [Google Scholar] [CrossRef]
- Pradip; Rai, B. Molecular modeling and rational design of flotation reagents. Int. J. Miner. Process. 2003, 72, 95–110. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Zhong, H. Molecular design of flotation collectors: A recent progress. Adv. Colloid Interface Sci. 2017, 246, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xie, A.G.; Tan, S.Z.; Cai, X. Antimicrobial effects of quaternary phosphonium salt intercalated clay minerals on Escherichia coli and Staphylococci aureus. Colloids Surf. B Biointerfaces 2011, 86, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Liu, B.L.; Lin, S.J. Synthesis of an active quaternary phosphonium salt and its application to the Wittig reaction: Kinetic study. J. Chin. Inst. Chem. Eng. 2007, 38, 451–459. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Zeng, Q.Y.; Ao, N.J. Preparation and antimicrobial activity of quaternary phosphonium modified epoxidized natural rubber. Mater. Lett. 2013, 93, 145–148. [Google Scholar] [CrossRef]
- Rodrigues, O.M.S.; Peres, A.E.C.; Martins, A.H.; Pereira, C.A. Kaolinite and hematite flotation separation using etheramine and ammonium quaternary salts. Miner. Eng. 2013, 40, 12–15. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, P.; Sun, W.; Hu, Y.H.; Yue, T.; Lai, X.S. Evaluation of quaternary phosphonium salt as the collector in bauxite reverse flotation. J. Environ. Chem. Eng. 2017, 5, 4494–4496. [Google Scholar] [CrossRef]
- Paul, K.; Guchhait, N. A quantum chemical computational insight into theintramolecular hydrogen bond interaction in an antibacterial drugmolecule-2-acetylindan-1,3-dione. Comput. Theor. Chem. 2013, 1012, 20–26. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Zhao, G.; Wang, S.; Liu, G. Adsorption of α-hydroxyoctyl phosphonic acid to ilmenite/waterinterface and its application in flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 67–73. [Google Scholar] [CrossRef]
- Chai, J.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Somasundaran, P.; Fuerstenau, D.W. Mechanisms of Alkyl Sulfonate Adsorption at the Alumina-Water Interface. J. Phys. Chem. 1966, 70, 90–96. [Google Scholar] [CrossRef]
- Fan, A.; Somasundaran, A.P.; Turro, N.J. Adsorption of Alkyltrimethylammonium Bromides on Negatively Charged Alumina. Langmuir 1997, 13, 506–510. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. II. Overlap Populations, Bond Orders, and Covalent Bond Energies. J. Chem. Phys. 1955, 23, 1841–1846. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Shingu, H. A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons. J. Chem. Phys. 1952, 20, 1653. [Google Scholar] [CrossRef]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z.; Lai, X. Adsorption mechanism of lead ions at ilmenite/water interface and its influence on ilmenite flotability. J. Ind. Eng. Chem. 2017, 53, 285–293. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, P.; Wang, H.; Hu, Y.; Sun, W. Flotability improvement of ilmenite using attrition-scrubbing as a pretreatment method. Minerals 2017, 7, 13. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, G.; Xia, L.; Lu, Y.; Hu, Y.; Zhao, S.; Yu, X. Flotation separation of diaspore from kaolinite, pyrophyllite and illite using three cationic collectors. Miner. Eng. 2008, 21, 1055–1061. [Google Scholar] [CrossRef]
- Liu, C.; Hu, Y.; Cao, X. Substituent effects in kaolinite flotation using dodecyl tertiary amines. Miner. Eng. 2009, 22, 849–852. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, Z.; Xu, L.; Hu, Y.; Huang, K.; Zhu, S. A comparison study of the flotation and adsorption behaviors of diaspore and kaolinite with quaternary ammonium collectors. Miner. Eng. 2014, 65, 124–129. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, L.H.; Hu, Y.H.; Wang, D.Z.; Li, C.K.; Meng, W.; Wang, X.J. Flotation and adsorption of quaternary ammonium cationic collectors on diaspore and kaolinite. Trans. Nonferr. Met. Soci. China 2011, 21, 2528–2534. [Google Scholar] [CrossRef]
- Dash, M.; Dwari, R.K.; Biswal, S.K.; Reddy, P.S.R.; Chattopadhyay, P.; Mishra, B.K. Studies on the effect of flocculant adsorption on the dewatering of iron ore tailings. Chem. Eng. J. 2011, 173, 318–325. [Google Scholar] [CrossRef]
- Evenäs, L.; Furó, I.; Stilbs, A.P.; Valiullin, R. Adsorption isotherm and aggregate properties of fluorosurfactants on alumina measured by 19F NMR. Langmuir 2002, 18, 8096–8101. [Google Scholar] [CrossRef]
- Kamyshny, A.; Lagerge, S.; Partyka, S.; Relkin, P.; Magdassi, S. Adsorption of native and hydrophobized human IgG onto silica: Isotherms, calorimetry, and biological activity. Langmuir 2001, 17, 8242–8248. [Google Scholar] [CrossRef]
- Salako, O.; Lo, C.; Zhang, J.S.; Couzis, A.; Somasundaran, P.; Lee, J.W. Adsorption of sodium dodecyl sulfate onto clathrate hydrates in the presence of salt. J. Colloid Interface Sci. 2012, 386, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Somasundaran, P. Synergistic adsorption of mixtures of cationic gemini and nonionic sugar-based surfactant on silica. J. Colloid Interface Sci. 2009, 331, 288. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, D.; Liu, Q.; Liu, W.; Zheng, J.; Sun, H. Flotation separation of copper–molybdenum sulfides using chitosan as a selective depressant. Miner. Eng. 2015, 83, 217–222. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Jia, R. The adsorption of alkylpyridinium chlorides and their effect on the interfacial behavior of quartz. Colloids Surf. A Physicochem. Eng. Asp. 2004, 250, 223–231. [Google Scholar] [CrossRef]
- Somasundaran, P.; Krishnakumar, S. Adsorption of surfactants and polymers at the solid-liquid interface. Colloids Surf. A Physicochem. Eng. Asp. 1997, 123, 491–513. [Google Scholar] [CrossRef]
- Koopal, L.K.; Lee, E.M.; Böhmer, M.R. Adsorption of cationic and anionic surfactants on charged metal oxide surfaces. J. Colloid Interface Sci. 1995, 170, 85–97. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry (IUPAC). Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar]


| Sample | Total Fe | SiO2 | Al2O3 | CaO | MgO |
|---|---|---|---|---|---|
| Quartz | 0.57 | 96.97 | 1.88 | 0.05 | 0.03 |
| Magnetite | 68.64 | 2.66 | 1.31 | 0.43 | 0.20 |
| Basis Set | DTA+ | TTP+ | ||
|---|---|---|---|---|
| HOMO | LUMO | HOMO | LUMO | |
| Def2-TZVP | −272.2 | −37.2 | −262.7 | −23.8 |
| Def2-SVP | −273.9 | −30.3 | −265.3 | −19.9 |
| Basis Set | DTA+ | TTP+ | ||
|---|---|---|---|---|
| Mulliken | NPA | Mulliken | NPA | |
| Atom (N) Atom (P) | ||||
| Def2-TZVP | 0.075 | −0.170 | 0.512 | 1.665 |
| Def2-SVP | −0.399 | −0.369 | 0.326 | 1.581 |
| Group (N+(CH3)3) Group (P+(C4H9)) | ||||
| Def2-TZVP | 0.800 | 0.690 | 0.865 | 1.158 |
| Def2-SVP | 0.650 | 0.650 | 0.816 | 1.14 |
| Collector | Product | Weight | Grade | Recovery | ||
|---|---|---|---|---|---|---|
| Fe | SiO2 | Fe | SiO2 | |||
| DTAC | Concentrate | 80.27 | 65.41 | 7.41 | 95.88 | 25.57 |
| Tailings | 19.73 | 11.43 | 87.74 | 4.12 | 74.43 | |
| Feeds | 100.00 | 54.76 | 23.26 | 100.00 | 100.00 | |
| TTPC | Concentrate | 78.34 | 67.83 | 5.38 | 97.03 | 18.35 |
| Tailings | 21.66 | 7.49 | 91.92 | 2.97 | 81.65 | |
| Feeds | 100.00 | 54.76 | 23.26 | 100.00 | 100.00 | |
| Collector | Product | Weight | Grade | Recovery | ||
|---|---|---|---|---|---|---|
| Fe | SiO2 | Fe | SiO2 | |||
| DTAC | Concentrate | 77.65 | 65.34 | 5.62 | 93.20 | 23.40 |
| Tailings | 22.35 | 16.57 | 63.92 | 6.80 | 76.60 | |
| Feeds | 100.00 | 54.44 | 18.65 | 100.00 | 100.00 | |
| TTPC | Concentrate | 78.63 | 66.42 | 4.21 | 95.63 | 17.71 |
| Tailings | 21.37 | 11.16 | 71.97 | 4.37 | 82.29 | |
| Feeds | 100.00 | 54.61 | 18.69 | 100.00 | 100.00 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Hu, Y.; Gao, Z.; Zhai, J.; Fang, D.; Yue, T.; Zhang, C.; Sun, W. Discovery of a Novel Cationic Surfactant: Tributyltetradecyl-Phosphonium Chloride for Iron Ore Flotation: From Prediction to Experimental Verification. Minerals 2017, 7, 240. https://doi.org/10.3390/min7120240
Chen P, Hu Y, Gao Z, Zhai J, Fang D, Yue T, Zhang C, Sun W. Discovery of a Novel Cationic Surfactant: Tributyltetradecyl-Phosphonium Chloride for Iron Ore Flotation: From Prediction to Experimental Verification. Minerals. 2017; 7(12):240. https://doi.org/10.3390/min7120240
Chicago/Turabian StyleChen, Pan, Yuehua Hu, Zhiyong Gao, Jihua Zhai, Dong Fang, Tong Yue, Chenyang Zhang, and Wei Sun. 2017. "Discovery of a Novel Cationic Surfactant: Tributyltetradecyl-Phosphonium Chloride for Iron Ore Flotation: From Prediction to Experimental Verification" Minerals 7, no. 12: 240. https://doi.org/10.3390/min7120240