Utilization of the MgO-Rich Residue Originated from Ludwigite Ore: Hydrothermal Synthesis of MHSH Whiskers
Abstract
:1. Introduction
2. Experimental
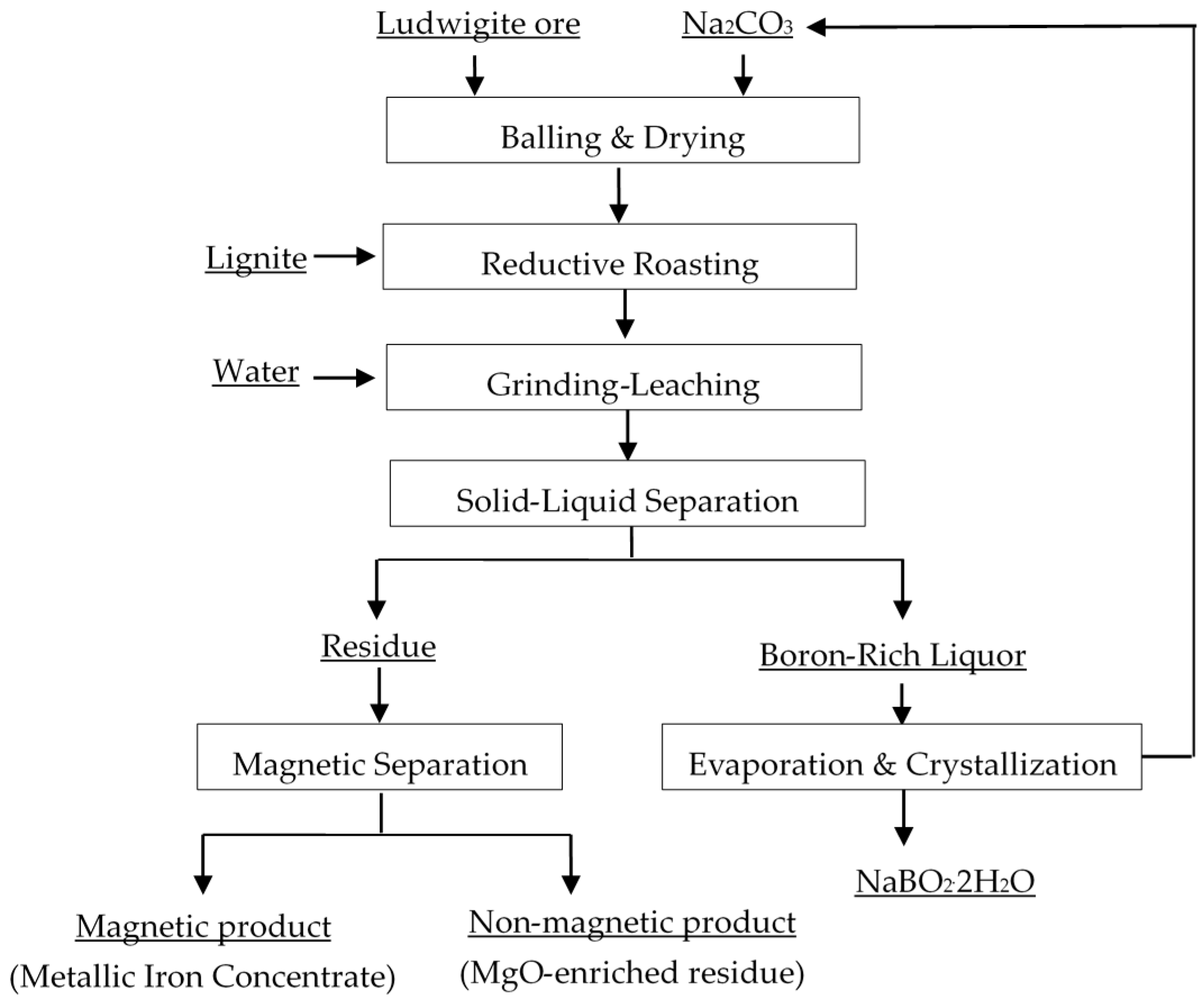
2.1. Materials
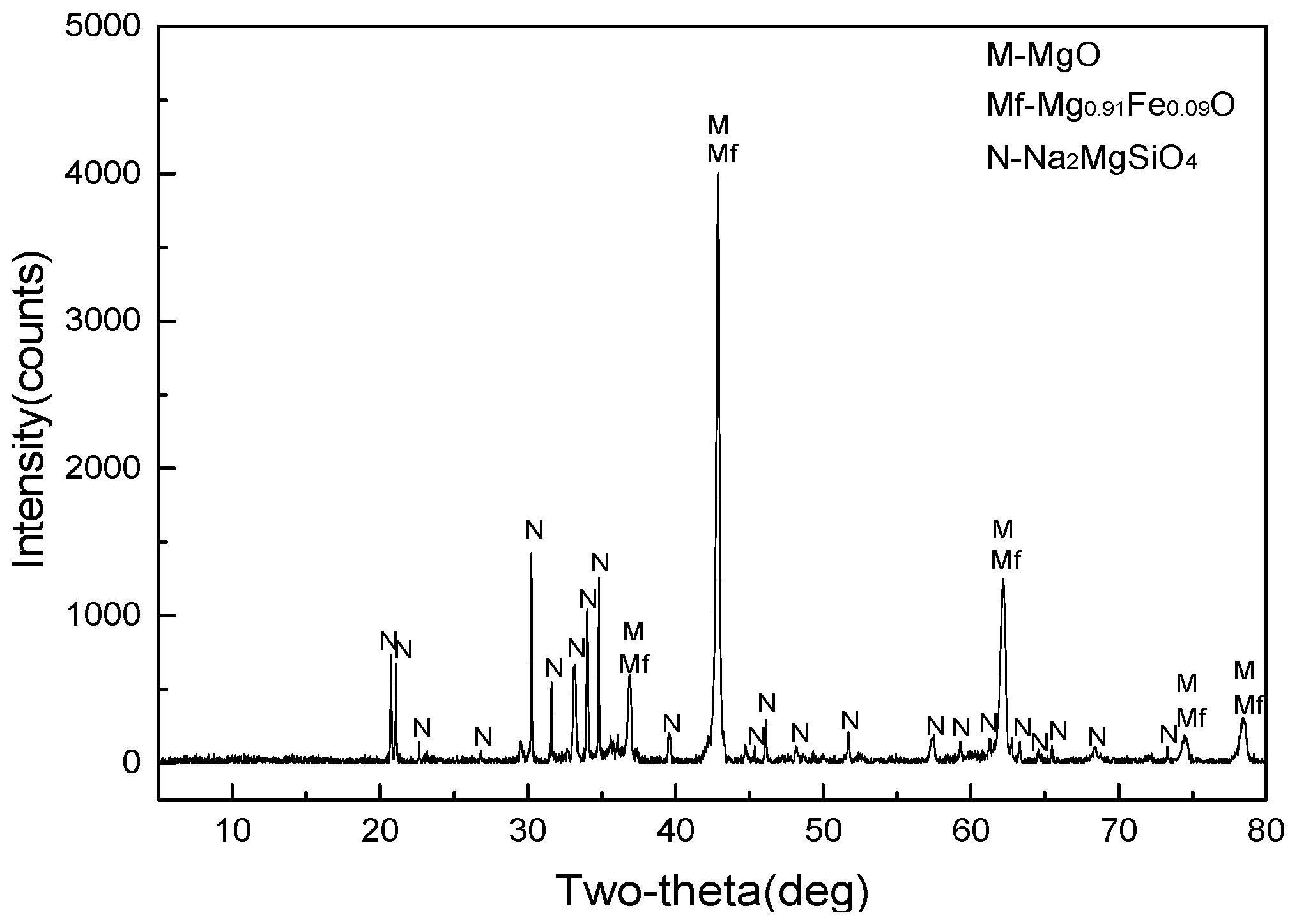
2.1.1. MgO-Enriched Residue
2.1.2. Chemical Regents
2.2. Methods
2.2.1. Experimental Procedure
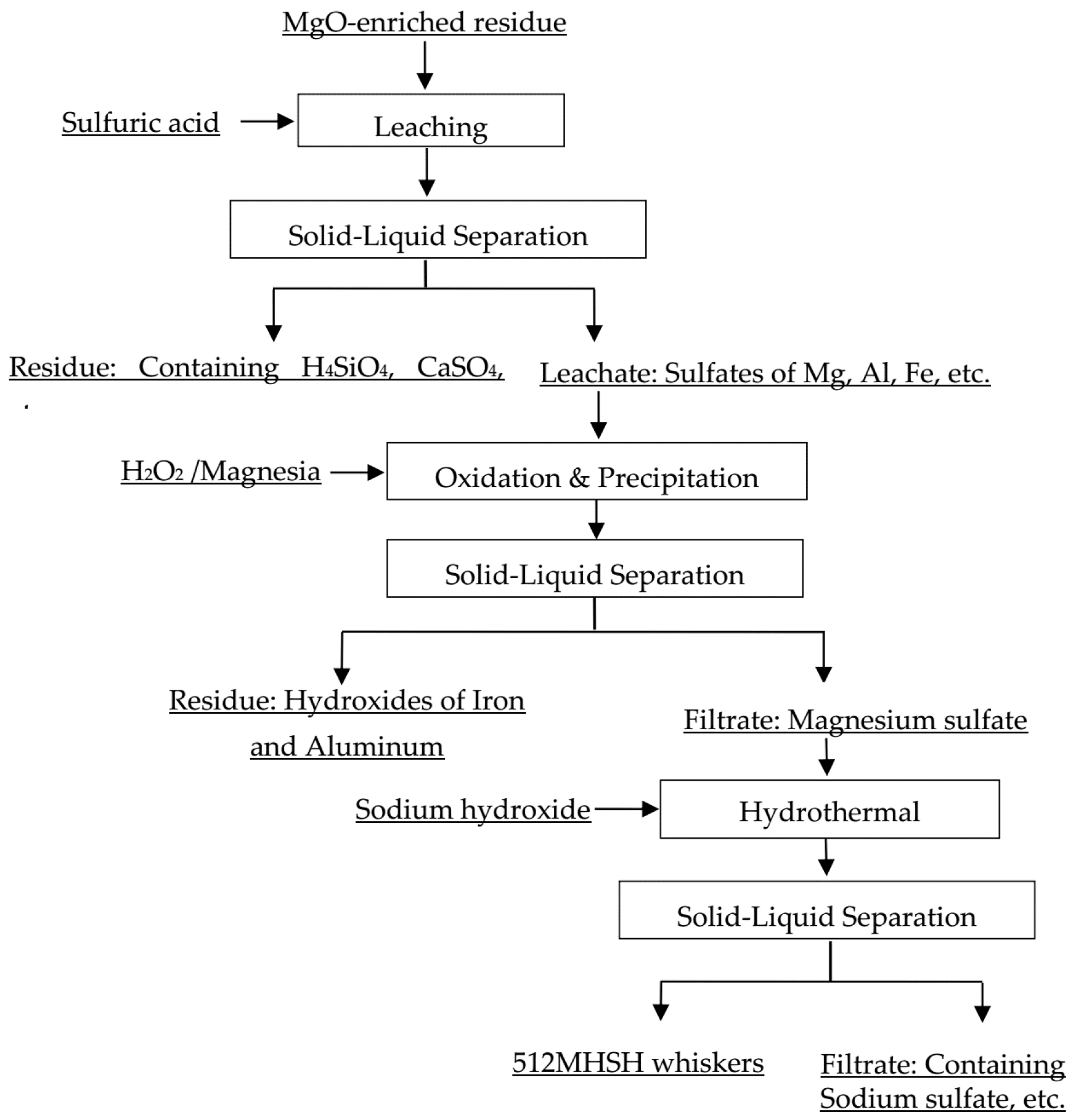
2.2.2. Sulfuric Acid Leaching of MgO-Enriched Residue
2.2.3. Purification of Acidic Leachate
2.2.4. Hydrothermal Synthesis
2.2.5. Instrumental Analyses
3. Results and Discussion
3.1. Separation and Purification of Magnesium Sulfate from MgO-Enriched Residue
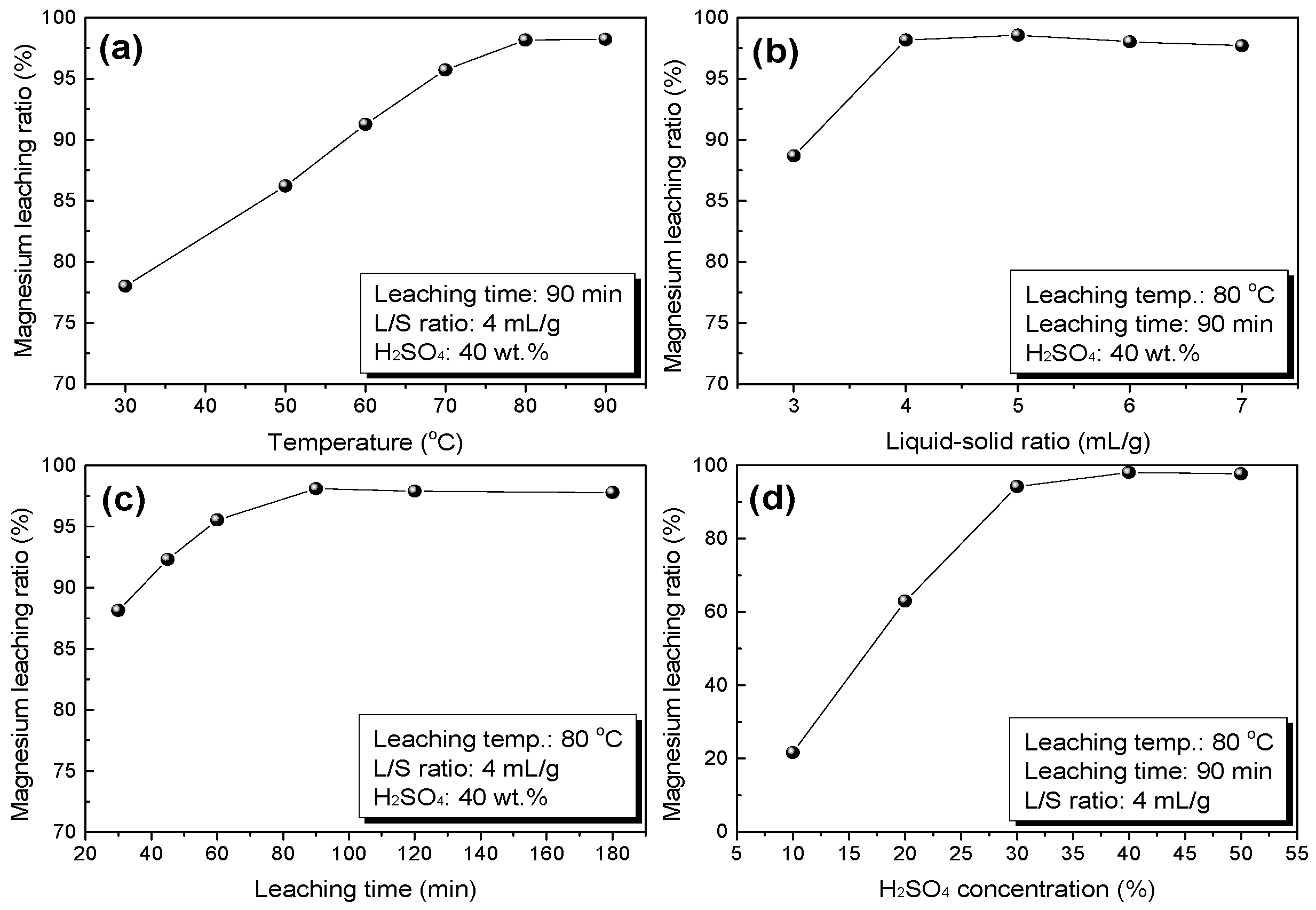
3.1.1. Sulfuric-Acid Leaching Behavior of Magnesium from MgO-Enriched Residue
3.1.2. Precipitating Impurities of Acidic Leachate
3.2. Preparing MHSH Whiskers from Acidic Leachate
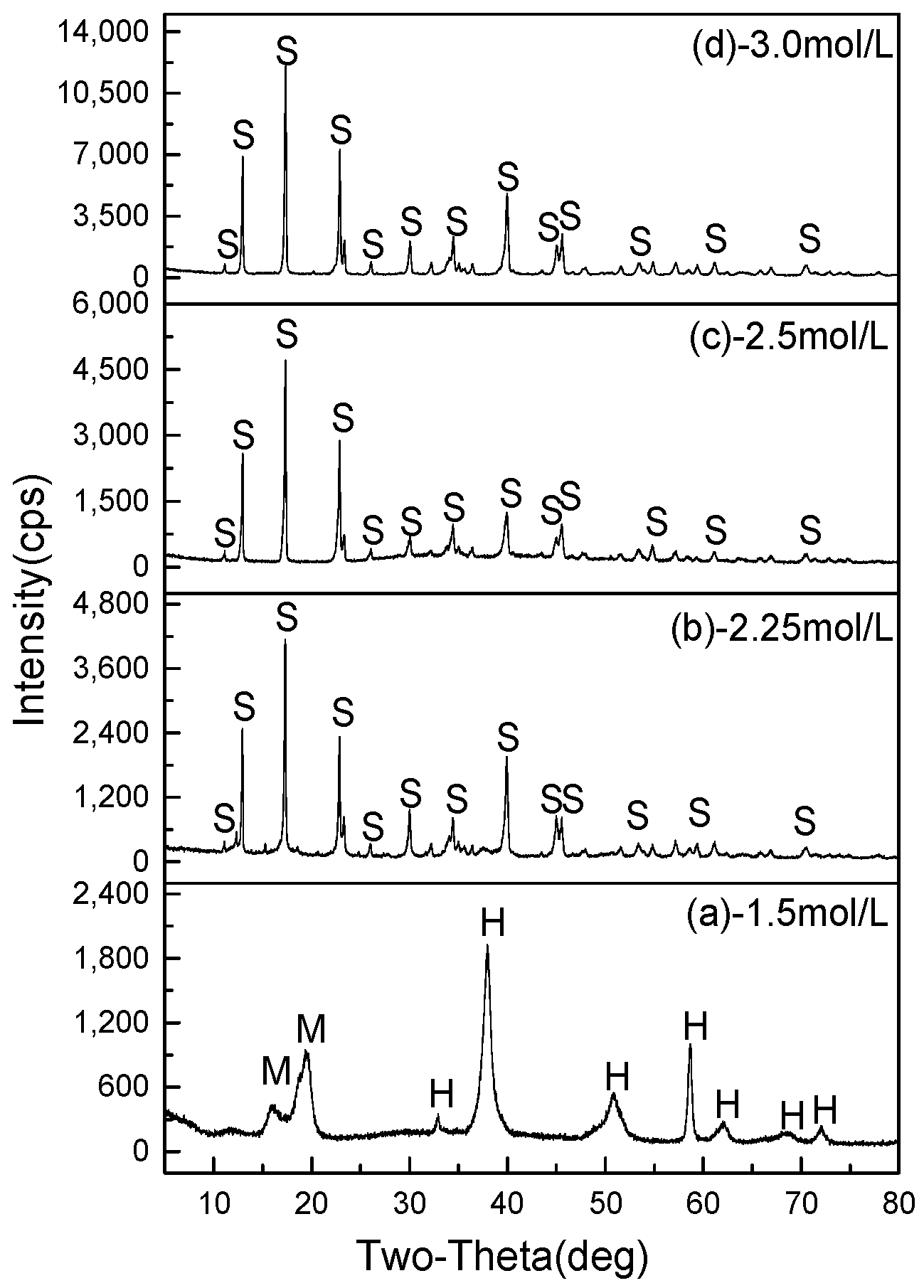
3.2.1. Effect of MgSO4 Concentration
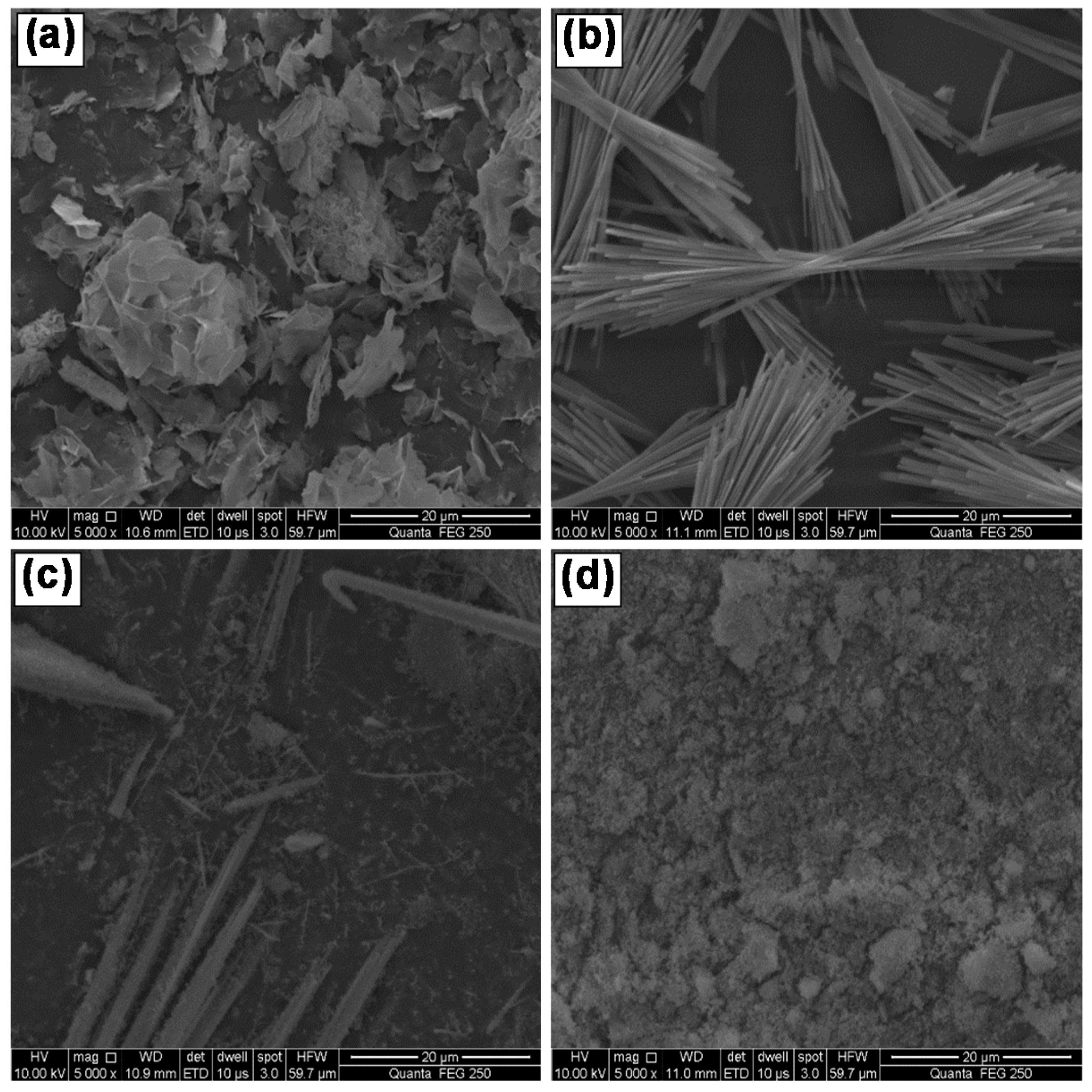
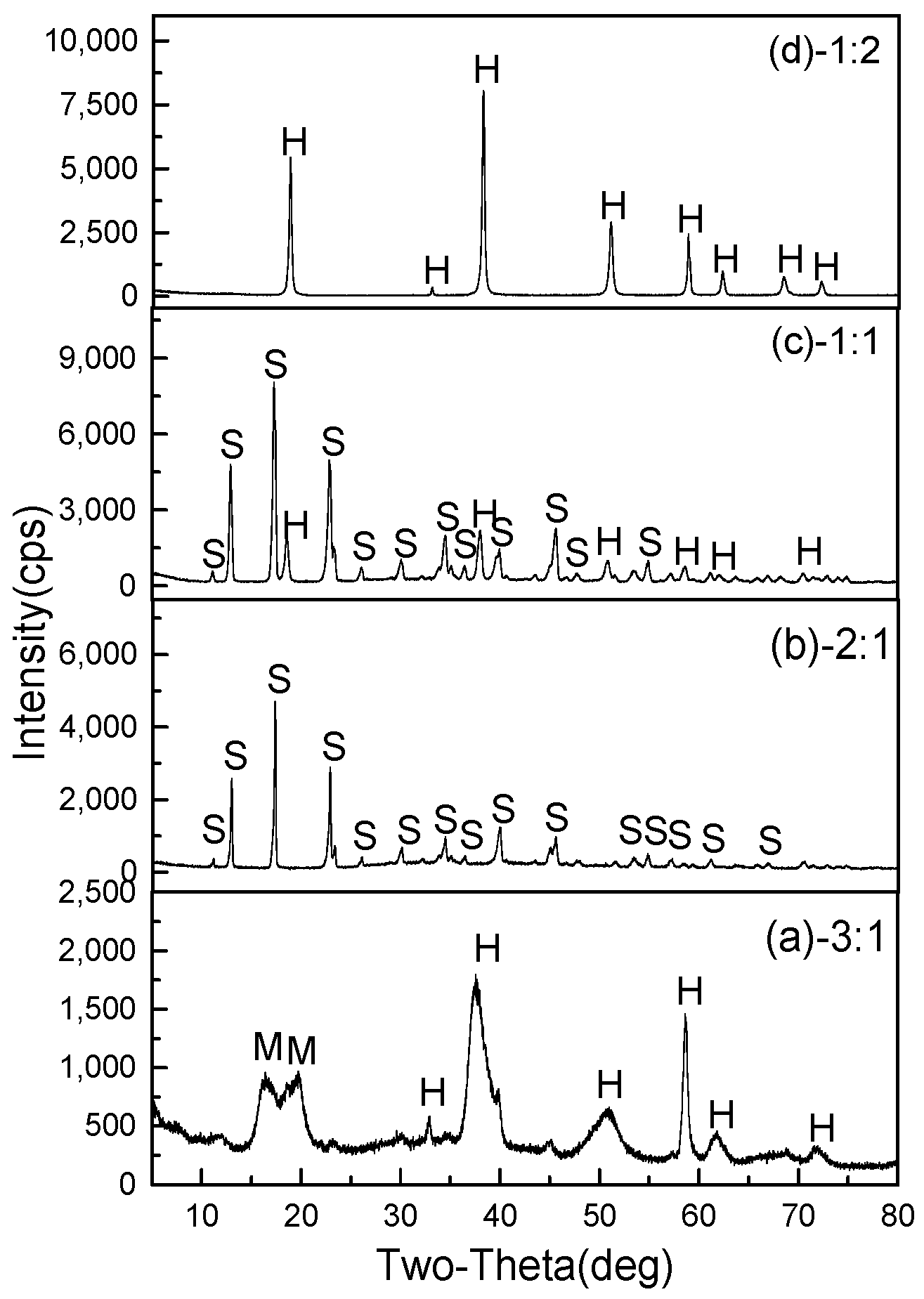
3.2.2. Effect of Mole Ratio of MgSO4/NaOH
3.2.3. Effect of Hydrothermal Temperature
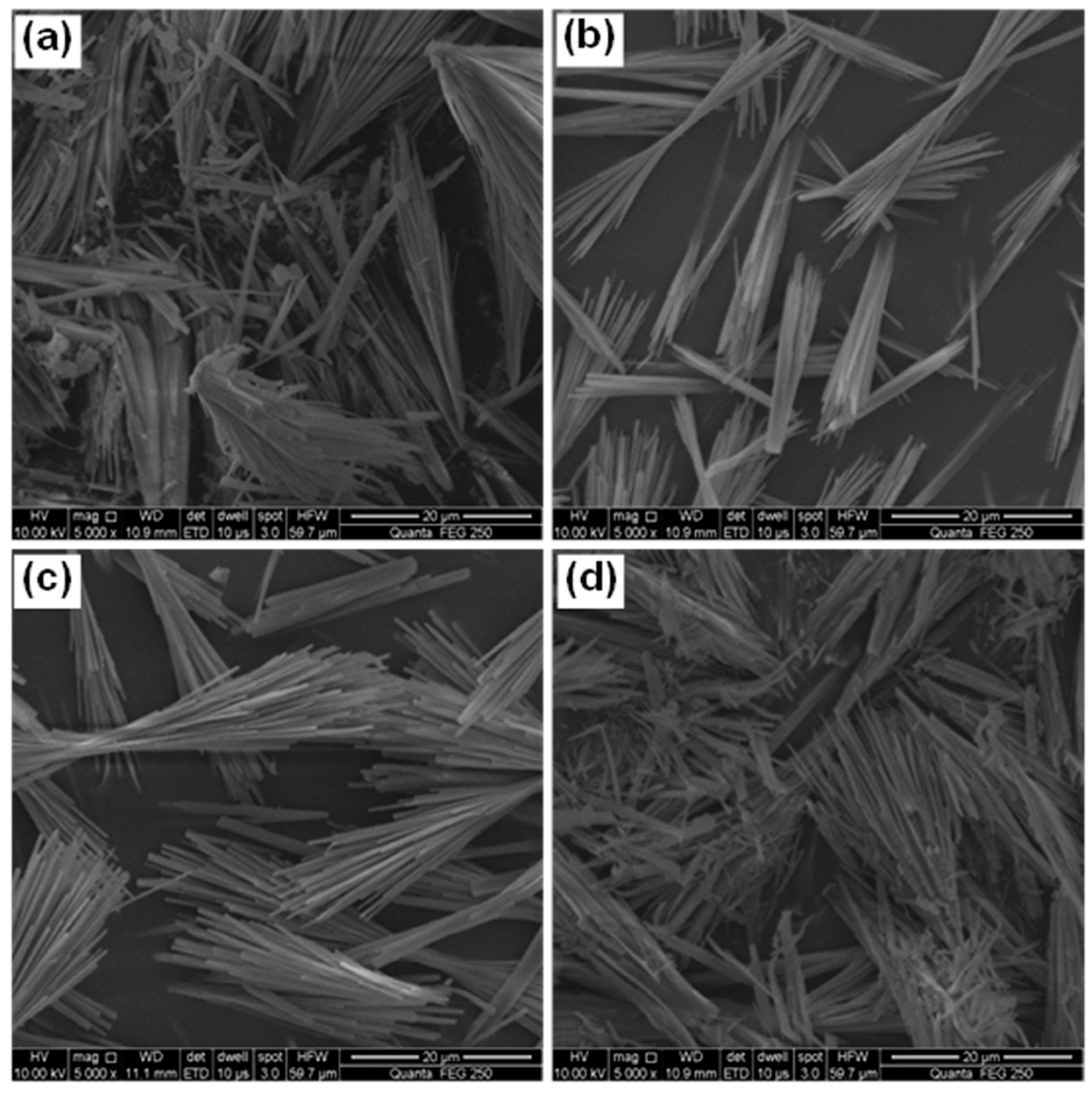
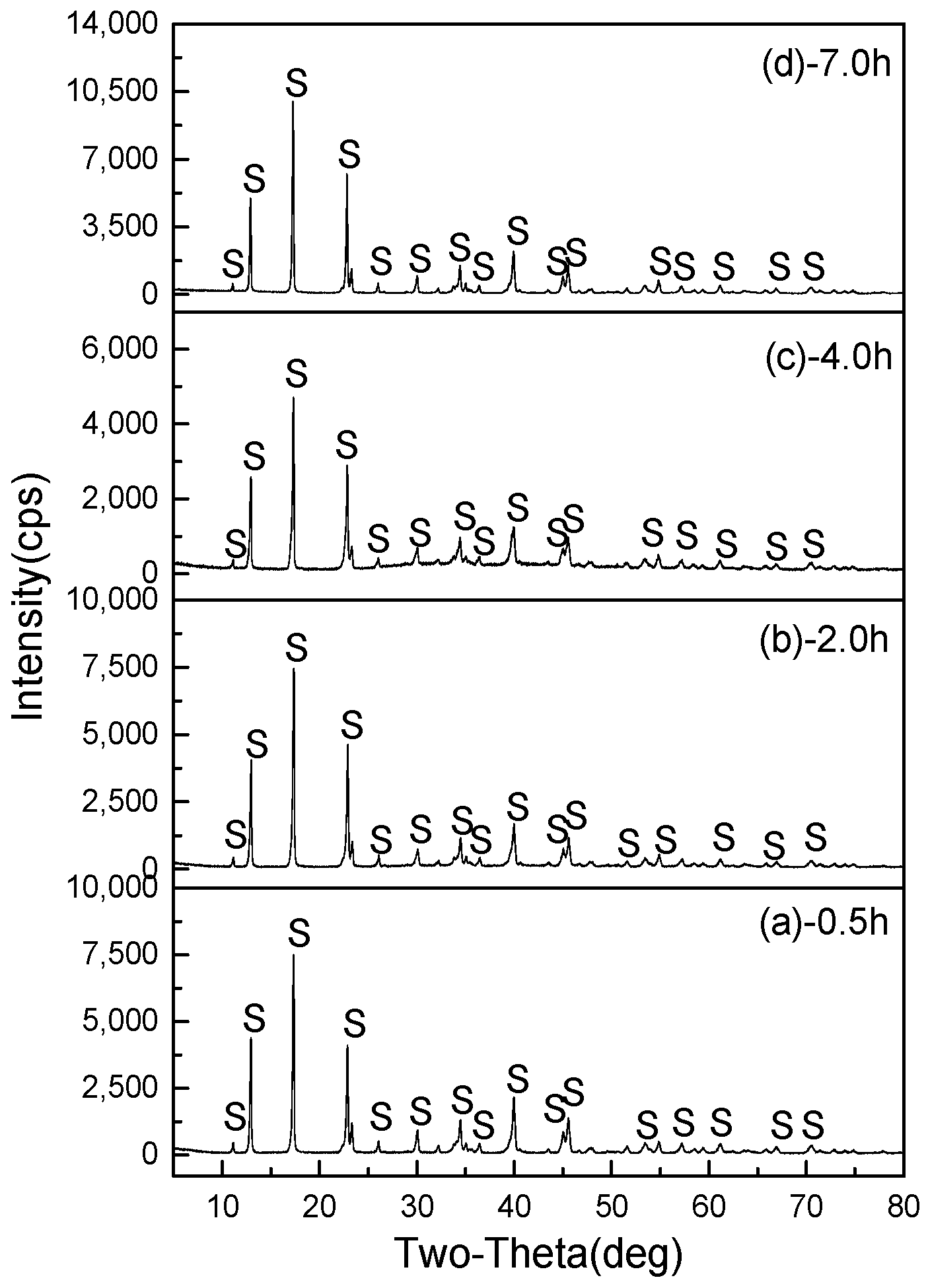
3.2.4. Effect of Hydrothermal Duration
3.3. Characterization of MHSH Whiskers
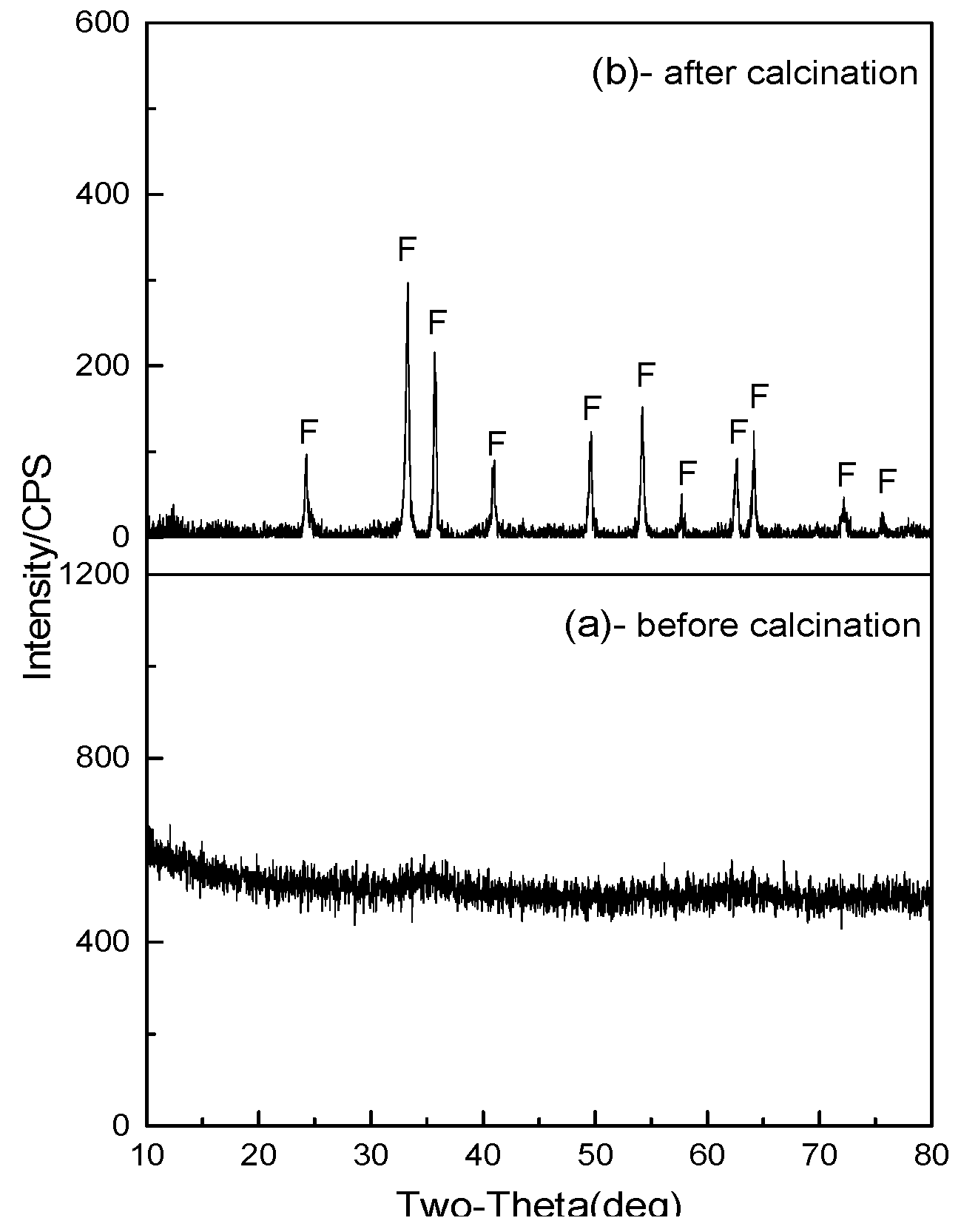
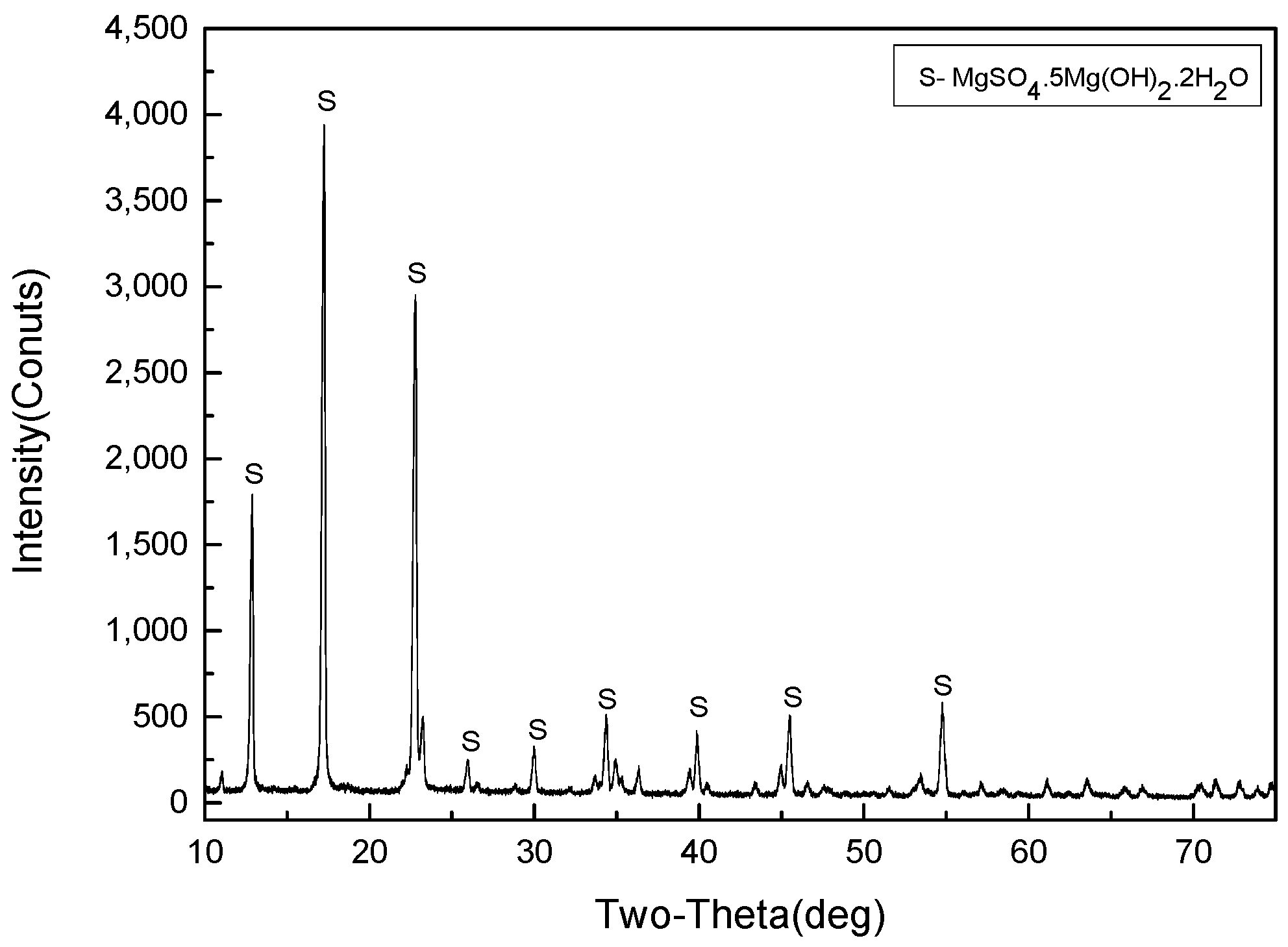
3.3.1. XRD
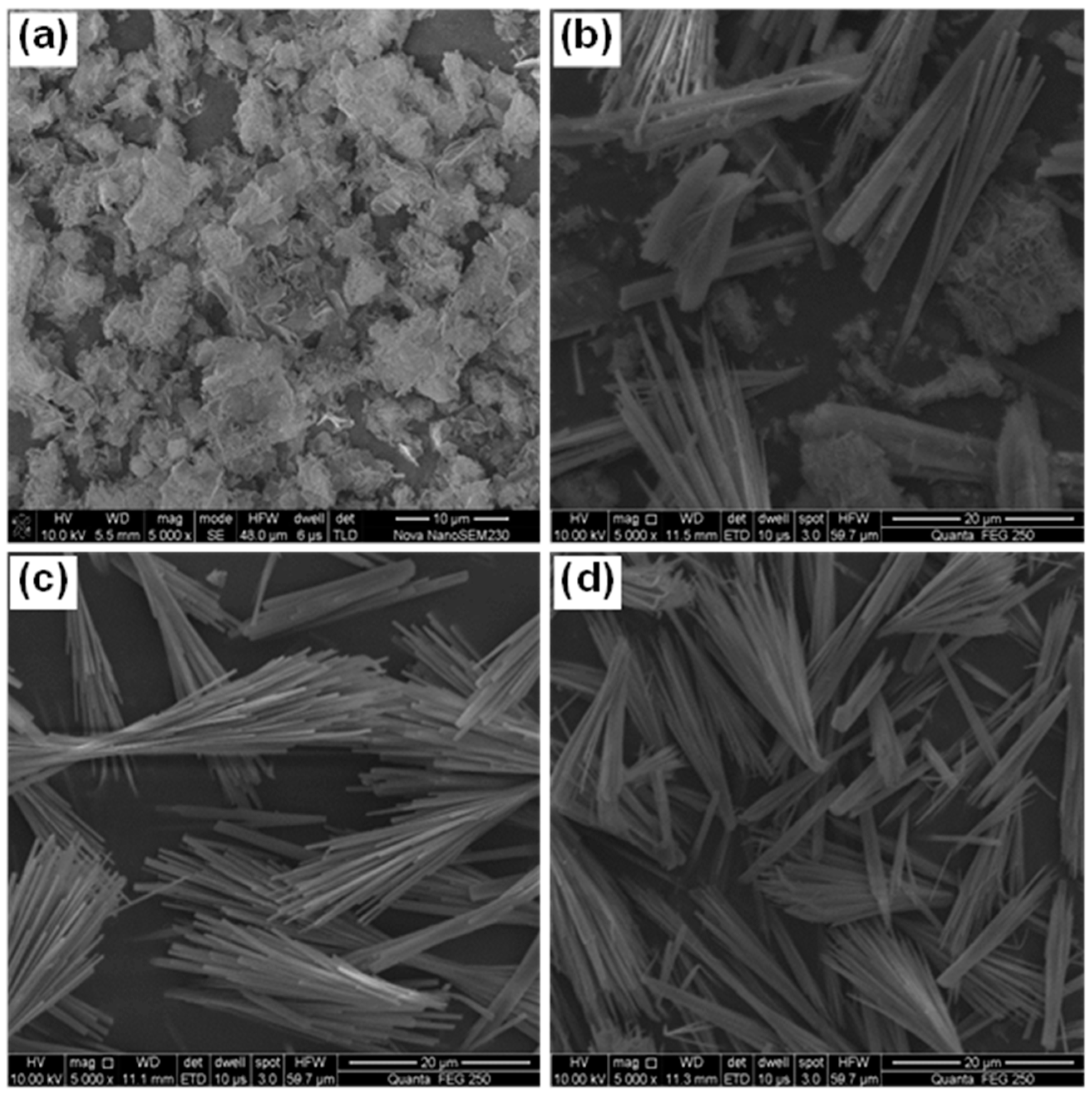
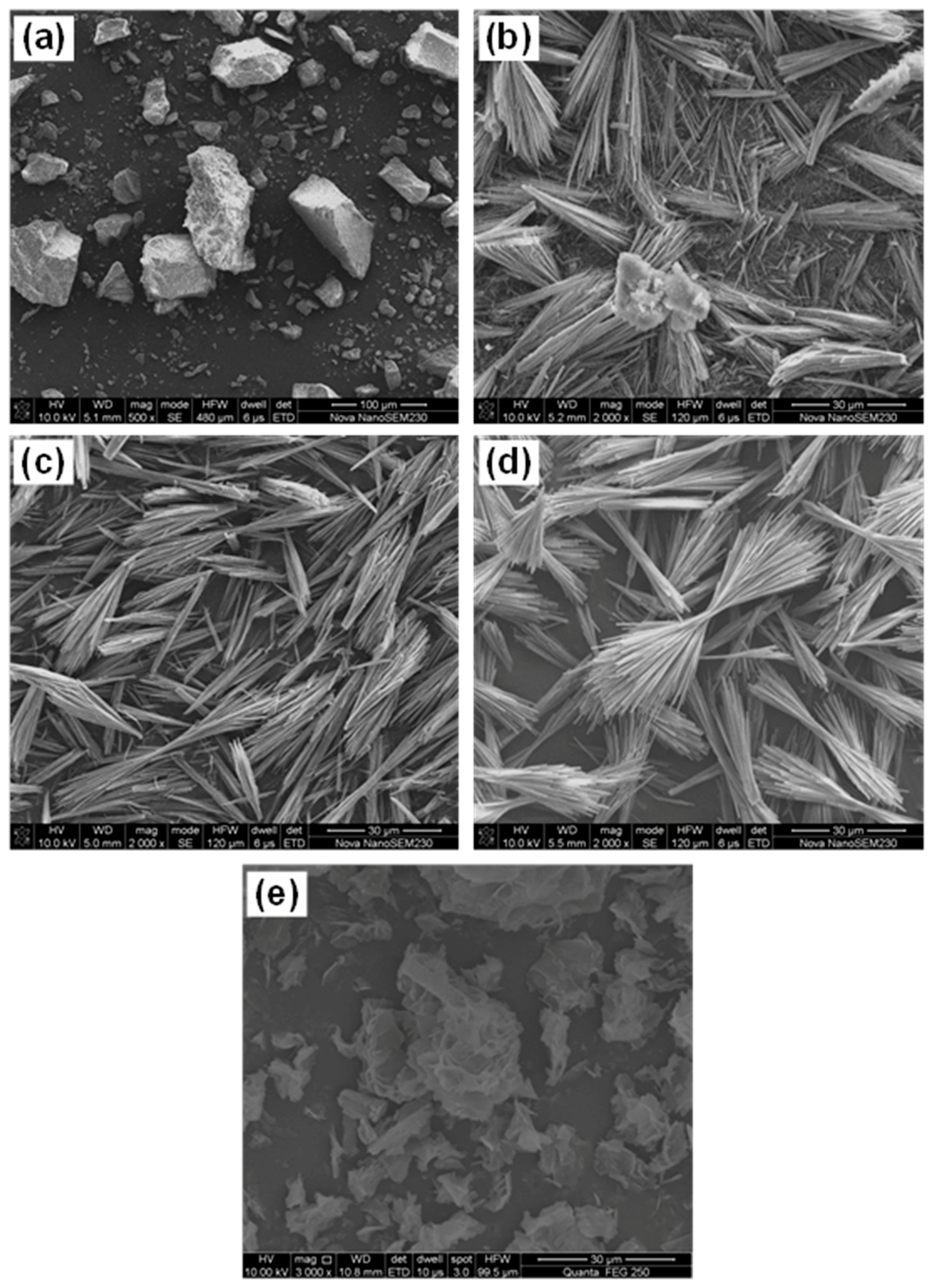
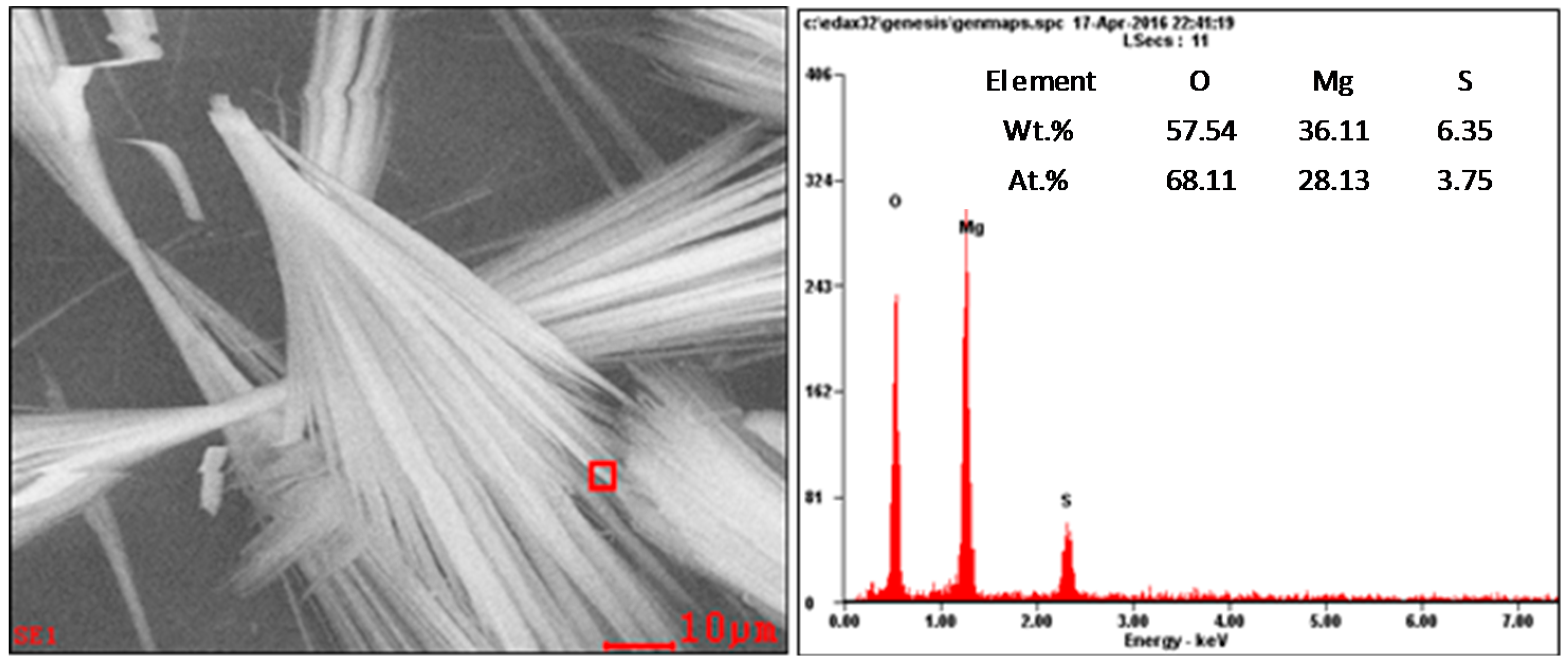
3.3.2. SEM-EDS
3.3.3. FT-IR
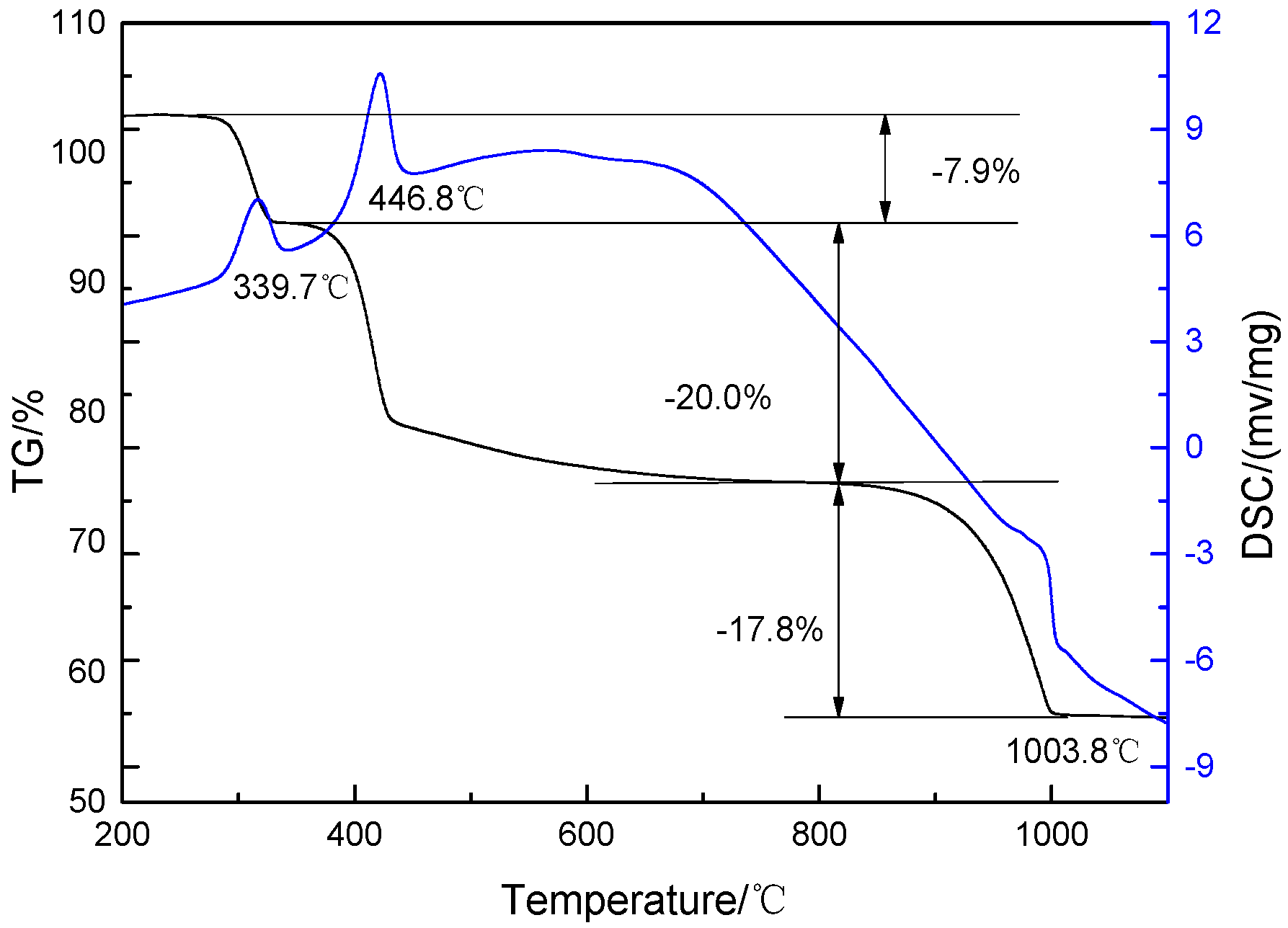
3.3.4. TG-DSC
4. Conclusions
- During the sulfuric acid leaching of MgO-enriched residue, silica could be effectively separated from magnesium by leaching with 40 wt % sulfuric acid at 80 °C for 90 min with a liquid-solid ratio of 4 mL/g. The leaching ratio of magnesium reached about 98.2% while 99.6% of silica was removed and enriched in the leaching residue. Furthermore, value-added products of silica gel with 67.7% SiO2 was prepared from the acid leaching residue.
- The impurities in acidic leachate could be well removed under the conditions of pH of 5.7, reaction time of 30 min and reaction temperature of 60 °C. The magnesium retention ratio was 98.1%, and the iron removal ratio was 99.6 %. Iron oxide red could be obtained as a byproduct from the precipitate.
- 512MHSH whiskers with a length of 30–100 μm, an average diameter of 0.5–1.0 μm and an aspect ratio of 100 were obtained by hydrothermal synthesis at 160–180 °C for 2.0–4.0 h using the mixed aqueous solution of 2.5–3.0 mol/L MgSO4 and 1.0 mol/L NaOH solution with a fixed MgSO4/NaOH mole ratio of 2. The whiskers were featured by smooth surface and small diameter.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kang, K.H.; Lee, D.K. Synthesis of magnesium oxysulfate whiskers using triethanolamine as a morphology control agent. J. Ind. Eng. Chem. 2014, 20, 2580–2583. [Google Scholar] [CrossRef]
- Yan, X.X.; Xu, D.L.; Xue, D.F. SO42− ions direct the one-dimensional growth of 5Mg(OH)2·MgSO4·2H2O. Acta Mater. 2007, 55, 5747–5757. [Google Scholar] [CrossRef]
- Dang, L.; Nai, X.Y.; Zhu, D.H.; Jing, Y.W.; Liu, X.; Dong, Y.P.; Li, W. Study on the mechanism of surface modification of magnesium oxysulfate whisker. Appl. Surf. Sci. 2014, 317, 325–331. [Google Scholar] [CrossRef]
- Zhu, D.H.; Nai, X.; Lan, S.; Bian, S.; Liu, X.; Li, W. Surface modification of magnesium hydroxide sulfate hydrate whiskers using a silane coupling agent by dry process. Appl. Surf. Sci. 2016, 390, 25–30. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, G.; Zhang, S.; Huang, X.; Yu, W.; Qian, Y. Preparation and characterization of magnesium hydroxide sulfate hydrate whiskers. Chem. Mater. 2000, 12, 2845–2852. [Google Scholar] [CrossRef]
- Ma, X.L.; Ning, G.Q.; Qi, C.L.; Gao, J.S. One-step synthesis of basic magnesium sulfate whiskers by atmospheric pressure reflux. Particuology 2016, 24, 191–196. [Google Scholar] [CrossRef]
- Sun, X.T.; Xiang, L. Hydrothermal conversion of magnesium oxysulfate whiskers to magnesium hydroxide nanobelts. Mater. Chem. Phys. 2008, 109, 381–385. [Google Scholar]
- Wang, X.L.; Xue, D.F. Direct observation of the shape evolution of MgO whiskers in a solution system. Mater. Lett. 2006, 60, 3160–3164. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Qi, H.; Ma, P.H.; Bao, J.Q. A new route to prepare magnesium oxide whisker. Inorg. Chem. Commun. 2002, 5, 147–149. [Google Scholar] [CrossRef]
- Gao, C.H.; Li, X.G.; Feng, L.J.; Lu, S.Y.; Liu, J.Y. Surface modification and characterization of magnesium hydroxide sulfate hydrate nanowhiskers. Appl. Surf. Sci. 2010, 256, 3234–3239. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, F.; Li, J.; Jin, Y. Hydrothermal formation and characterization of magnesium oxysulfate whiskers. Mater. Chem. Phys. 2004, 87, 424–429. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Yang, J.J.; Jia, J.P.; You, C.; Chen, M.F. Effects of modifying agents on surface modifications of magnesium oxide whiskers. Appl. Surf. Sci. 2016, 388, 370–375. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; You, C.; Chen, M.F. Effects of MgO whiskers on mechanical properties and crystallization behavior of PLLA/MgO composites. Mater. Des. 2016, 89, 573–581. [Google Scholar] [CrossRef]
- Liu, C.J.; Zhao, Q.; Wang, Y.G.; Shi, P.Y.; Jiang, M.F. Hydrothermal synthesis of calcium sulfate whisker from flue gas desulfurization gypsum. Chin. J. Chem. Eng. 2016, 24, 1552–1560. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Deng, Y.L. Solution synthesis of magnesium hydroxide sulfate hydrate nanobelts using sparingly soluble carbonate salts as supersaturation control agents. J. Colloid Interface Sci. 2007, 316, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhao, H.; Sun, Y.; Zhang, G.; Wu, H.; Qian, Y. Superstructured magnesium hydroxide sulfate hydrate fibres—Photoluminescence study. Int. J. Inorg. Mater. 2001, 3, 151–156. [Google Scholar] [CrossRef]
- Gao, C.H.; Li, X.G.; Feng, L.J.; Xiang, Z.C.; Zhang, D.H. Preparation and thermal decomposition of 5Mg(OH)2·MgSO4·2H2O nanowhiskers. J. Chem. Eng. 2009, 150, 551–554. [Google Scholar] [CrossRef]
- Su, X.L.; Sun, C.M.; Chen, Y.X.; Zeng, T. Preparation of basic magnesium sulfate whisker by serpentine mine tailings solid wastes. Bull. Chin. Ceram. Soc. 2014, 33, 92–96. (In Chinese) [Google Scholar]
- An, J.; Xue, X.X. Life cycle environmental impact assessment of borax and boric acid production in China. J. Clean. Prod. 2014, 66, 121–127. [Google Scholar] [CrossRef]
- Ning, Z.Q.; Zhai, Y.C.; Song, Q.S. Extracting B2O3 from calcined boron mud using molten sodium hydroxide. Rare Met. 2015, 34, 744–751. [Google Scholar] [CrossRef]
- Wang, G.; Xue, Q.G.; Wang, J.S. Effect of Na2CO3 on reduction and melting separation of ludwigite/coal composite pellet and property of boron-rich slag. Trans. Nonferrous Met. Soc. China 2016, 26, 282–293. [Google Scholar] [CrossRef]
- Fu, X.J.; Zhao, J.Q.; Chen, S.Y.; Liu, Z.G.; Guo, T.L.; Chu, M.S. Comprehensive Utilization of Ludwigite Ore Based on Metallizing Reduction and Magnetic Separation. J. Iron Steel Res. Int. 2015, 22, 672–680. [Google Scholar] [CrossRef]
- Li, Y.L.; Qu, J.K.; Wei, G.Y.; Qi, T. Influence of Na2CO3 as Additive on Direct Reduction of Boron-bearing Magnetite Concentrate. J. Iron Steel Res. Int. 2016, 23, 103–108. [Google Scholar] [CrossRef]
- Ren, W.; Ye, J.; Wang, X.; Gong, W.; Lin, Y.; Ning, G. Study on preparation of basic magnesium sulfate whiskers from boron slurry. Inorg. Chem. Ind. 2010, 42, 56–59. (In Chinese) [Google Scholar]
- Jie, L.I.; Fan, Z.G.; Liu, Y.L.; Liu, S.L.; Tao, J.; Xi, Z.P. Preparation of boric acid from low-grade ascharite and recovery of magnesium sulfate. Trans. Nonferrous Met. Soc. China 2010, 20, 1161–1165. [Google Scholar]
- Liang, B.; Li, G.; Rao, M.; Peng, Z.; Zhang, Y.; Jiang, T. Water leaching of boron from soda-ash-activated ludwigite ore. Hydrometallurgy 2017, 167, 101–106. [Google Scholar] [CrossRef]
- Li, G.; Liang, B.; Rao, M.; Zhang, Y.; Jiang, T. An innovative process for extracting boron and simultaneous recovering metallic iron from ludwigite ore. Miner. Eng. 2014, 56, 57–60. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, J.; Ma, H.W.; Liu, M.T.; Ma, X. Recovery of magnesium and potassium from biotite by sulfuric acid leaching and alkali precipitation with ammonia. Hydrometallurgy 2015, 157, 188–193. [Google Scholar] [CrossRef]



| MgO | SiO2 | Na2O | Fe | B2O3 | Al2O3 | CaO | 1 LOI |
|---|---|---|---|---|---|---|---|
| 43.54 | 16.07 | 12.03 | 5.91 | 1.58 | 0.98 | 2.52 | 15.18 |
| Compositions | SiO2 | MgO | Fe2O3 | Al2O3 | CaSO4 | Na2O | B2O3 | LOI |
|---|---|---|---|---|---|---|---|---|
| content/% | 67.76 | 1.63 | 2.54 | 0.28 | 13.98 | 0.42 | 0.07 | 12.21 |
| Composition | Si | Mg | Fe | Al | Ca | Na | B |
|---|---|---|---|---|---|---|---|
| Acid leaching solution (g/L) | 0.054 | 51.29 | 11.29 | 0.96 | 1.65 | 17.59 | 0.92 |
| Leaching ratio (%) | 0.4 | 98.2 | 95.5 | 93.2 | 45.8 | 98.5 | 98.9 |
| Ions | Fe3+ | Fe2+ | Al3+ | Mg2+ |
|---|---|---|---|---|
| Starting precipitation pH | 1.6 | 6.5 | 3.3 | 9.4 |
| Complete precipitation pH | 3.2 | 9.7 | 5.2 | 12.4 |
| Ksp | 4.0 × 10−38 | 8.0 × 10−16 | 1.9 × 10−36 | 9.6 × 10−3 |
| Mg | Na | Fe | Al | Si | B |
|---|---|---|---|---|---|
| 64.40 | 7.27 | 2.75 × 10−3 | 0.05 × 10−3 | 0.17 × 10−3 | 0.686 |
| Al2O3 | SiO2 | Fe2O3 | CaO | MgO | Na2O | S |
|---|---|---|---|---|---|---|
| 4.30 | 0.7 | 60.63 | 0.13 | 1.27 | 0 | 2.02 |
| Step | Decomposition Temperature/°C | Theoretical Weight Loss/% | Hydrothermal Product Weight Loss/wt % |
|---|---|---|---|
| 1 | 306–377 | 8.04 | 7.9 |
| 2 | 386–641 | 20.10 | 20.0 |
| 3 | 897–1029 | 17.89 | 17.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Fang, L.; Zhang, X.; Liang, B.; Rao, M.; Peng, Z.; Jiang, T. Utilization of the MgO-Rich Residue Originated from Ludwigite Ore: Hydrothermal Synthesis of MHSH Whiskers. Minerals 2017, 7, 138. https://doi.org/10.3390/min7080138
Li G, Fang L, Zhang X, Liang B, Rao M, Peng Z, Jiang T. Utilization of the MgO-Rich Residue Originated from Ludwigite Ore: Hydrothermal Synthesis of MHSH Whiskers. Minerals. 2017; 7(8):138. https://doi.org/10.3390/min7080138
Chicago/Turabian StyleLi, Guanghui, Li Fang, Xin Zhang, Binjun Liang, Mingjun Rao, Zhiwei Peng, and Tao Jiang. 2017. "Utilization of the MgO-Rich Residue Originated from Ludwigite Ore: Hydrothermal Synthesis of MHSH Whiskers" Minerals 7, no. 8: 138. https://doi.org/10.3390/min7080138





