Fe–Ti(–V) Oxide Deposits of the Kunene Anorthosite Complex (SW Angola): Mineralogy and Thermo-Oxybarometry
Abstract
:1. Introduction
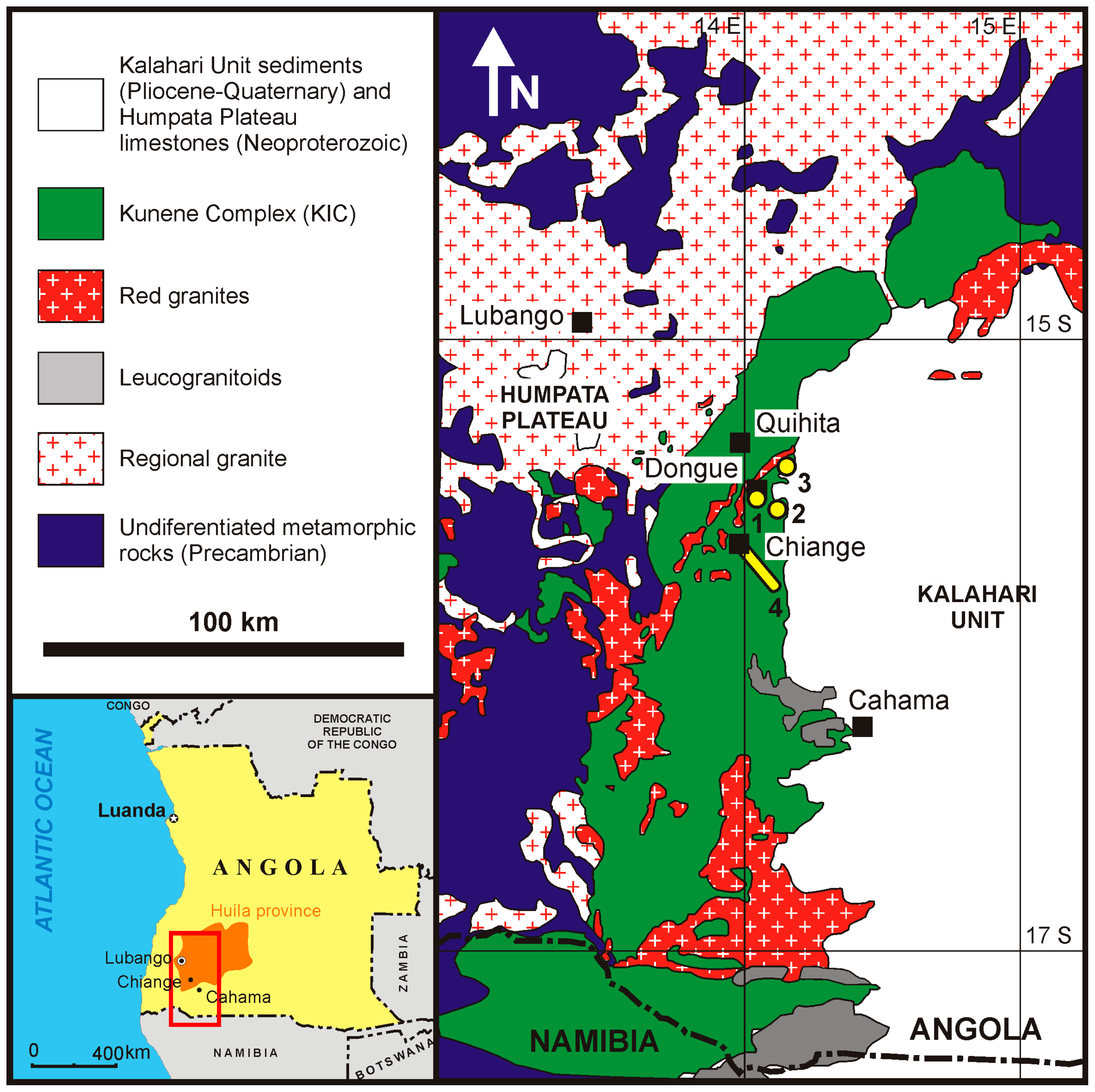
2. Geological Setting
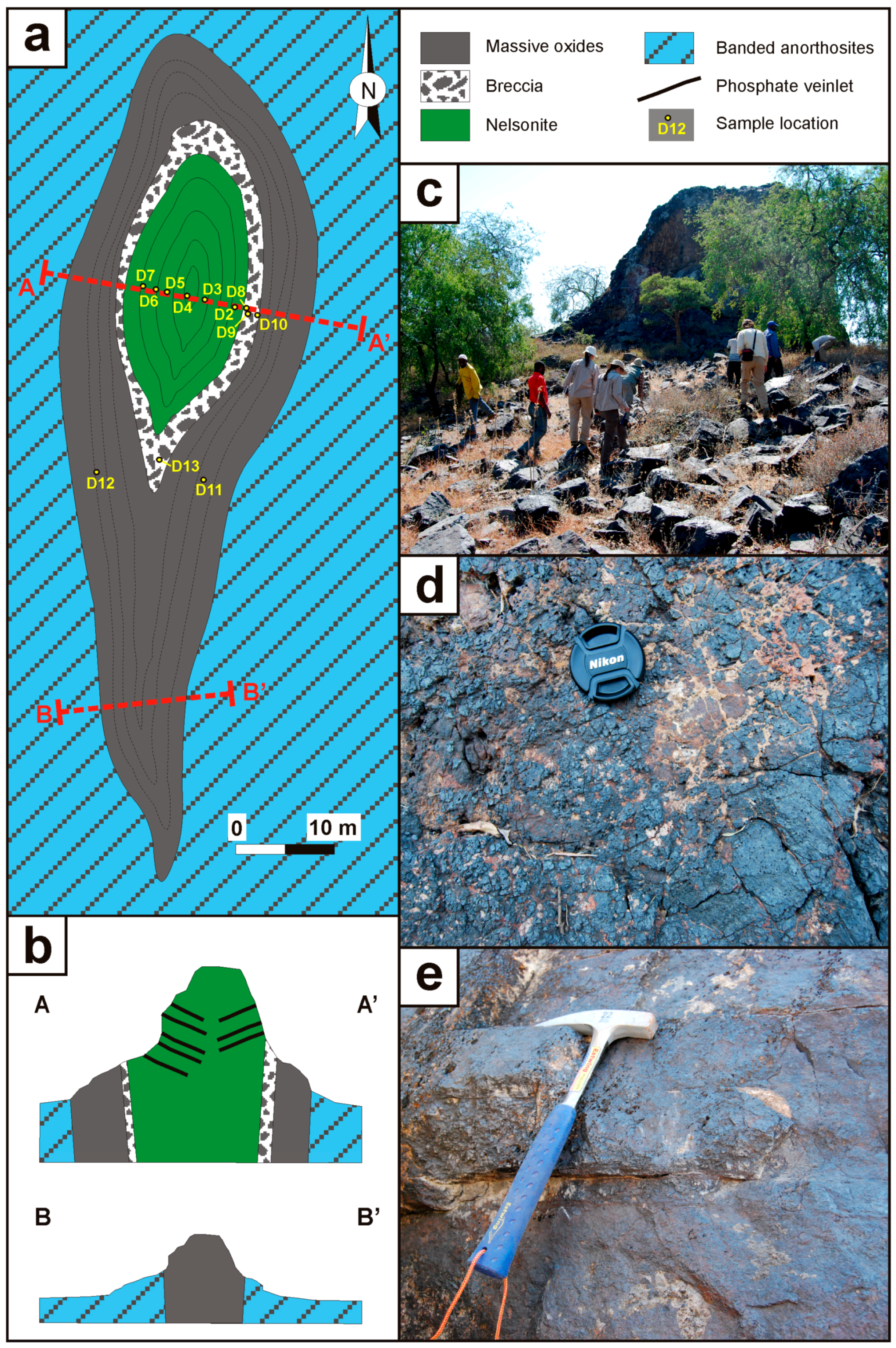
2.1. Structure of the Fe–Ti(–V) Oxide Bodies
2.1.1. Dongue Sul
2.1.2. Tchimbueio
2.1.3. Tchingunguanganda
2.1.4. Chiange Velho
3. Materials and Methods
3.1. Sampling
3.2. Bulk Rock Geochemistry
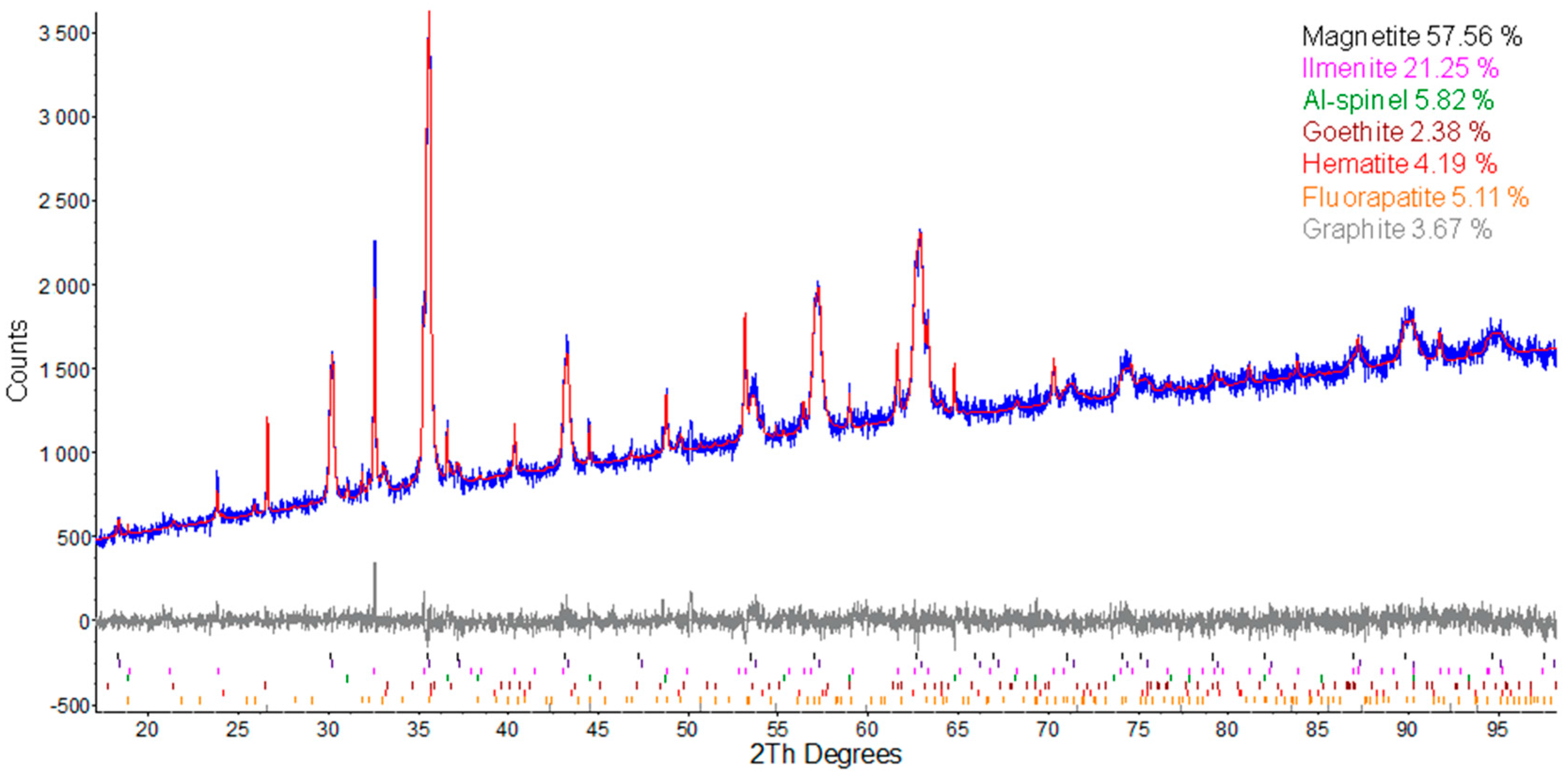
3.3. Powder X-ray Diffraction (PXRD)
3.4. Scanning Electron Microscopy (SEM)
3.5. Electron Microprobe Analyses (EMP)
Geothermometry
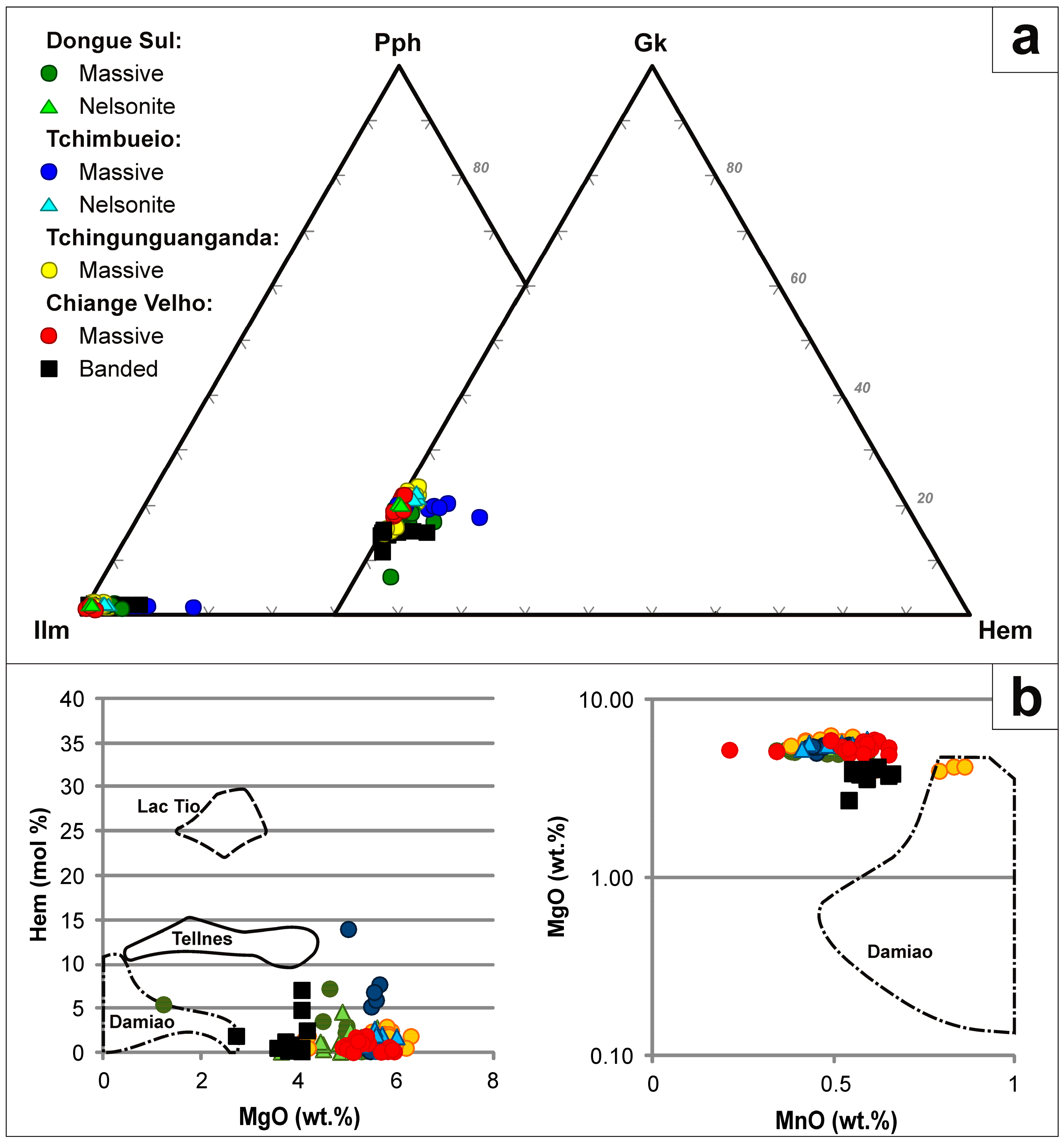
4. Results
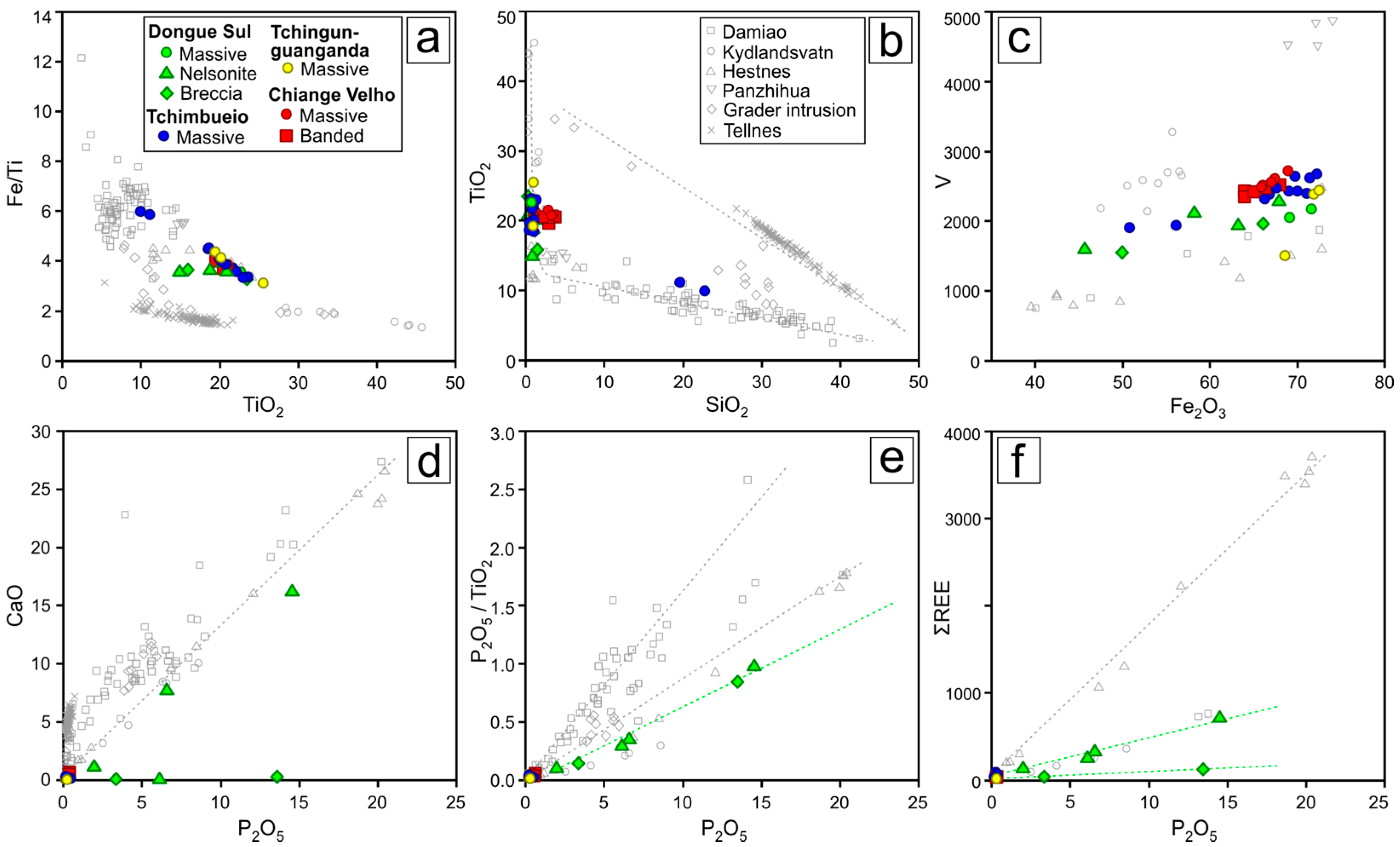
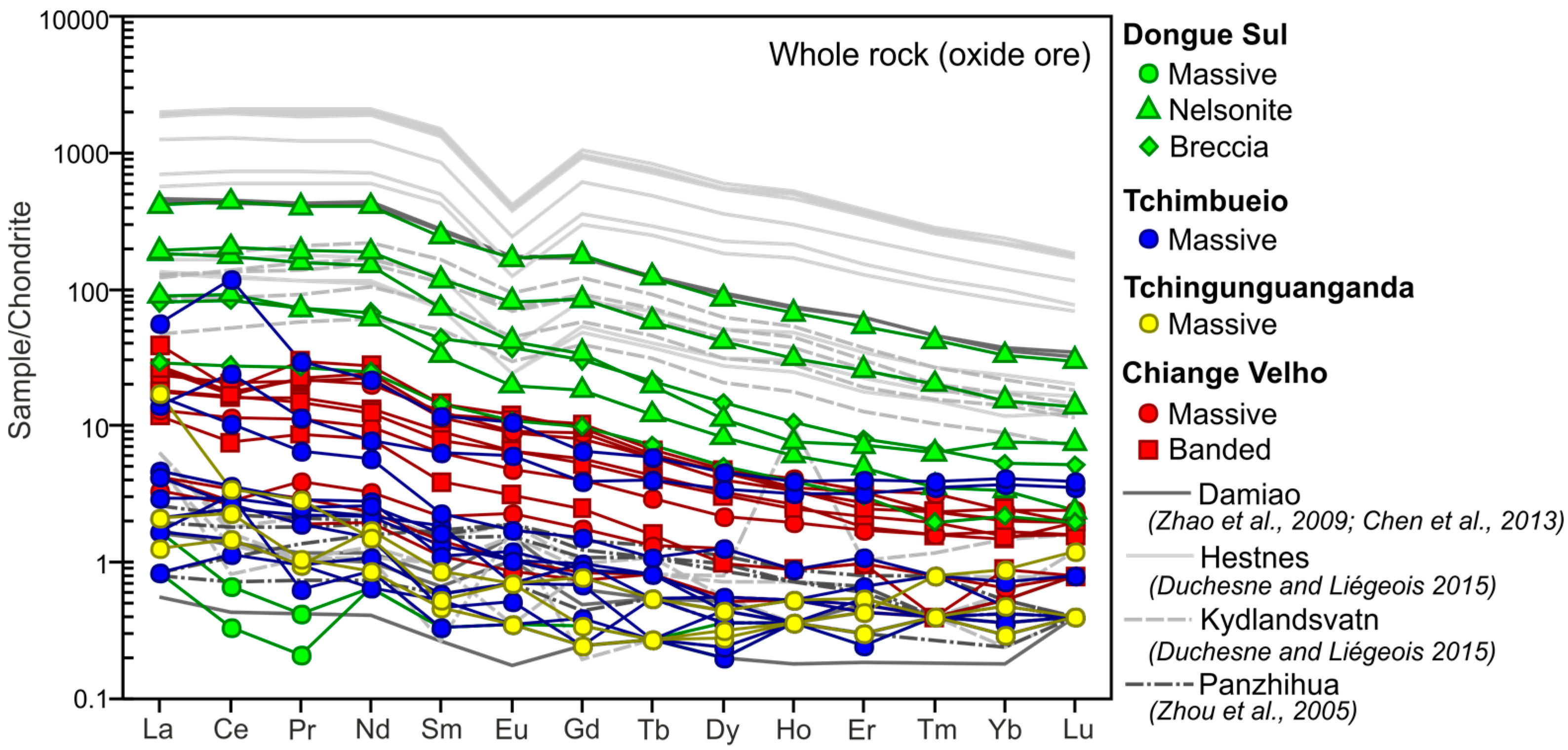
4.1. Bulk Rock Geochemistry
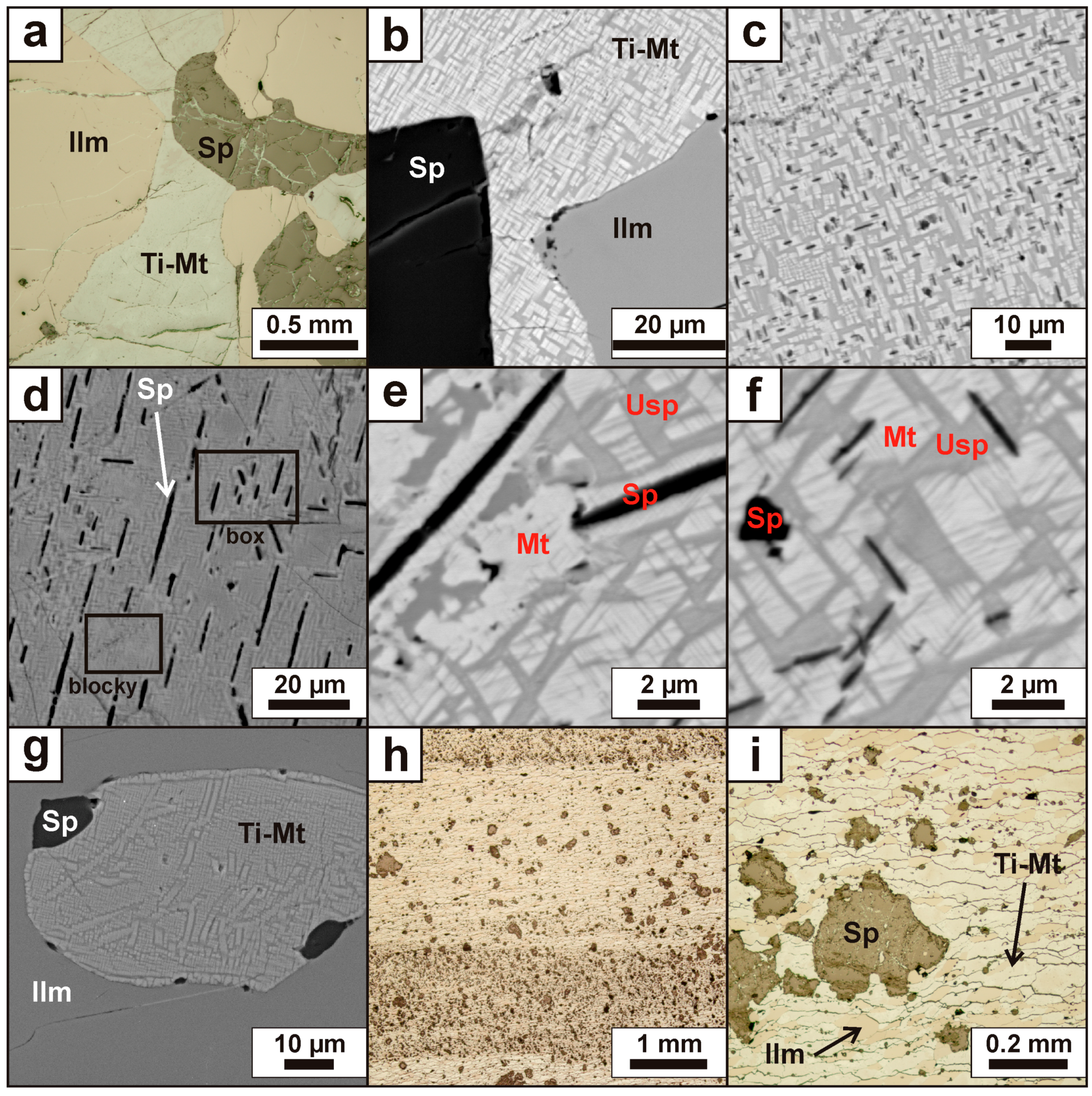
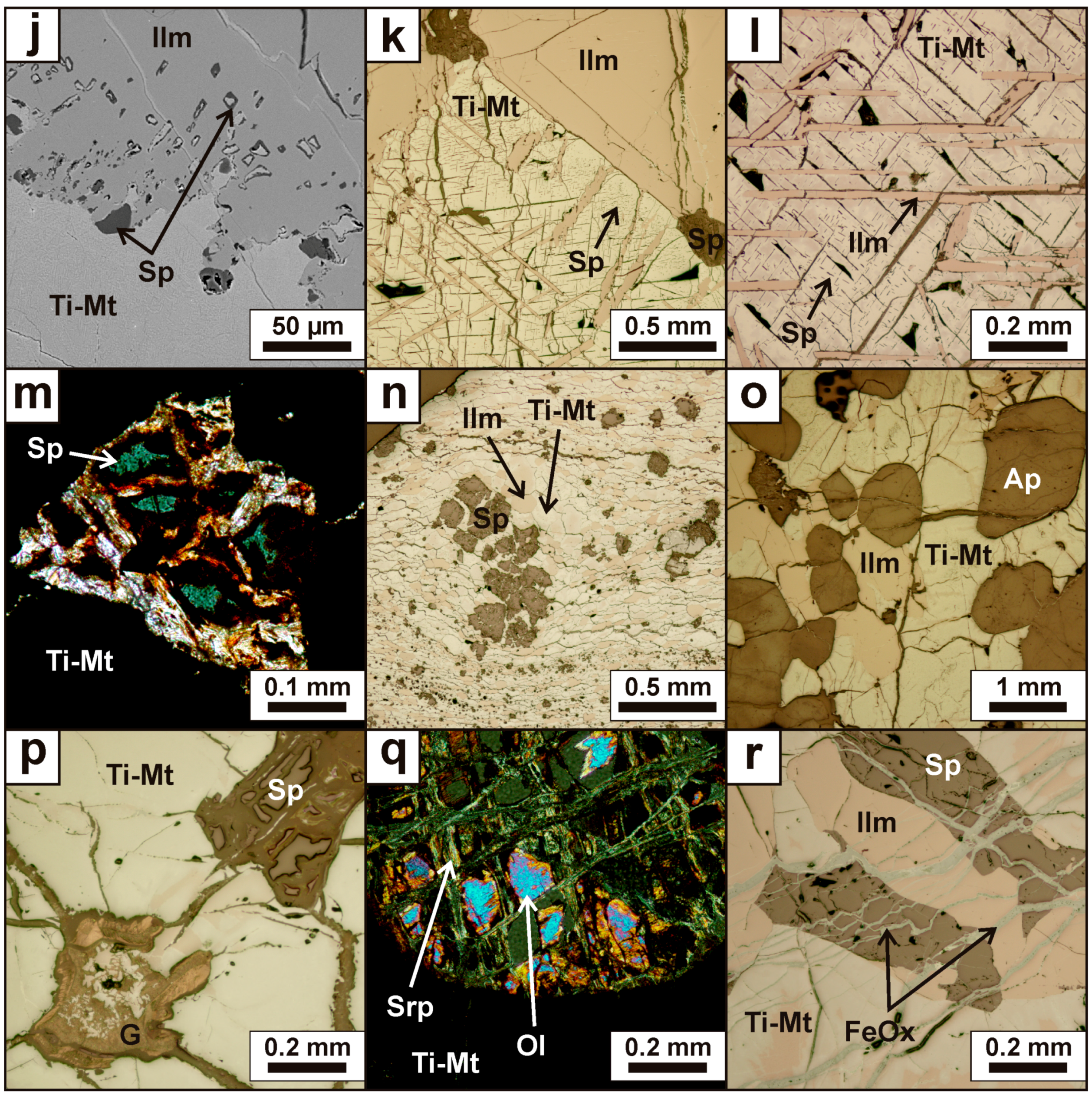
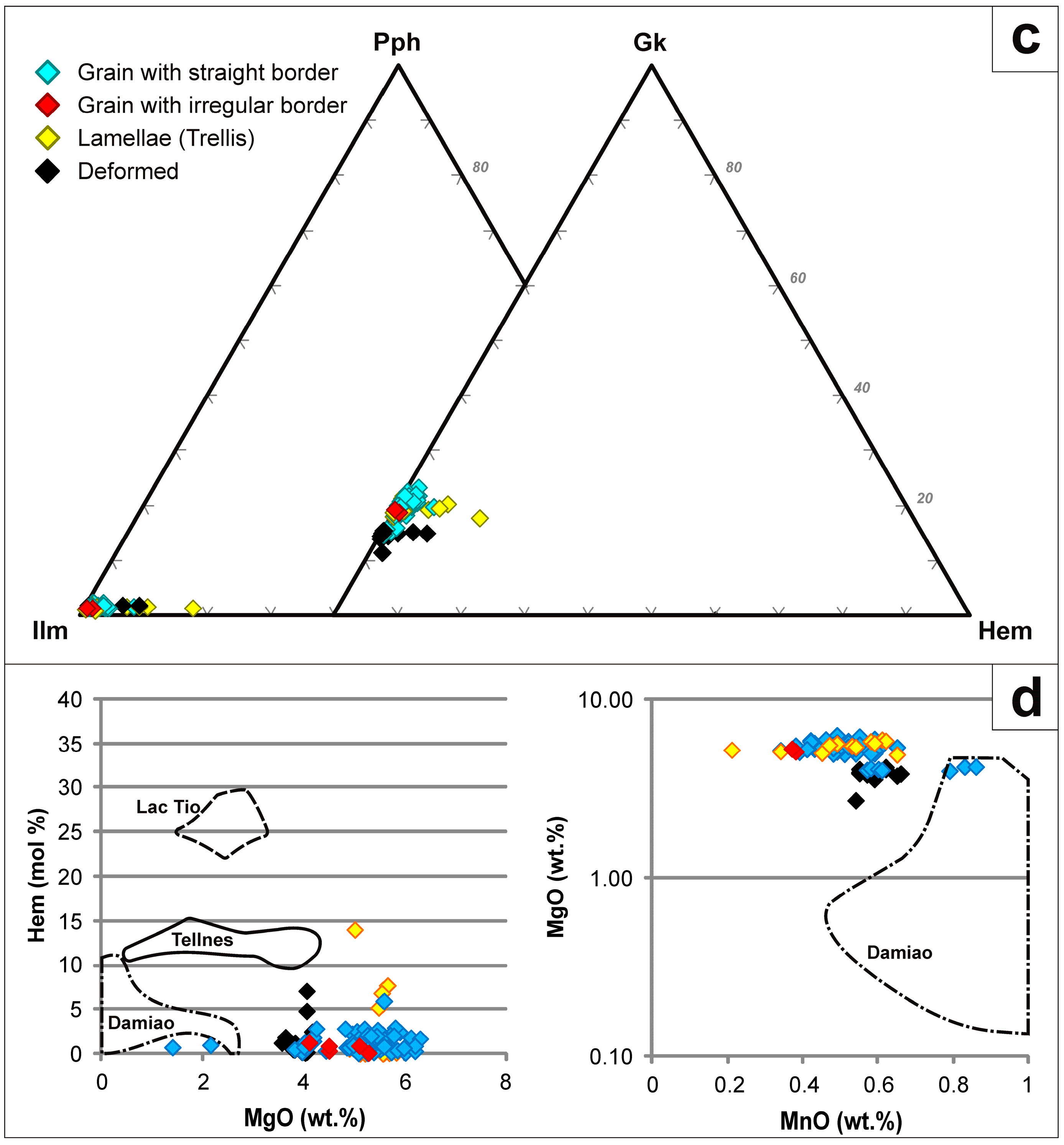
4.2. Mineralogy and Textures
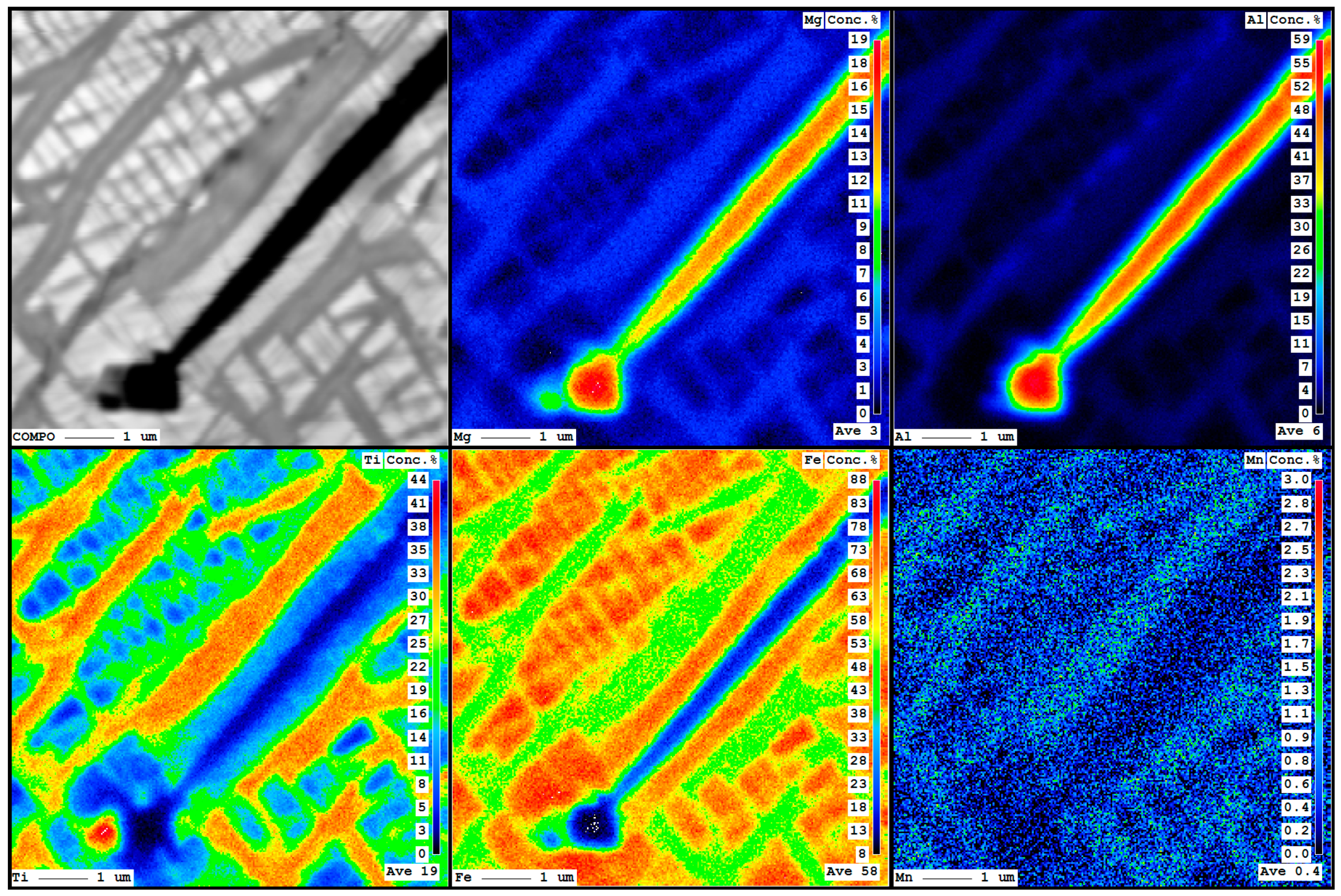
4.2.1. Titaniferous Magnetite
4.2.2. Ilmenite
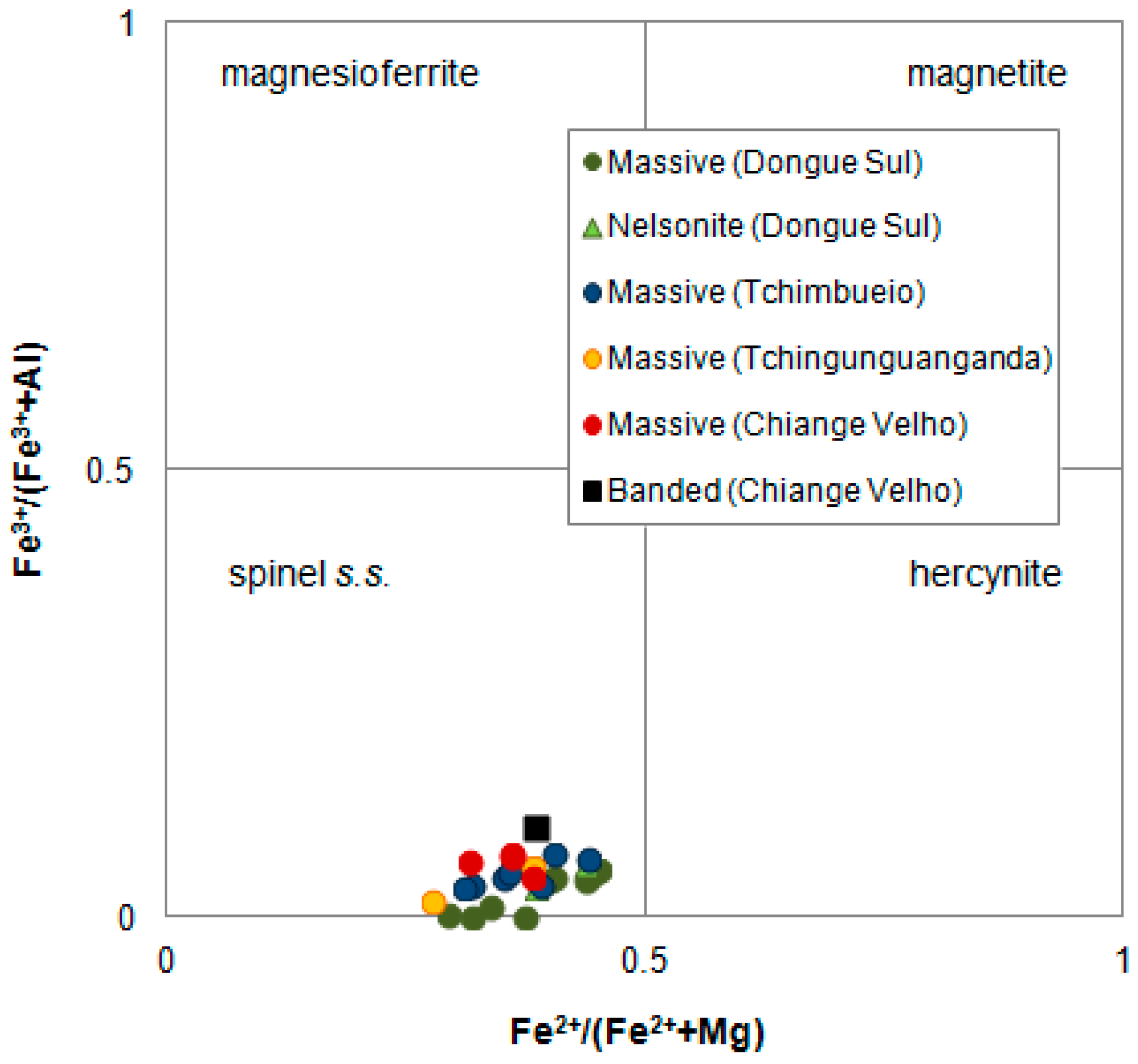
4.2.3. Aluminous Spinel
4.2.4. Accessory Minerals
4.3. Mineral Chemistry
4.3.1. Titaniferous Magnetite
4.3.2. Ilmenite
4.3.3. Aluminous Spinel
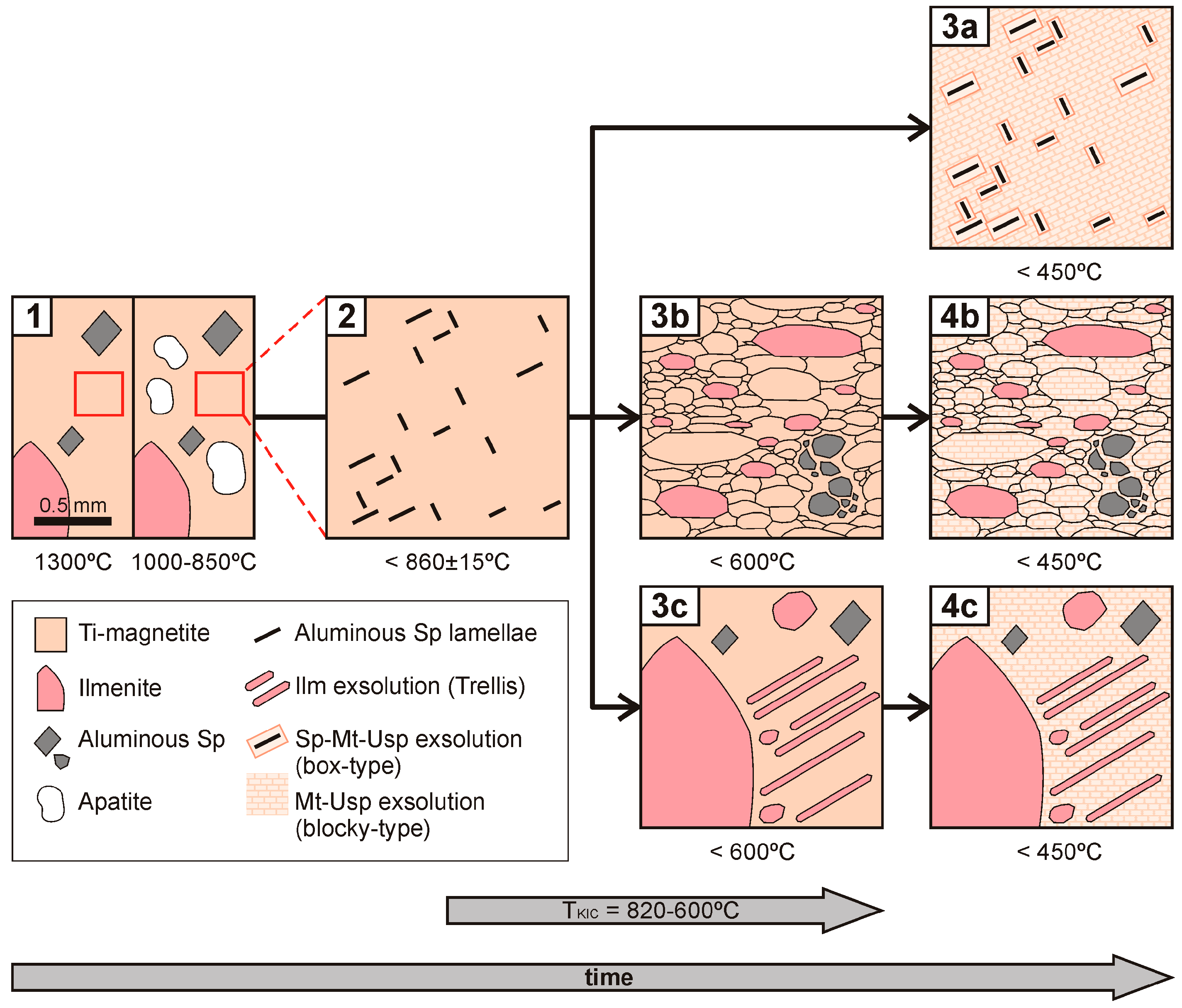
4.4. Geothermobarometry
5. Discussion
5.1. T-fO2 Evolution of the Studied KIC Fe–Ti(–V) Oxide Bodies
5.2. Genesis of the Fe–Ti(–V) Oxide Bodies in the KIC
5.3. Massif-Type Anorthosite Fe–Ti(–V) Oxide Bodies in the Angolan KIC and Economic Significance
6. Conclusions and Future Developments
- According to the calculated temperatures and the observed textures, the thermal history and crystallization sequence of the KIC mineralizations included: (1) Crystallization in equilibrium of titaniferous magnetite, ilmenite and spinel below 1300 °C (between 850 °C and 1000 °C in nelsonites); (2) exsolution of aluminous spinel in titaniferous magnetite below 850 °C; (3) shear deformation in Chiange Velho that produced mylonitic fabrics in some rocks and recrystallization below 600 °C; (4) exsolution of titaniferous magnetite in ulvöspinel and magnetite below 450 °C (Figure 12). This suggests that the Fe–Ti(–V) oxide bodies associated to the massif-type anorthosite may have remained long time at a certain depth (8 kbar~24 km).
- The Fe–Ti(–V) oxide bodies of the Angolan KIC contain remarkable reserves of Ti, V, P and REE that may represent an economic resource.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gross, G.A.; Gower, C.F.; Lefebure, D.V. Magmatic Ti-Fe ± V Oxide Deposits, in: Geological Fieldwork; Paper 1998-1; British Columbia Ministry of Employment and Investment: Vancouver, BC, Canada, 1997; pp. 24J-1–24J-3. [Google Scholar]
- Kesler, S.E.; Simon, A.C. Mineral Resources, Economics and the Environment, 2nd ed.; Cambridge University Press: Cambridge, UK, 2015; 446p. [Google Scholar]
- Chen, W.T.; Zhou, M.F.; Zhao, T.P. Differentiation of nelsonitic magmas in the formation of the ~1.74 Ga Damaio Fe-Ti-P ore deposit, North China. Contrib. Mineral. Petrol. 2013, 165, 1341–1362. [Google Scholar] [CrossRef]
- Polyak, D.E. USGS Mineral Commodity Summaries, Vanadium. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/vanadium/mcs-2017-vanad.pdf (accessed on 13 December 2017).
- Zhou, M.F.; Chen, W.T.; Wang, C.Y.; Prevec, S.A.; Liu, P.P.; Howarth, G.H. Two stages of immiscible liquid separation in the formation of Panzhihua-type Fe-Ti-V oxide deposits, SW China. Geosci. Front. 2013, 4, 481–502. [Google Scholar] [CrossRef]
- Charlier, B.; Namur, O.; Bolle, O.; Latypov, R.; Duchesne, J.C. Fe-Ti-V-P ore deposits associated with Proterozoic massif-type anorthosites and related rocks. Earth Sci. Rev. 2015, 141, 56–81. [Google Scholar] [CrossRef]
- Hébert, C.; Cadieux, A.M.; van Breemen, O. Temporal evolution and nature of Ti-Fe-P mineralization in the anorthosite-mangerite-charnockite-granite (AMCG) suites of the south-central Grenville Province, Saguenay-Lac St. Jean area, Quebec, Canada. Can. J. Earth Sci. 2005, 42, 1865–1880. [Google Scholar] [CrossRef]
- Ashwal, L.D.; Twist, D. The Kunene complex, Angola/Namibia: A composite massif-type anorthosite complex. Geol. Mag. 1994, 131, 579–591. [Google Scholar] [CrossRef]
- Mayer, A.; Hofmann, A.W.; Sinigoi, S.; Morais, E. Mesoproterozoic Sm–Nd and U–Pb ages for the Kunene Anorthosite Complex of SW Angola. Precambrian Res. 2004, 133, 187–206. [Google Scholar] [CrossRef]
- Menge, G.F.W. The antiformal structure and general aspects of the Kunene Complex. Z. Dtsch. Geol. Ges. 1998, 149, 431–448. [Google Scholar]
- Drüppel, K.; Von Seckendorff, V.; Okrusch, M. Subsolidus reaction textures in the anorthositic rocks of the southern part of the Kunene Intrusive Complex, NW Namibia. Eur. J. Mineral. 2001, 13, 289–309. [Google Scholar] [CrossRef]
- Drüppel, K.; Littmann, R.L.; Romer, R.L.; Okrusch, M. Petrology and isotope geochemistry of the Mesoproterozoic anorthosite and related rocks of the Kunene Intrusive Complex, NW Namibia. Precambrian Res. 2007, 156, 1–31. [Google Scholar] [CrossRef]
- Gleißner, P.; Drüppel, K.; Taubald, H. Magmatic evolution of anorthosites of the Kunene Intrusive Complex, NW Namibia: Evidence from oxygen isotope data and trace element zoning. J. Petrol. 2010, 51, 897–919. [Google Scholar] [CrossRef]
- Morais, E.; Sinigoi, S.; Mayer, A.; Mucana, A.; Miguel, L.G.; Rufino Neto, J. The Kunene gabbro-anorthosite Complex: Preliminary results based on new field and chemical data. Afr. Geosci. Rev. 1998, 5, 485–498. [Google Scholar]
- Cardoso, S.M.; Marques, J.M. Notícia Explicativa da Folha n° 378 (Chibemba), Carta Geológica de Angola—Escala 1:100,000; Direcção Provincial dos Serviços de Geologia e Minas de Angola: Luanda, Angola, 1971. [Google Scholar]
- De Carvalho, H. Notícia Explicativa da Folha n° 377 (Vila de Almoster), Carta Geológica de Angola—Escala 1:100,000; Direcção Provincial dos Serviços de Geologia e Minas de Angola: Luanda, Angola, 1972. [Google Scholar]
- Lorena, S.A.P. Ocorrências de ilmenites no sul de Angola. Bol. Serv. Geol. Minas Angola 1969, 20, 21–23. [Google Scholar]
- De Carvalho, H.; Alves, P.H. Gabbro-Anorthosite Complex of SW Angola/NW Namibia; Serie de Ciencias da Terra; Comunicações Instituto Investigação Científica Tropical: Lisboa, Portugal, 1990; pp. 5–64. [Google Scholar]
- De Carvalho, H.; Alves, P.H. The Precambrian of SW Angola and NW Namibia; General Remarks, Correlation Analysis, Economic Geology; Comunicações Instituto Investigação Científica Tropical: Lisboa, Portugal, 1993; Volume 4, p. 38. [Google Scholar]
- De Carvalho, H.; Tassinari, C.; Alves, P.H.; Guimarães, F.; Simões, M.C. Geochronological review of the Precambrian in western Angola: Links with Brazil. J. Afr. Earth Sci. 2000, 31, 383–402. [Google Scholar] [CrossRef]
- Silva, Z.C.G. Geochemistry of the gabbro-anorthosite Complex of Angola. J. Afr. Earth Sci. 1990, 10, 683–692. [Google Scholar] [CrossRef]
- Silva, Z.C.G. Mineralogy and cryptic layering of the Kunene anorthosite complex of SW Angola and Namibia. Mineral. Mag. 1992, 56, 319–327. [Google Scholar] [CrossRef]
- Ramdohr, P. Ulvöspinel and its significance in titaniferous iron ores. Econ. Geol. 1953, 48, 677–688. [Google Scholar] [CrossRef]
- Simpson, E.S.W. The anorthosite of southern Angola: A review of present data. In African Magmatism and Tectonics; Clifford, T.N., Gass, I.G., Eds.; Oliver and Boyd: Edinburgh, UK, 1970; pp. 89–96. [Google Scholar]
- Roman’ko, E.F.; Titov, V.I. Iron-titanium ore mineralization in the Kunene intrusive massif. Izv. Vyssh. Uchebn. Zaved. Geol. Razved. 2005, 6, 35–38. [Google Scholar]
- Simpson, E.S.W.; Otto, J.D.T. On the Precambrian anorthosite mass of southern Angola. In Proceedings of the 21st International Geological Congress; Det Berlingske Bogtrykkeri: Copenhagen, Denmark, 1960; Volume 13, pp. 216–227. [Google Scholar]
- Vermaak, C.F. Kunene Anorthosite Complex. In Precambrian of the Southern Hemisphere, Developments in Precambrian Geology; Hunter, D.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1981; Volume 2, pp. 578–599. ISBN 0444418628. [Google Scholar]
- Stone, P.; Brown, G.M. The Quihita-Cunene layered gabbroic intrusion of South-West Angola. Geol. Mag. 1958, 95, 195–206. [Google Scholar] [CrossRef]
- Slejko, F.; Demarchi, G.; Morais, E. Mineral chemistry and Nd isotopic composition of two anorthositic rocks from the Kunene Complex (SW Angola). J. Afr. Earth Sci. 2002, 35, 77–88. [Google Scholar] [CrossRef]
- Maier, W.D.; Rasmussen, B.; Fletcher, I.R.; Li, C.; Barnes, S.-J.; Huhma, H. The Kunene anorthosite complex, Namibia, and its satellite intrusions: Geochemistry, geochronology, and economic potential. Econ. Geol. 2013, 108, 953–986. [Google Scholar] [CrossRef]
- Brandt, S.; Klemd, R.; Okrusch, M. Ultrahigh-Temperature Metamorphism and Multistage Evolution of Garnet–Orthopyroxene Granulites from the Proterozoic Epupa Complex, NW Namibia. J. Petrol. 2003, 44, 1121–1144. [Google Scholar] [CrossRef]
- Brandt, S.; Klemd, R. Upper-amphibolite facies partial melting of paragneisses from the Epupa Complex, NW Namibia, and relations to Mesoproterozoic anorthosite magmatism. J. Metamorph. Geol. 2008, 26, 871–893. [Google Scholar] [CrossRef]
- Seth, B.; Armstrong, R.A.; Brandt, S.; Villa, I.M.; Kramers, J.D. Mesoproterozoic U-Pb and Pb-Pb ages of granulites in NW Namibia: Reconstructing a complete orogenic cycle. Precambrian Res. 2003, 126, 147–168. [Google Scholar] [CrossRef]
- Seth, B.; Armstrong, R.A.; Büttner, A.; Villa, I.M. Time constraints for Mesoproterozoic upper amphibolite facies metamorphism in NW Namibia: A multi-isotopic approach. Earth Planet. Sci. Lett. 2005, 230, 355–378. [Google Scholar] [CrossRef]
- Kröner, A.; Rojas-Agramonte, Y.; Wong, J.; Wilde, S.A. Zircon reconnaissance dating of Proterozoic gneisses along the Kunene River of Northwestern Namibia. Tectonophysics 2015, 662, 125–139. [Google Scholar] [CrossRef]
- Torquato, J.R.; Silva, A.T.S.F.; da Cordani, U.G.; Kawashita, K. A evolução geológica do Cinturão Móvel do Quipungo no Ocidente de Angola. An. Acad. Brasil. Geocienc. 1979, 51, 133–143. [Google Scholar]
- De Carvalho, H.; Fernandez, A.; Vialette, Y. Chronologie absolue du Précambrien du Sud-Ouest de l’Angola. C. R. Acad. Sci. Paris 1979, 288, 1647–1650. [Google Scholar]
- Maier, W.D.; Teigler, B.; Miller, R. The Kunene anorthosite complex and its satellite intrusions. In The Geology of Namibia; Miller, R.M.G., Ed.; Geological Survey of Namibia: Windhoek, Namibia, 2008; pp. 9-1–9-18. ISBN 9780869767313. [Google Scholar]
- Mayer, A.; Sinigoi, S.; Miguel, L.G.; Morais, E.; Petrini, R. Kibaran ages in the Kunene anorthositic complex. Abstr. Geoluanda 2000, 106. [Google Scholar]
- Morais, E.; Sinigoi, S.; Mayer, A.; Miguel, L.G. The Kunene gabbro-anorthosite Complex: Coalescence of discrete crystal mush intrusions. Abstr. Geoluanda 2000, 110. [Google Scholar]
- Drüppel, K.; Okrusch, M. Geo-und isotopenchemische Untersuchungen der Anorthosite des Kunene-Intrusiv-Komplexes (KIC) in NW-Namibia. Eur. J. Mineral. 2000, 12, 37. [Google Scholar]
- Bassot, J.P.; Pascal, M.; Vialette, Y. Données nouvelles sur la stratigraphie, la géochemie et la géochronologie des formations precambriennes de la partie meridionale du Haut Plateau angolais. Bull. BRGM sec 4 Géol. Gén. 1981, 4, 285–309. [Google Scholar]
- De Carvalho, H.; Crasto, J.; Silva, Z.C.; Vialette, Y. The Kibaran cycle in Angola: A discussion. Afr. Geol. Rev. Geol. J. 1987, 22, 85–102. [Google Scholar] [CrossRef]
- Von Seckendorff, V.; Drüppel, K.; Okrusch, M.; Littmann, S.; Cook, N. Oxide-sulphide relationships in sodalite-bearing metasomatites of the Empembe-Swartbooisdrif Alkaline Province, North-West Namibia. Miner. Deposita 2000, 35, 430–450. [Google Scholar] [CrossRef]
- Franke, H.; Drüppel, K.; Brätz, H. Formation of anorthosite-related Fe-Ti deposits, Namibia. Geochim. Cosmochim. Acta 2009, 73 (Suppl. 1), A394. [Google Scholar]
- Gleiβner, P.; Drüppel, K.; Becker, H. Osmium isotopes and highly siderophile element fractionation in the massif-type anorthosites of the Mesoproterozoic Kunene Intrusive Complex, NW Namibia. Chem. Geol. 2012, 302–303, 33–47. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F. Quantitative analysis of homogeneous or stratified microvolumes applying the model PAP. In Electron Probe Quantitation; Heinrich, K.J., Newbury, D.E., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 31–75. ISBN 978-1-4899-2619-7. [Google Scholar]
- Carmichael, I.S.E. The iron-titanium oxides of salic volcanic rocks and their associated ferromagnesian silicates. Contrib. Mineral. Petrol. 1967, 14, 36–64. [Google Scholar] [CrossRef]
- Torres Roldán, R.L.; García-Casco, A.; García-Sánchez, P.A. CSpace: An integrated workplace for the graphical and algebraic analysis of phase assemblages on 32-bit Wintel platforms. Comput. Geosci. 2000, 26, 779–793. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Villanova-de-Benavent, C.; Galí, S.; Melgarejo, J.C.; Gonc̦alves, A.O. Fe-Ti(-V) oxide mineralogy and thermo-oxybarometry from the Kunene anorthosite Complex deposits (SW Angola). In Let's Talk Ore Deposits: Proceedings of the 11th Biennial SGA Meeting; Barra, F., Reich, M., Campos, E., Tornos, F., Eds.; Universidad Católica del Norte: Antofagasta, Chile, 2011; pp. 465–467. ISBN 9789562873291. [Google Scholar]
- Zhao, T.P.; Chen, W.; Zhou, M.F. Geochemical and Nd-Hf isotopic constraints on the origin of the ~1.74-Ga Damiao anorthosite complex, North Xina Craton. Lithos 2009, 113, 673–690. [Google Scholar] [CrossRef]
- Zhou, M.F.; Robinson, P.T.; Lesher, C.M.; Keays, R.R.; Zhang, C.J.; Malpas, J. Geochemistry, petrogenesis and metallogenesis of the Panzhihua gabbroic layered intrusion and associated Fe-Ti-V oxide deposits, Sichuan Province, SW China. J. Petrol. 2005, 46, 2253–2280. [Google Scholar] [CrossRef]
- Duchesne, J.C.; Liégeois, J.P. The origin of nelsonite and high-Zr ferrodiorite associated with Proterozoic anorthosite. Ore Geol. Rev. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Zhou, M.-F.; Arndt, N.T.; Malpas, J.; Wang, C.Y.; Kennedy, A.K. Two magma series and associated ore deposit types in the Permian Emeishan large igneous province, SW China. Lithos 2008, 103, 352–368. [Google Scholar] [CrossRef]
- Charlier, B.; Sakoma, E.; Sauvé, M.; Stanaway, K.; Vander Auwera, J.; Duchesne, J.C. The Grader layered intrusion (Havre-Saint Pierre anorthosite, Quebec) and genesis of nelsonite and other Fe-Ti-P ores. Lithos 2008, 101, 359–378. [Google Scholar] [CrossRef]
- Waychunas, G.A. Crystal chemistry of oxides and oxyhydroxides. In Oxide Minerals: Petrologic and Magnetic Significance; Lindsley, D.H., Ed.; Mineralogical Society of America: Chantilly, VA, USA, 1991; Volume 25, pp. 11–68. ISBN 0-939950-30-8. [Google Scholar]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Haggerty, S.E. Oxide textures-a mini atlas. Rev. Mineral. 1991, 25, 303–321. [Google Scholar]
- Duchesne, J.C. Iron-titanium oxide minerals in the Bjerkrem-Sokndal Massif, South-western Norway. J. Petrol. 1972, 13, 57–81. [Google Scholar] [CrossRef]
- Von Gruenewaldt, G.; Klemm, D.D.; Henckel, J.; Dehm, R.M. Exolution features in titanomagnetites from massive magnetite layers and their host rocks of the Upper Zone, Eastern Bushveld Complex. Econ. Geol. 1985, 80, 1041–1061. [Google Scholar] [CrossRef]
- Ivanyuk, G.Y.; Kalashnikov, A.O.; Pakhomovsky, Y.A.; Bazai, A.V.; Goryainov, P.M.; Mikhailova, J.A.; Yakovenchuk, V.N.; Konopleva, N.G. Subsolidus Evolution of the Magnetite-Spinel-Ulvöspinel Solid Solutions in the Kovdor Phoscorite-Carbonatite Complex, NW Russia. Minerals 2017, 7, 215. [Google Scholar] [CrossRef]
- Duchesne, J.C. Les gisements d’oxydes de fer et titane dans les roches anorthositiques du Rogaland (Norvège méridionale). Colloq. Sci. Int. Eugène Raguin 1973, 241–248. [Google Scholar]
- Sauerzapf, U.; Lattard, D.; Burchard, M.; Engelmann, R. The titanomagnetite-ilmenite equilibrium: New experimental data and thermo-oxybarometric application to the crystallization of basic to intermediate rocks. J. Petrol. 2008, 49, 1161–1185. [Google Scholar] [CrossRef]
- Spencer, K.J.; Lindsley, D.H. A solution model for coexisting iron-titanium oxides. Am. Mineral. 1981, 66, 1189–1201. [Google Scholar]
- Lister, G.F. The composition and origin of selected iron-titanium deposits. Econ. Geol. 1966, 61, 275–310. [Google Scholar] [CrossRef]
- Buddington, A.F.; Lindsley, D.H. Iron-titanium oxide minerals and their synthetic equivalents. J. Petrol. 1964, 5, 310–357. [Google Scholar] [CrossRef]
- Frost, B.R. Introduction to oxygen fugacity and its petrologic importance. Rev. Mineral. Geochem. 1991, 25, 303–321. [Google Scholar]
- Jakobsson, S.; Oskarsson, N. The system C-O in equilibrium with graphite at high pressure and temperature: An experimental study. Geochim. Cosmochim. Acta 1994, 58, 9–17. [Google Scholar] [CrossRef]
- Duchesne, J.C. Microstructures of Fe-Ti oxide minerals in the South Rogaland anorthositic complex (Norway). Ann. Soc. Geol. Belg. 1970, 93, 527–544. [Google Scholar]
- Price, G.D. Subsolidus phase relations in the titanomagnetite solid solution series. Am. Mineral. 1981, 66, 751–758. [Google Scholar]
- Haggerty, S.E. Oxidation of opaque mineral oxides in basalts. In Oxide Minerals; Rumble, D., Ed.; Mineralogical Society of America: Chantilly, VA, USA, 1976; Volume 3, pp. Hg1–Hg100. ISBN 0-939950-03-0. [Google Scholar]
- Haggerty, S.E. Opaque mineral oxides in terrestrial igneous rocks. In Oxide Minerals; Rumble, D., Ed.; Mineralogical Society of America: Chantilly, VA, USA, 1976; Volume 3, pp. Hg101–Hg300. ISBN 0-939950-03-0. [Google Scholar]
- Turnock, A.C.; Eugster, H.P. Fe-Al oxides: Phase relationships below 1000 °C. J. Petrol. 1962, 3, 533–565. [Google Scholar] [CrossRef]
- Vincent, E.A. Ulvöspinel in the Skaergaard intrusion, East Greenland. Neues Jahrb. Mineral. Abh. 1960, 94, 992–1016. [Google Scholar]
- Morse, S.A. The feldspar/magma density paradox. In The Nain Anorthosite Project, Labrador: Field Report 1971; Morse, S.A., Ed.; University of Massachusetts at Amherst: Amherst, MA, USA, 1971; Volume 9, pp. 113–116. [Google Scholar]
- Campbell, I.H.; Roeder, P.L.; Dixon, J.M. Plagioclase buoyancy in basaltic liquids as determined with a centrifuge furnace. Contrib. Mineral. Petrol. 1978, 67, 369–377. [Google Scholar] [CrossRef]
- Scoates, J.S. The plagioclase-magma density paradox re-examined and the crystallization of Proterozoic anorthosites. J. Petrol. 2000, 41, 627–649. [Google Scholar] [CrossRef]
- Charlier, B.; Duchesne, J.C.; Vander Auwera, J. Magma chamber processes in the Tellnes ilmenite deposit (Rogaland anorthosite province; SW Norway) and the formation of Fe-Ti ores in massif-type anorthosites. Chem. Geol. 2006, 234, 264–290. [Google Scholar] [CrossRef]
- Philpotts, A.R. Origin of certain iron-titanium oxide and apatite rocks. Econ. Geol. 1967, 62, 303–315. [Google Scholar] [CrossRef]
- Charlier, B.; Namur, O.; Malpas, S.; De Marneffe, C.; Duchesne, J.C.; Vander Auwera, J.; Bolle, O. Origin of the giant Allard Lake ilmenite ore deposit (Canada) by fractional crystallization, multiple magma pulses and mixing. Lithos 2010, 117, 119–134. [Google Scholar] [CrossRef]
- Kohlstedt, D.L.; Zimmerman, M.E.; Mackwell, S.J. Stress-driven melt segregation in partially molten feldspathic rocks. J. Petrol. 2010, 51, 9–19. [Google Scholar] [CrossRef]
- Paludan, J.; Hansen, U.B.; Olesen, N.Ø. Structural evolution of the Precambrian Bjerkreim–Sokndal intrusion, South Norway. Nor. Geol. Tidsskr. 1994, 74, 185–198. [Google Scholar]
- Wilson, J.R.; Robins, B.; Nielsen, F.; Duchesne, J.C.; Vander Auwera, J. The Bjerkreim-Sokndal layered intrusion, Southwest Norway. In Layered Intrusions; Cawthorn, R.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 231–256. ISBN 0444540644. [Google Scholar]
- Duchesne, J.C. Liquid ilmenite or liquidus ilmenite: A comment on the nature of ilmenite vein deposits. In Petrology and Geochemistry of Magmatic Suites of Rocks in the Continental and Oceanic Crusts (a Volume Dedicated to Professor Jean Michot); Demaiffe, D., Ed.; Royal Museum for Central Africa: Tervuren, Belgium; Université Libre de Bruxelles: Bruxelles, Belgium, 1996; pp. 73–82. [Google Scholar]
- Duchesne, J.C.; Shumlyanskyy, L.; Charlier, B. The Fedorivka layered intrusion (Korosten Pluton, Ukraine): An example of highly differentiated ferrobasaltic evolution. Lithos 2006, 89, 353–376. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Chen, Z.; Wang, D.; Chen, W. Hydrothermal mineralization and fluid inclusion study on the Heishan iron deposit, Chengde County, Hebei Province, China. Acta Petrol. Sin. 2010, 26, 858–870. [Google Scholar]
- Li, H.; Li, L.; Zhang, Z.; Santosh, M.; Liu, M.; Cui, Y.; Yang, X.; Chen, J.; Yao, T. Alteration of the Damiao anorthosite complex in the northern North China Craton: Implications for high-grade iron mineralization. Ore Geol. Rev. 2014, 57, 574–588. [Google Scholar] [CrossRef]
- Emslie, R.F. Anorthosite massifs, rapakivi granites and late Proterozoic rifting of North America. Precambrian Res. 1978, 7, 61–98. [Google Scholar] [CrossRef]
- Ashwal, L.D. Anorthosites; Springer-Verlag: Berlin, Germany, 1993; ISBN 978-3-642-77442-3. [Google Scholar]
- Bybee, G.M.; Ashwal, L.D.; Shirey, S.B.; Horan, M.; Mock, T.; Andersen, T.B. Pyroxene megacrysts in Proterozoic anorthosites: Implications for tectonic setting, magma source and magmatic processes at the Moho. Earth Planet. Sci. Lett. 2014, 389, 74–85. [Google Scholar] [CrossRef]
- Duchesne, J.C. Fe–Ti deposits in Rogaland anorthosites (South Norway): Geochemical characteristics and problems of interpretation. Miner. Deposita 1999, 34, 182–198. [Google Scholar] [CrossRef]
- Longhi, J. A mantle or mafic crustal source for Proterozoic anorthosites? Lithos 2005, 83, 183–198. [Google Scholar] [CrossRef]
- Longhi, J.; Vander Auwera, J.; Fram, M.S.; Duchesne, J.C. Some phase equilibrium constraints on the origin of Proterozoic (massif) anorthosites and related rocks. J. Petrol. 1999, 40, 339–362. [Google Scholar] [CrossRef]
- Martignole, J.; Schrijver, K. The level of anorthosites and its tectonic pattern. Tectonophysics 1970, 10, 403–409. [Google Scholar] [CrossRef]
- Kolker, A.; Lindsley, D.H. Geochemical evolution of the Maloin Ranch pluton, Laramie Anorthosite Complex, Wyoming: Petrology and mixing relations. Am. Mineral. 1989, 74, 307–324. [Google Scholar]
- Toplis, M.J.; Carroll, M.R. An experimental study of the influence of oxygen fugacity on Fe–Ti oxide stability, phase relations, and mineral-melt equilibria in ferro-basaltic systems. J. Petrol. 1995, 36, 1137–1170. [Google Scholar] [CrossRef]
- Vander Auwera, J.; Longhi, J. Experimental study of a jotunite (hypersthene monzodiorite): Constraints on the parent magma composition and crystallization conditions (P, T, fO2) of the Bjerkreim-Sokndal layered intrusion (Norway). Contrib. Mineral. Petrol. 1994, 118, 60–78. [Google Scholar] [CrossRef]
- Frost, C.D.; Chamberlain, K.R.; Frost, K.R.; Scoates, J.R. The 1.76 Ga Horse Creek anorthosite complex, Wyoming: A massif anorthosite emplaced late in the Medicine Bow orogeny. Rocky Mt. Geol. 2000, 35, 71–90. [Google Scholar] [CrossRef]
- Corriveau, L.; Perreault, S.; Davidson, A. Prospective metallogenic settings of the Grenville Province. In Mineral Deposits of Canada: A Synthesis of Major Deposit-Types, District Metallogeny, The Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Mineral Deposits Division, Geological Survey of Canada: Ottawa, ON, Canada, 2007; pp. 819–847. [Google Scholar]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanova-de-Benavent, C.; Torró, L.; Castillo-Oliver, M.; Campeny, M.; Melgarejo, J.C.; Llovet, X.; Galí, S.; Gonçalves, A.O. Fe–Ti(–V) Oxide Deposits of the Kunene Anorthosite Complex (SW Angola): Mineralogy and Thermo-Oxybarometry. Minerals 2017, 7, 246. https://doi.org/10.3390/min7120246
Villanova-de-Benavent C, Torró L, Castillo-Oliver M, Campeny M, Melgarejo JC, Llovet X, Galí S, Gonçalves AO. Fe–Ti(–V) Oxide Deposits of the Kunene Anorthosite Complex (SW Angola): Mineralogy and Thermo-Oxybarometry. Minerals. 2017; 7(12):246. https://doi.org/10.3390/min7120246
Chicago/Turabian StyleVillanova-de-Benavent, Cristina, Lisard Torró, Montgarri Castillo-Oliver, Marc Campeny, Joan Carles Melgarejo, Xavier Llovet, Salvador Galí, and Antonio Olimpio Gonçalves. 2017. "Fe–Ti(–V) Oxide Deposits of the Kunene Anorthosite Complex (SW Angola): Mineralogy and Thermo-Oxybarometry" Minerals 7, no. 12: 246. https://doi.org/10.3390/min7120246




