Separation of Molybdenite from Chalcopyrite in the Presence of Novel Depressant 4-Amino-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. FT-IR Spectra Measurement
2.3. Zeta Potential Measurement
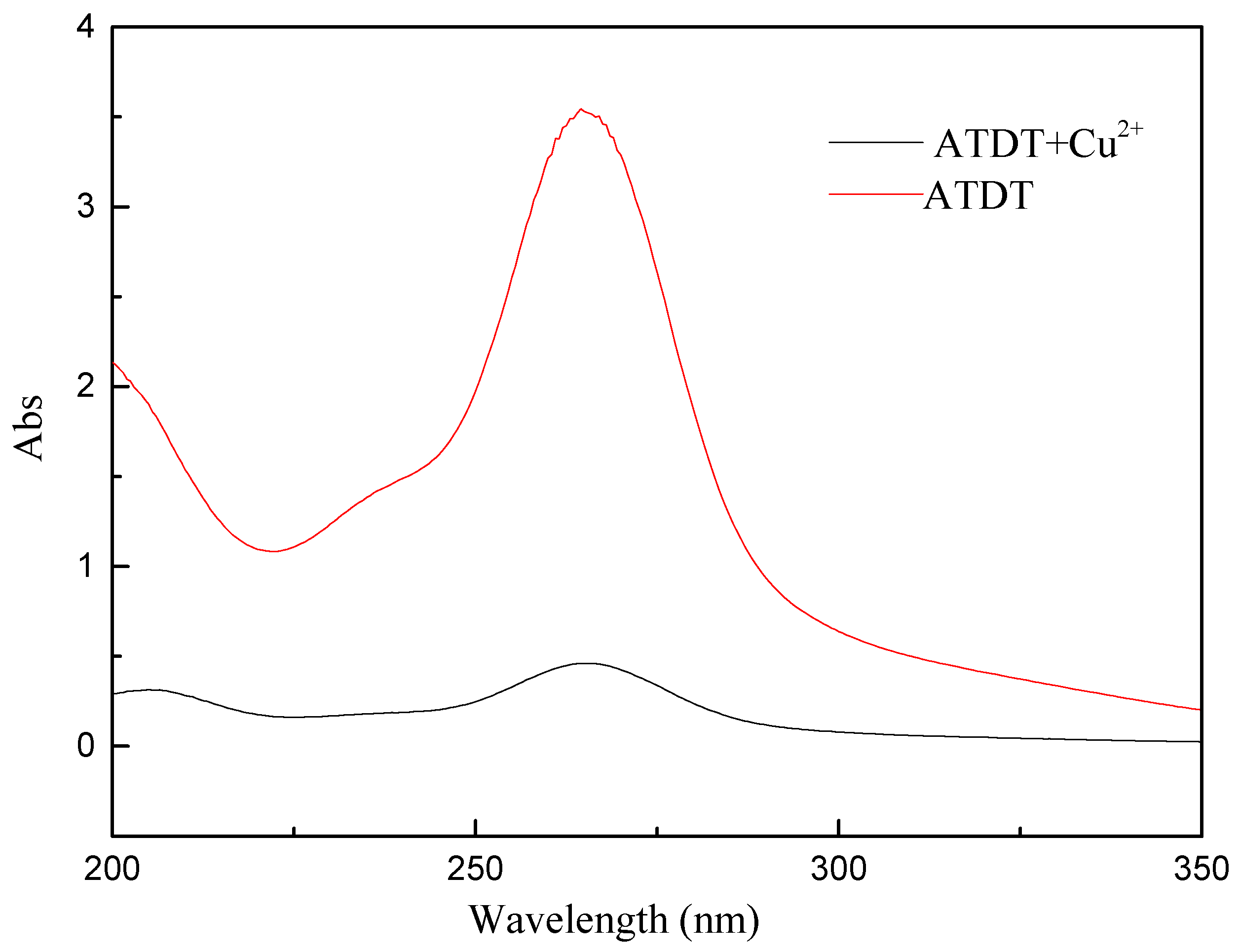
2.4. Evaluation of Reaction between ATDT and Cu2+ Ions
2.5. X-ray Photoelectron Spectroscopy Measurements
2.6. Flotation Tests
2.6.1. Single Mineral Flotation
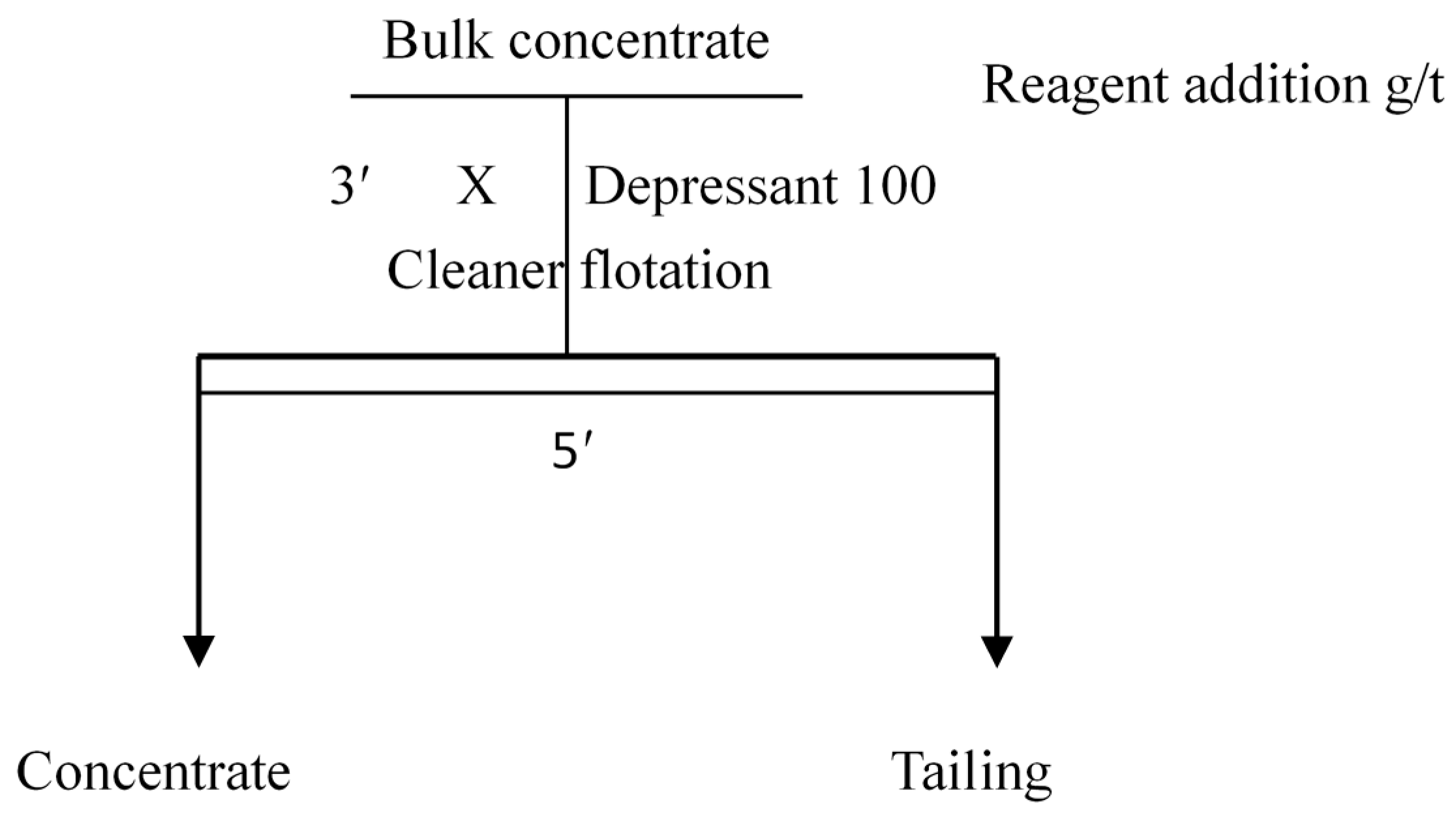
2.6.2. Bench-Scale Flotation Tests
3. Results and Discussion
3.1. Synthesis and Characterization of ATDT
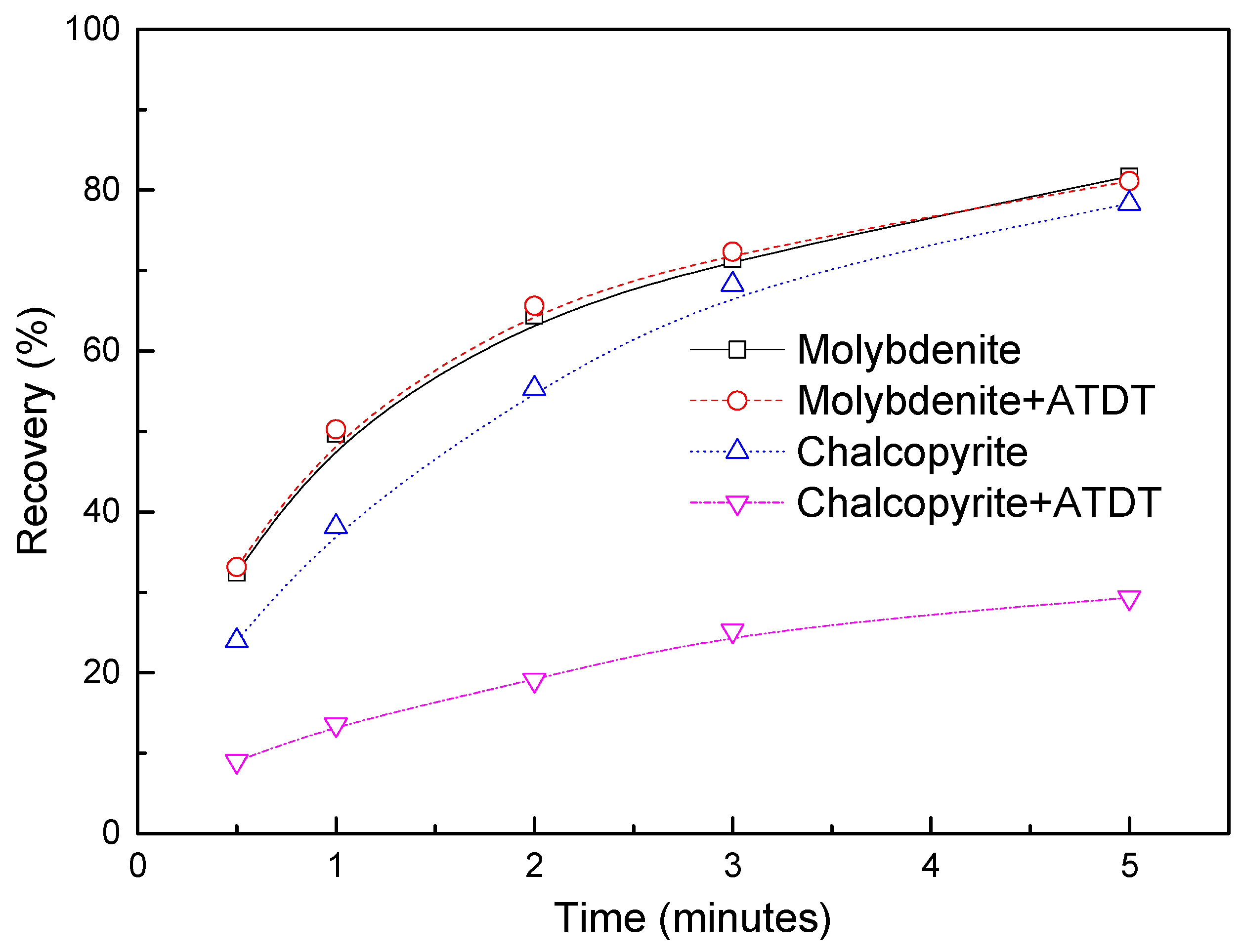
3.2. Flotation Tests
3.2.1. Effect of pH Value
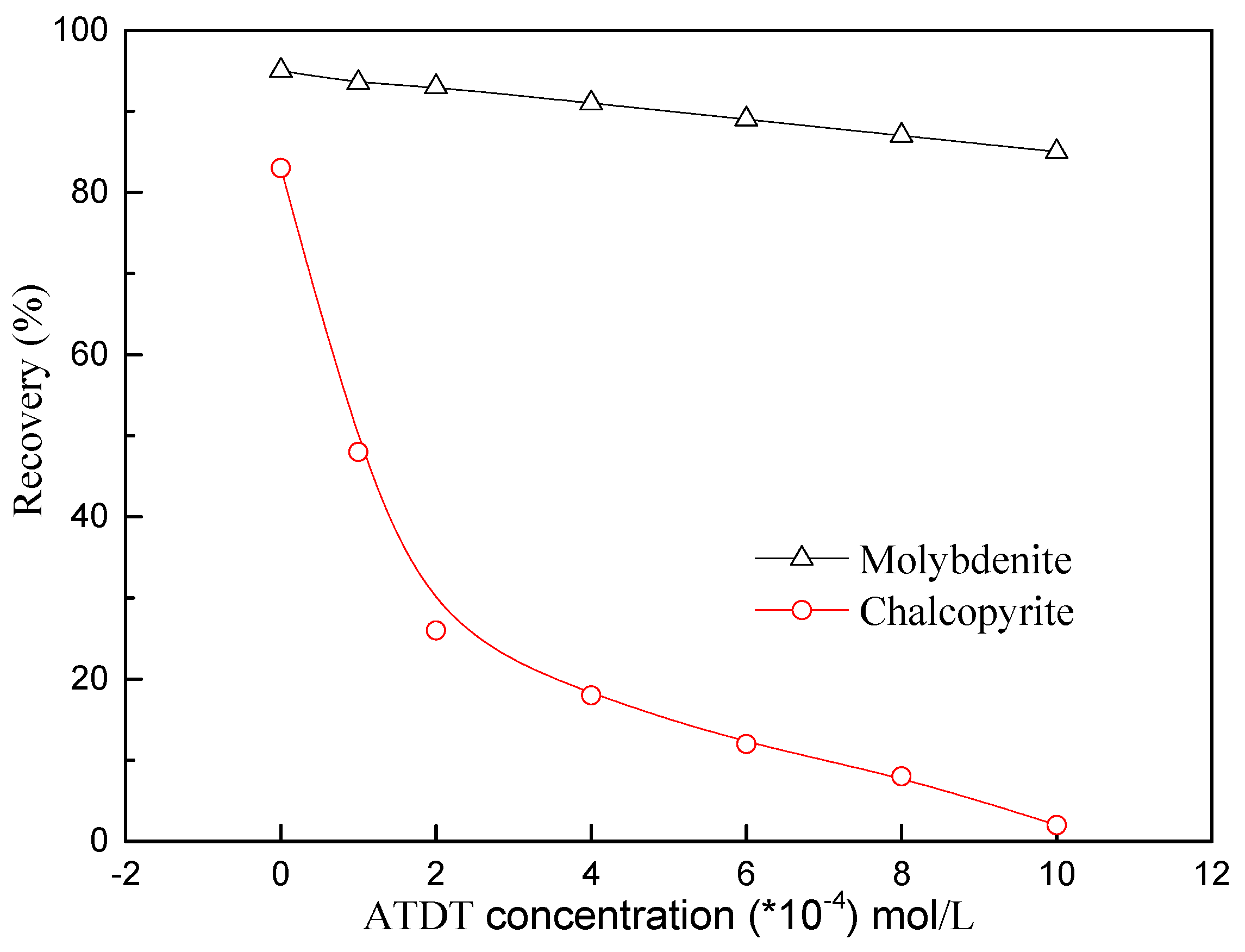
3.2.2. Effect of Depressant Concentration
3.2.3. Bench-Scale Flotation Tests
3.3. UV Spectroscopic Analysis
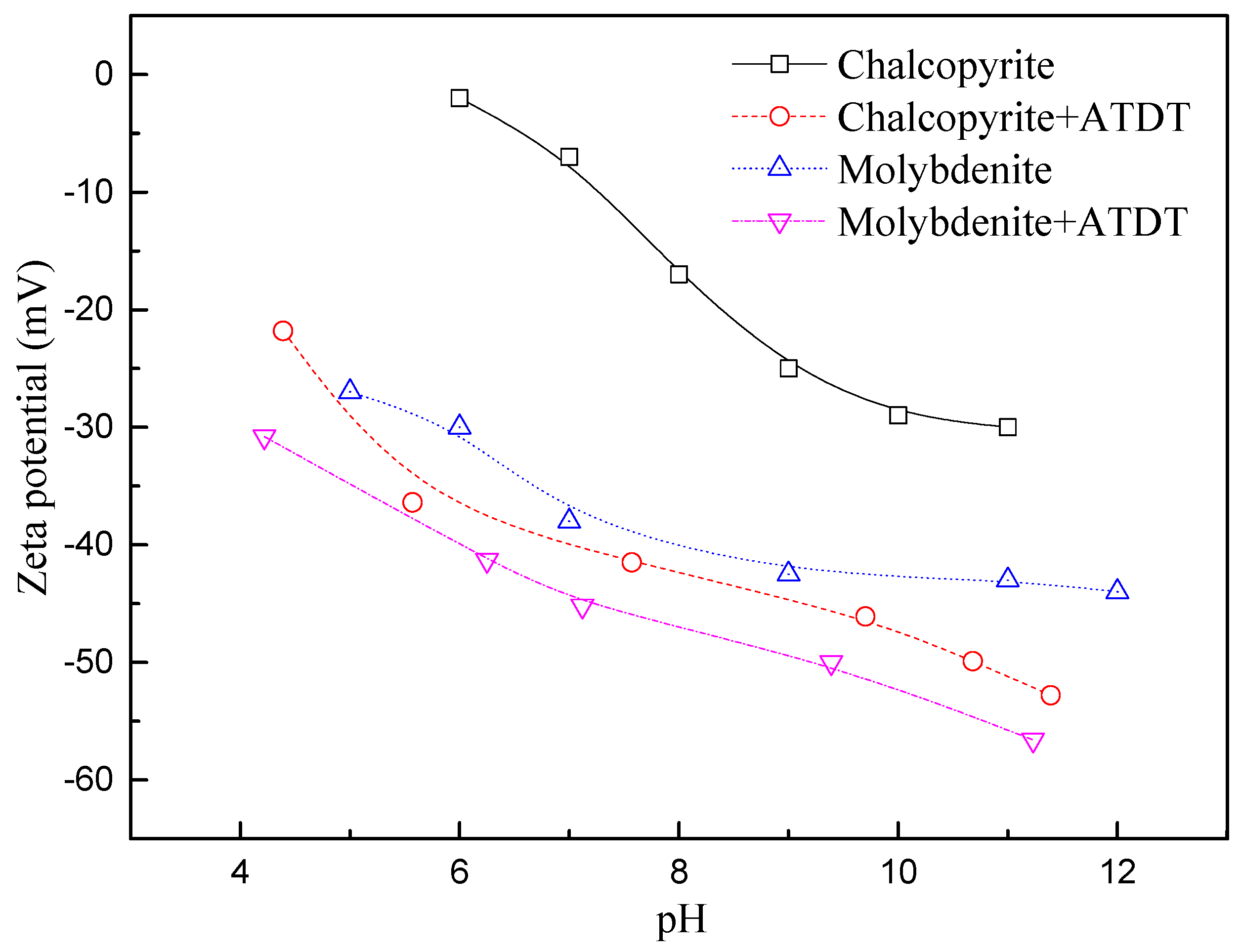
3.4. Zeta Potential Measurement
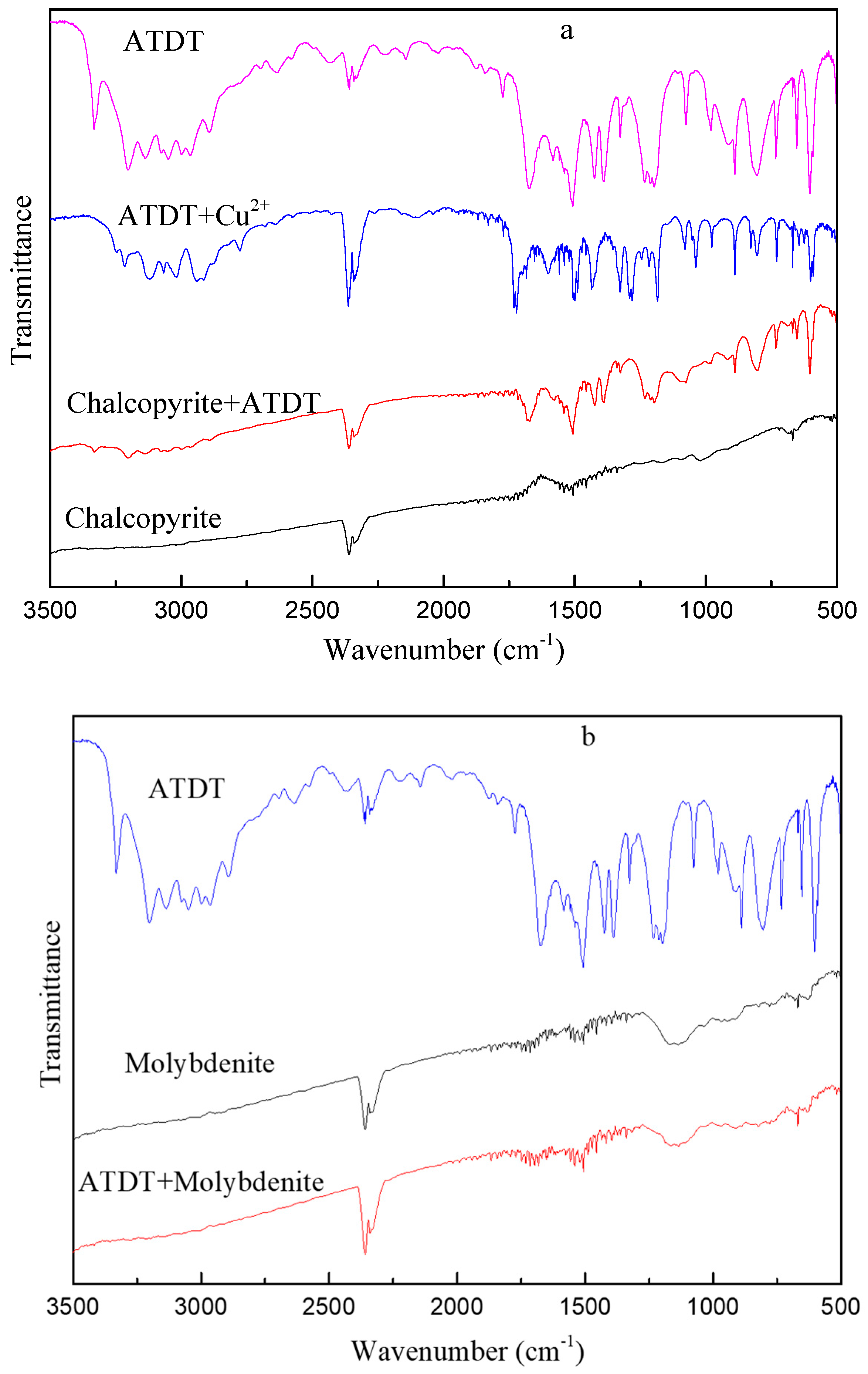
3.5. FT-IR Analysis
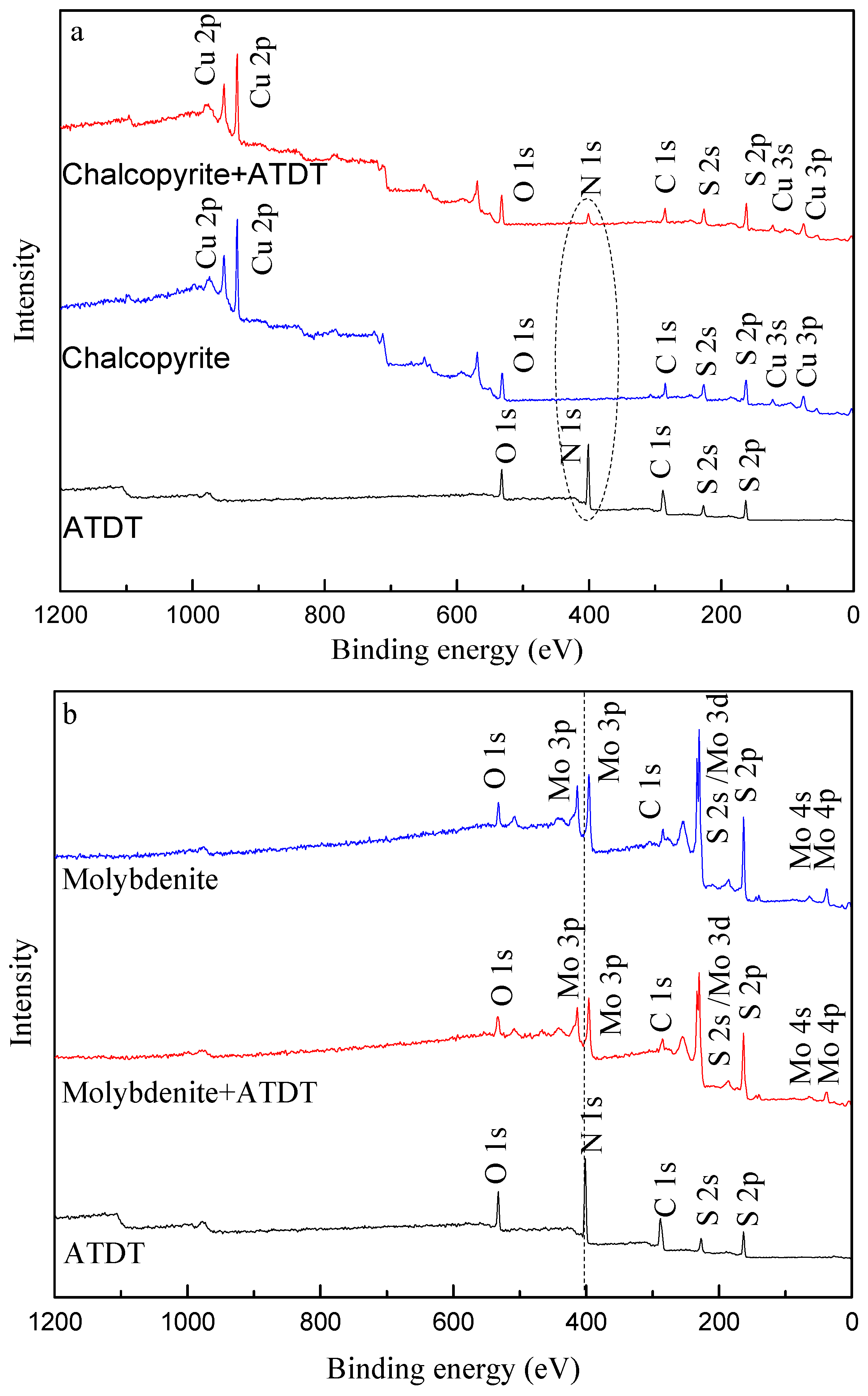
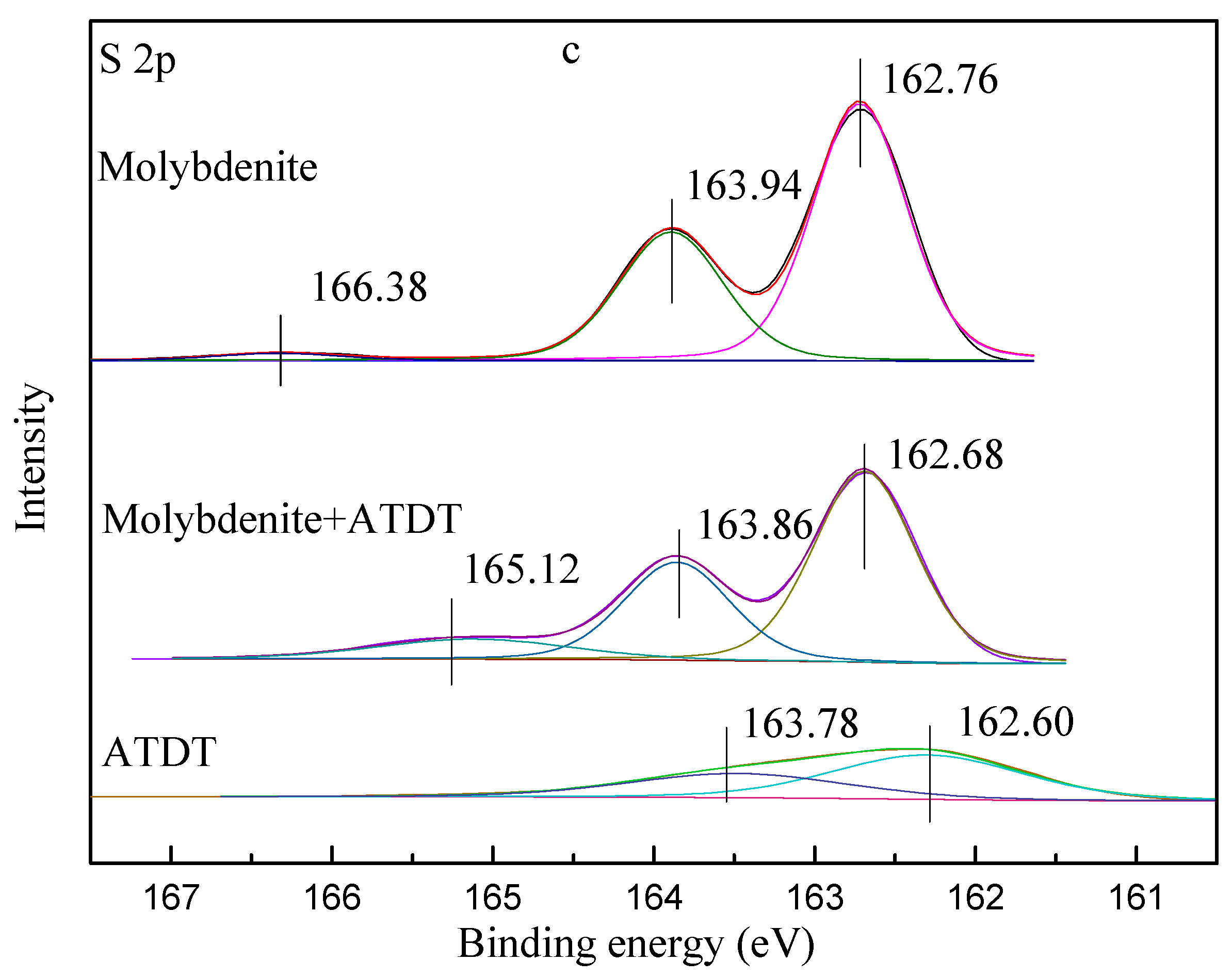
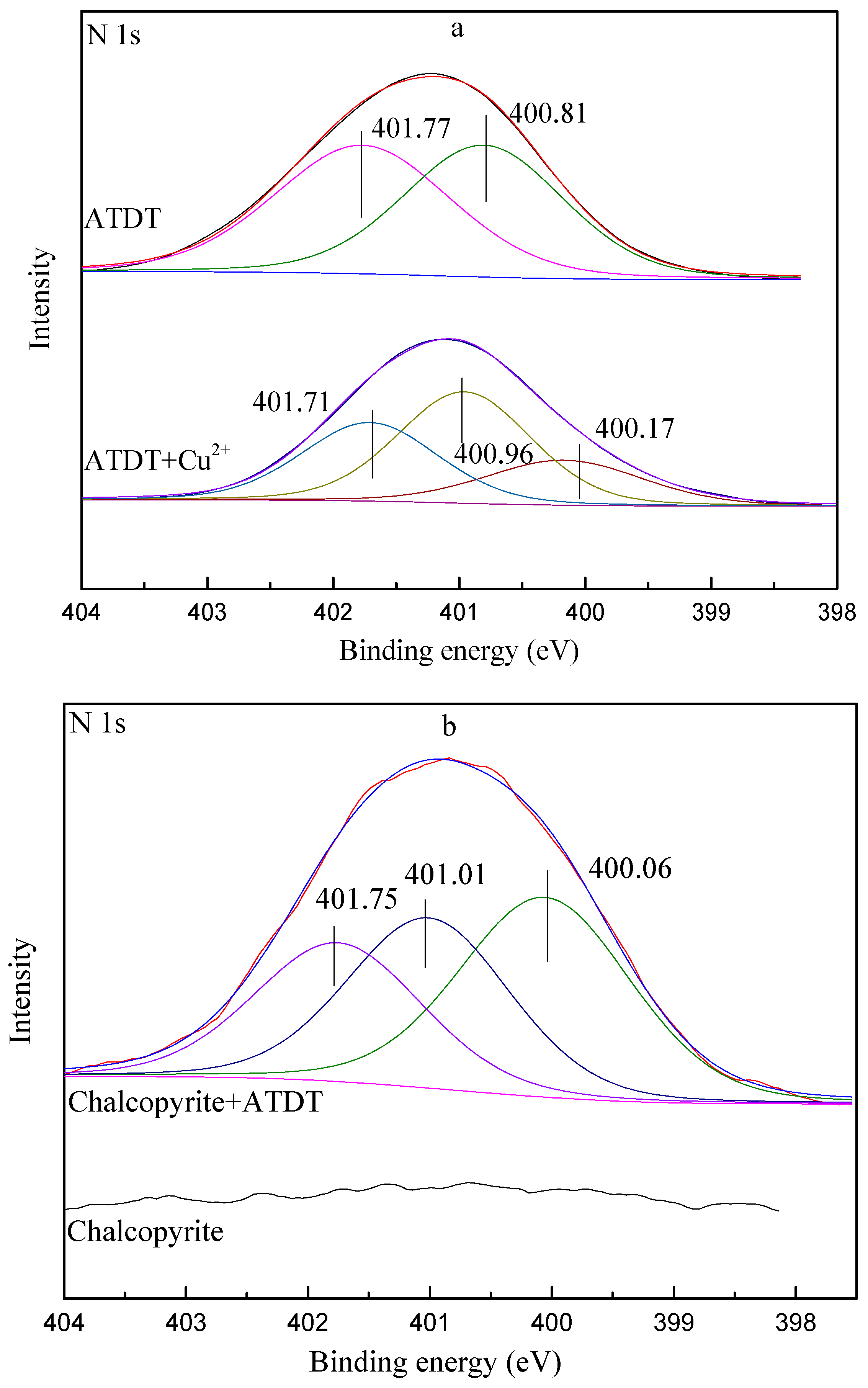
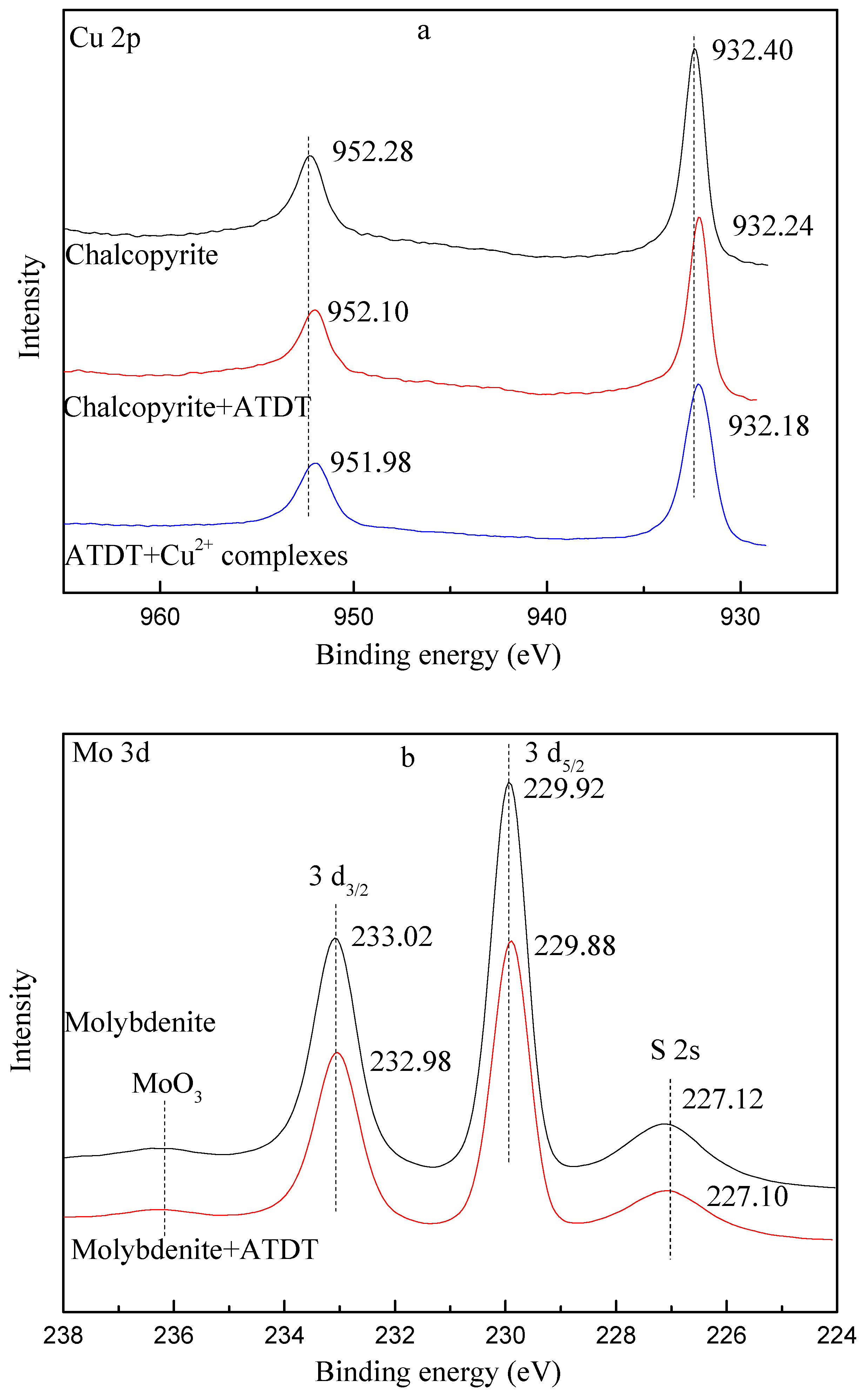
3.6. XPS Measurement
3.7. The Adsorption Model
4. Conclusions
- (1)
- The micro flotation results demonstrated that ATDT exhibited superior depressing power to chalcopyrite, thus the flotation separation of molybdenite from chalcopyrite could be realized under weak alkaline conditions.
- (2)
- The results of bench flotation indicated that the selective index of Mo/Cu improved significantly while ATDT was used as a potential chalcopyrite depressant in bench scale Cu-Mo flotation separation tests.
- (3)
- The results of the Zeta potential, UV spectra, FT-IR, and XPS analysis demonstrated that ATDT might react with Cu ions through N and thiol S atoms through the formation of copper complexes with the breakage of S-H bonds. However, for molybdenite, the possible physisorption mechanism of ATDT on molybdenite might be dominated by hydrogen bonds or Van der Waals' force electrostatic forces.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wie, J.M.; Fuerstenau, D.W. The effect of dextrin on surface properties and the flotation of molybdenite. Int. J. Miner. Process. 1974, 1, 17–32. [Google Scholar] [CrossRef]
- Hadi, A.; Zahra, M.; Ziaedin, S.S.; Mohammad, N.; Navid, M.M. Removal of copper from molybdenite concentrate by mesophilic and extreme thermophilic microorganisms. Int. J. Min. Sci. Technol. 2013, 23, 827–834. [Google Scholar]
- Yin, Z.; Sun, W.; Hu, Y.; Zhai, J.; Guan, Q. Evaluation of the replacement of NaCN with depressant mixtures in the separation of copper–molybdenum sulphide ore by flotation. Sep. Purif. Technol. 2017, 173, 9–16. [Google Scholar] [CrossRef]
- Peterson, J.A.; Saran, M.S.; Wisnouskas, J.S. Differential flotation reagent for molybdenum separation. U.S. Patent 4,575,419 A, 11 March 1986. [Google Scholar]
- Srivastava, A.K.; Kumar, A.; Misra, N.; Manjula, P.S.; Sarojini, B.K.; Narayana, B. Synthesis, spectral (FT-IR, UV-Visible, NMR) features, biological activity prediction and theoretical studies of 4-Amino-3-(4-hydroxybenzyl)-1H-1,2,4-triazole-5(4H)-thione and its tautomer. J. Mol. Struct. 2016, 1107, 137–144. [Google Scholar] [CrossRef]
- Ghosh, S.; Verma, A.; Mukerjee, A.; Mandal, M.K. Synthesis, characterization and antimicrobial evaluation of some novel 1,2,4-triazolo[3,4-b][1,3,4]thiadiazine bearing substituted phenylquinolin-2-one moiety. Arab. J. Chem. 2015, in press. [Google Scholar] [CrossRef]
- Singh, K.; Raparia, S.; Surain, P. Co(II), Ni(II), Cu(II) and Zn(II) Complexes of 4-(4-cyanobenzylideneamino)-3-mercapto-5-oxo-1,2,4-triazine: Synthesis, characterization and biological studies. Med. Chem. Res. 2014, 24, 2336–2346. [Google Scholar] [CrossRef]
- Rathi, P.; Singh, D.P. Template engineered biopotent macrocyclic complexes involving furan moiety: Molecular modeling and molecular docking. J. Mol. Struct. 2015, 1093, 201–207. [Google Scholar] [CrossRef]
- Singh, R.N.; Rawat, P.; Sahu, S. Vibrational spectra, electronic absorption, nonlinear optical properties, evaluation of bonding, chemical reactivity and thermodynamic properties of ethyl 4-(1-(2-(hydrazinecarbonothioyl)hydrazono)ethyl)-3,5-dimethyl-1H-pyrrole-2-carboxylate molecule by ab initio HF and density functional methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 1162. [Google Scholar]
- Subashchandrabose, S.; Thanikachalam, V.; Manikandan, G.; Saleem, H.; Erdogdu, Y. Synthesis and spectral characterization of bis(4-amino-5-mercapto-1,2,4-triazol-3-yl)propane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 157, 96. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Manzoor, K.; Ahmed, S.; Ikram, S. Synthesis and characterization of terepthalaldehyde–thiocarbohydrazide polymer doped with Cu(II) and Zn(II) Metal ions for solar cell applications. Opt. Int. J. Light Electron Opt. 2016, 127, 4329–4332. [Google Scholar] [CrossRef]
- Esmaeili, N.; Neshati, J.; Yavari, I. Corrosion inhibition of new thiocarbohydrazides on the carbon steel in hydrochloric acid solution. J. Ind. Eng. Chem. 2015, 22, 159–163. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Isatin derivatives as a non-toxic corrosion inhibitor for mild steel in 20% H2SO4. Corros. Sci. 2015, 95, 62–70. [Google Scholar] [CrossRef]
- John, S.; Joseph, A.; Sajini, T.; Jose, A. Corrosion inhibition properties of 1,2,4-Hetrocyclic Systems: Electrochemical, theoretical and Monte Carlo simulation studies. J. Egypt. J. Pet. 2016, in press. [Google Scholar] [CrossRef]
- Bahadır, Z.; Bulut, V.N.; Ozdes, D.; Duran, C.; Bektas, H.; Soylak, M. Separation and preconcentration of lead, chromium and copper by using with the combination coprecipitation-flame atomic absorption spectrometric determination. J. Ind. Eng. Chem. 2014, 20, 1030–1034. [Google Scholar] [CrossRef]
- Nalawade, R.A.; Nalawade, A.M.; Kamble, G.S.; Anuse, M.A. Rapid, synergistic extractive spectrophotometric determination of copper(II) by using sensitive chromogenic reagent N″,N″′-bis[(E)-(4-fluorophenyl) methylidene]thiocarbonohydrazide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 146, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.M.; Moustafa, M.E.; Nassar, M.Y.; Abdelrahman, E.A. Synthesis and characterization of novel Cu (II) complexes with 3-substituted-4-amino-5-mercapto-1,2,4-triazole Schiff bases: A new route to CuO nanoparticles. J. Mol. Struct. 2015, 1086, 223–231. [Google Scholar] [CrossRef]
- Yurt, A.; Balaban, A.; Kandemir, S.U.; Bereket, G.; Erk, B. Investigation on some Schiff bases as HCl corrosion inhibitors for carbon steel. Mater. Chem. Phys. 2004, 85, 420–426. [Google Scholar] [CrossRef]
- Gürten, A.A.; Keleş, H.; Bayol, E.; Kandemirli, F. The effect of temperature and concentration on the inhibition of acid corrosion of carbon steel by newly synthesized Schiff base. J. Ind. Eng. Chem. 2014, 27, 68–78. [Google Scholar] [CrossRef]
- Mccarrick, R.M.; Eltzroth, M.J.; Squattrito, P.J. Coordination geometries of bis(4-amino-3-alkyl-1,2,4-triazole-5-thione) complexes of first-row transition metals: Crystal structures of complexes with propyl and hydrogen in the 3-position. Relationship to the 3-methyl and 3-ethyl analogs. Inorg. Chim. Acta 2000, 311, 95–105. [Google Scholar] [CrossRef]
- Clark, R.W.; Squattrito, P.J.; Sen, A.K.; Dubey, S.N. Structural trends in a series of divalent transition metal triazole complexes. Inorg. Chim. Acta 1999, 293, 61–69. [Google Scholar] [CrossRef]
- Kajdan, T.W.; Squattrito, P.J.; Dubey, S.N. Coordination geometries of bis (4-amino-3-ethyl-1,2,4-triazole-5-thione) complexes of Mn, Fe, Co, Ni, Cu and Zn: Relationship to the 3-methyl analogs. Inorg. Chim. Acta 2000, 300, 1082–1089. [Google Scholar] [CrossRef]
- Zangrando, E.; Islam, M.T.; Islam, A.A.A.A.; Sheikh, M.C.; Tarafder, M.T.H.; Miyatake, R.; Zahan, R.; Hossain, M.A. Synthesis, characterization and bio-activity of nickel(II) and copper(II) complexes of a bidentate NS Schiff base of S-benzyl dithiocarbazate. Inorg. Chim. Acta 2015, 427, 278–284. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Y.; Qu, X.; Xiao, J.; Yang, X.; Xu, Z. Understanding the hydrophobic mechanism of 3-hexyl-4-amino-1, 2,4-triazole-5-thione to malachite by ToF-SIMS, XPS, FT-IR, contact angle, zeta potential and micro-flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 34–42. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Liu, R.; Chen, P.; Tian, M.J. Utilization of acetic acid-[(hydrazinylthioxomethyl)thio]-sodium as a novel selective depressant for chalcopyrite in the flotation separation of molybdenite. Sep. Purif. Technol. 2017, 179, 248–256. [Google Scholar] [CrossRef]
- Dixit, P.P.; Dixit, P.P.; Thore, S.N. Hybrid triazoles: Design and synthesis as potential dual inhibitor of growth and efflux inhibition in tuberculosis. Eur. J. Med. Chem. 2016, 107, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Fawzi, M.M. 1,2,4-Triazin-5-ones. U.S. Patent 4,346,220 A, 24 August 1982. [Google Scholar]
- Tandon, S.S.; Dul, M.C.; Lee, J.L.; Dawe, L.N.; Anwar, M.U.; Thompson, L.K. Complexes of ditopic carbo- and thio-carbohydrazone ligands – mononuclear, 1D chain, dinuclear and tetranuclear examples. Dalton Trans. 2011, 40, 3466–3475. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.F.A.; Chaves, A.P.; Luz, A.B.; França, S.C.A. The use of dextrin in purification by flotation of molybdenite concentrates. Int. J. Miner. Process. 2014, 127, 23–27. [Google Scholar] [CrossRef]
- Xu, M. Modified flotation rate constant and selectivity index. Miner. Eng. 1998, 11, 271–278. [Google Scholar] [CrossRef]
- Wise, C.F.; Liu, D.; Mayer, K.J.; Crossland, P.M.; Hartley, C.L.; Mcnamara, W.R. A nickel complex of a conjugated bis-dithiocarbazate Schiff base for the photocatalytic production of hydrogen. Dalton Trans. 2015, 44, 14265–14271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q. Flotation separation of carbonate from sulfide minerals, II: Mechanisms of flotation depression of sulfide minerals by thioglycollic acid and citric acid. Miner. Eng. 2004, 17, 865–878. [Google Scholar] [CrossRef]
- Mitchell, T.K.; Nguyen, A.V.; Evans, G.M. Heterocoagulation of chalcopyrite and pyrite minerals in flotation separation. Adv. Colloid Interface Sci. 2005, 114–115, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ge, Y.; Guo, X.; Guo, W. The depression effect and mechanism of NSFC on dolomite in the flotation of phosphate ore. Sep. Purif. Technol. 2016, 161, 88–95. [Google Scholar] [CrossRef]
- Subashchandrabose, S.; Babu, N.R.; Saleem, H.; Padusha, M.S.A. Vibrational studies on (E)-1-((pyridine-2-yl)methylene)semicarbazide using experimental and theoretical method. J. Mol. Struct. 2015, 1094, 254–263. [Google Scholar] [CrossRef]
- Centore, R.; Takjoo, R.; Capobianco, A.; Peluso, A. Ring to open-chain transformation induced by selective metal coordination in a new dithiocarbazate ligand. Inorg. Chim. Acta 2013, 404, 29–33. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, Y. The oxidation of copper sulfide minerals during grinding and their interactions with clay particles. Powder Technol. 2012, 230, 112–117. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Dixon, D.G.; Asselin, E. Electrochemical and XPS analysis of chalcopyrite (CuFeS 2) dissolution in sulfuric acid solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, Z.; Wang, J.; Liu, Q.; Xiao, J.; Zeng, H.; Zhong, H.; Xu, Z. Study of N-isopropoxypropyl-N′-ethoxycarbonyl thiourea adsorption on chalcopyrite using in situ SECM, ToF-SIMS and XPS. J. Coll. Interface Sci. 2015, 437, 42. [Google Scholar] [CrossRef] [PubMed]
- Sandström, Å.; Shchukarev, A.; Paul, J. XPS characterisation of chalcopyrite chemically and bio-leached at high and low redox potential. Miner. Eng. 2005, 18, 505–515. [Google Scholar] [CrossRef]
- Yang, Y.; Harmer, S.; Chen, M. Synchrotron X-ray photoelectron spectroscopic study of the chalcopyrite leached by moderate thermophiles and mesophiles. Miner. Eng. 2014, 69, 185–195. [Google Scholar] [CrossRef]
- Yang, Y.; Harmer, S.; Chen, M. Synchrotron-based XPS and NEXAFS study of surface chemical species during electrochemical oxidation of chalcopyrite. Hydrometallurgy 2015, 156, 89–98. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, C.; Zhang, K.; Chen, Y.; Li, C.; Gao, P.; Yang, P.; Ouyang, Q. Three-dimensional hierarchical MoS2 nanoflake array/carbon cloth as high-performance flexible lithium-ion battery anodes. J. Mater. Chem. A 2014, 2, 4551–4557. [Google Scholar] [CrossRef]
- Fairthorne, G.; Brinen, J.S.; Fornasiero, D.; Nagaraj, D.R.; Ralston, J. Spectroscopic and electrokinetic study of the adsorption of butyl ethoxycarbonyl thiourea on chalcopyrite. Int. J. Miner. Process. 1998, 54, 147–163. [Google Scholar] [CrossRef]
- Yoshida, T. An X-ray photoelectron spectroscopic study of several metal complexes of 2-mercaptobenzimidazole and 2-mercaptobenzoxazole. Bull. Chem. Soc. Jpn. 2006, 53, 1449–1450. [Google Scholar] [CrossRef]
- Owusu, C.; Fornasiero, D.; Addai-Mensah, J.; Zanin, M. Effect of regrinding and pulp aeration on the flotation of chalcopyrite in chalcopyrite/pyrite mixtures. Powder Technol. 2014, 267, 61–67. [Google Scholar] [CrossRef]
- Kalegowda, Y.; Chan, Y.L.; Wei, D.H.; Harmer, S.L. X-PEEM, XPS and ToF-SIMS characterisation of xanthate induced chalcopyrite flotation: Effect of pulp potential. Surf. Sci. 2015, 635, 70–77. [Google Scholar] [CrossRef]
- He, Z.; Liu, G.; Yang, X.; Liu, W. A novel surfactant, N, N-diethyl-N′-cyclohexylthiourea: Synthesis, flotation and adsorption on chalcopyrite. J. Ind. Eng. Chem. 2016, 37, 107–114. [Google Scholar] [CrossRef]
- Bera, P.; Kim, C.H.; Seok, S.I. Synthesis, spectroscopic characterization and thermal behavior of cadmium(II) complexes of S-methyldithiocarbazate (SMDTC) and S-benzyldithiocarbazate (SBDTC): X-ray crystal structure of [Cd(SMDTC)3]·2NO3. Polyhedron 2008, 27, 3433–3438. [Google Scholar] [CrossRef]
- Iranmahboob, J.; Gardner, S.D.; Toghiani, H.; Hill, D.O. XPS study of molybdenum sulfide catalyst exposed to CO and H2. J. Colloid Interface Sci. 2004, 270, 123–126. [Google Scholar] [CrossRef]
- Hirajima, T.; Miki, H.; Suyantara, G.P.W.; Matsuoka, H.; Elmahdy, A.M.; Sasaki, K.; Imaizumi, Y.; Kuroiwa, S. Selective flotation of chalcopyrite and molybdenite with H2O2 oxidation. Miner. Eng. 2017, 100, 83–92. [Google Scholar] [CrossRef]
- Baker, M.A.; Gilmore, R.; Lenardi, C.; Gissler, W. XPS investigation of preferential sputtering of S from MoS2 and determination of MoSx stoichiometry from Mo and S peak positions. Appl. Surf. Sci. 1999, 150, 255–262. [Google Scholar] [CrossRef]




| Parameters | Control Test | ATDT | ||
|---|---|---|---|---|
| Molybdenite | Chalcopyrite | Molybdenite | Chalcopyrite | |
| R∞ | 78.80 | 77.84 | 81.10 | 30.67 |
| K | 0.96 | 0.67 | 0.62 | 0.55 |
| KM | 0.76 | 0.52 | 0.50 | 0.17 |
| S.I. (Mo/Cu) | 1.45 | 2.98 | ||
| Compound | υC-N | υC=N | υC=S | υNH2 | υC=O | υ((CN) + (NH)) |
|---|---|---|---|---|---|---|
| ATDT | 1232(w)/1424(s) | 1582 | 1075 | 3201 | 1773 | 1389 |
| ATDT-Cu(II) | 1244(w)/1436(s) | 1558 | 1079 | 3215 | 1771 | 1427 |
| ATDT + Cp | 1447 | 1558 | 1077 | 3206 | 1423 |
| Samples | Atomic Concentration of Elements (Atomic %) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | N | S | Cu | Fe | Mo | |
| chalcopyrite | 25.45 | 20.73 | 0.00 | 27.48 | 18.98 | 7.36 | |
| Chalcopyrite + ATDT | 26.26 | 19.53 | 10.82 | 22.44 | 15.07 | 5.88 | |
| Δc | 0.81 | −1.20 | 10.82 | −5.04 | −3.91 | −1.48 | |
| Molybdenite | 17.43 | 12.31 | 0.00 | 47.94 | 22.30 | ||
| Molybdenite + ATDT | 24.21 | 15.29 | 0.84 | 42.37 | 17.30 | ||
| Δm | 6.78 | 2.98 | 0.84 | −5.57 | 0.00 | 0.00 | −5.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Zhang, C. Separation of Molybdenite from Chalcopyrite in the Presence of Novel Depressant 4-Amino-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one. Minerals 2017, 7, 146. https://doi.org/10.3390/min7080146
Yin Z, Sun W, Hu Y, Zhang C, Guan Q, Zhang C. Separation of Molybdenite from Chalcopyrite in the Presence of Novel Depressant 4-Amino-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one. Minerals. 2017; 7(8):146. https://doi.org/10.3390/min7080146
Chicago/Turabian StyleYin, Zhigang, Wei Sun, Yuehua Hu, Chenhu Zhang, Qingjun Guan, and Chenyang Zhang. 2017. "Separation of Molybdenite from Chalcopyrite in the Presence of Novel Depressant 4-Amino-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one" Minerals 7, no. 8: 146. https://doi.org/10.3390/min7080146




