A Review of the Carbon Footprint of Cu and Zn Production from Primary and Secondary Sources
Abstract
:1. Introduction
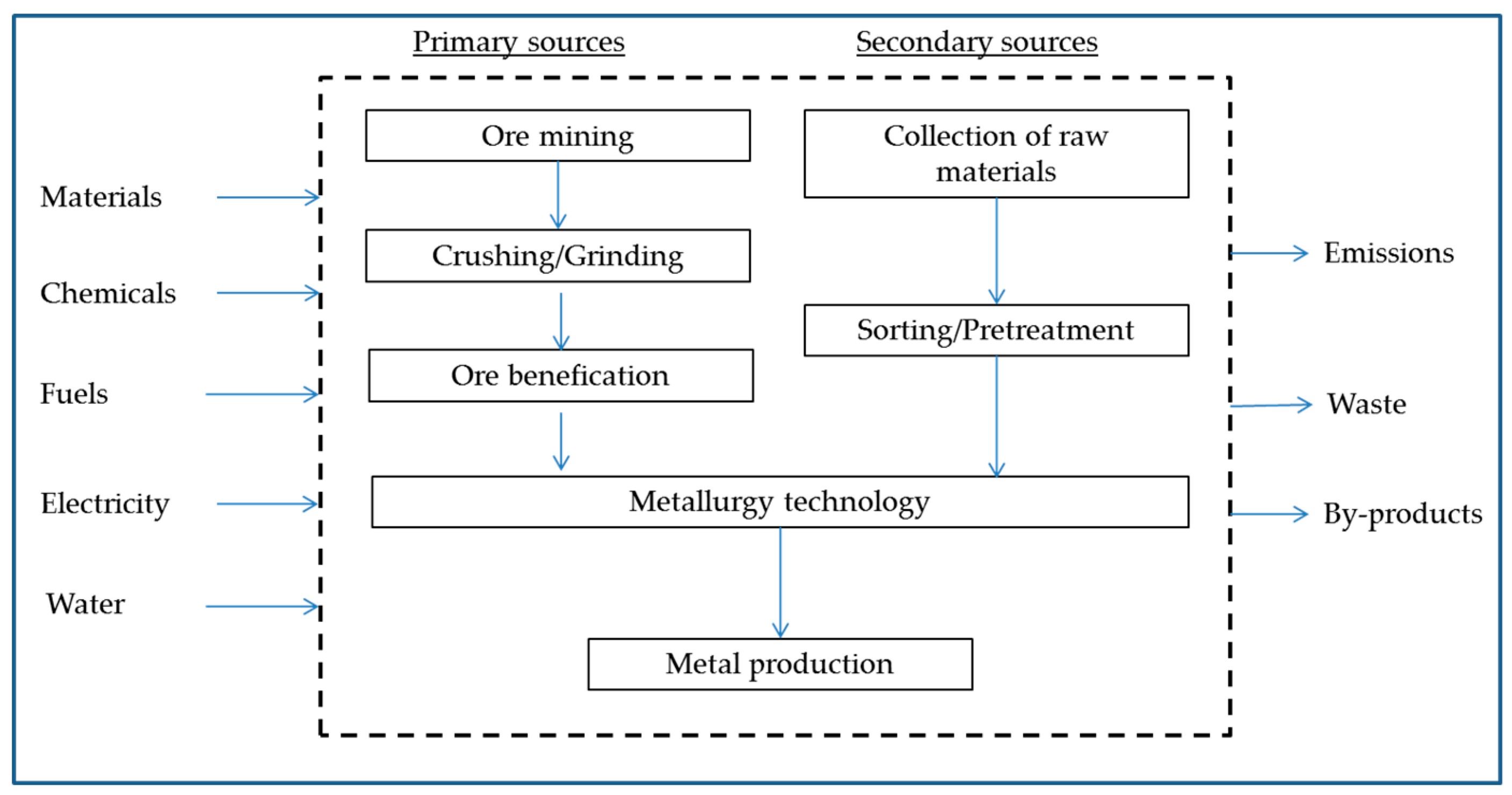
2. Carbon Footprint and LCA
Methodology
3. Results and Discussion
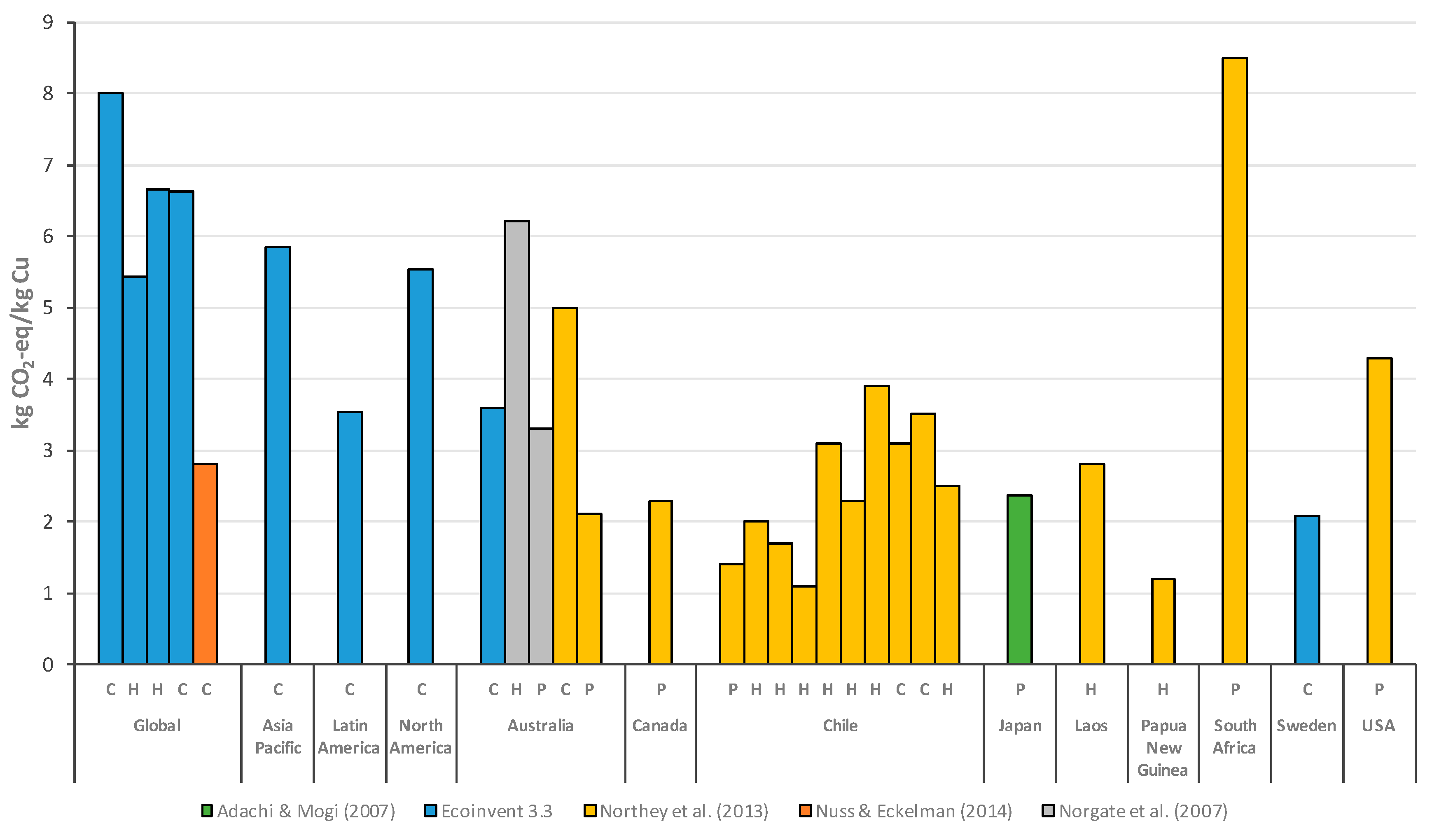
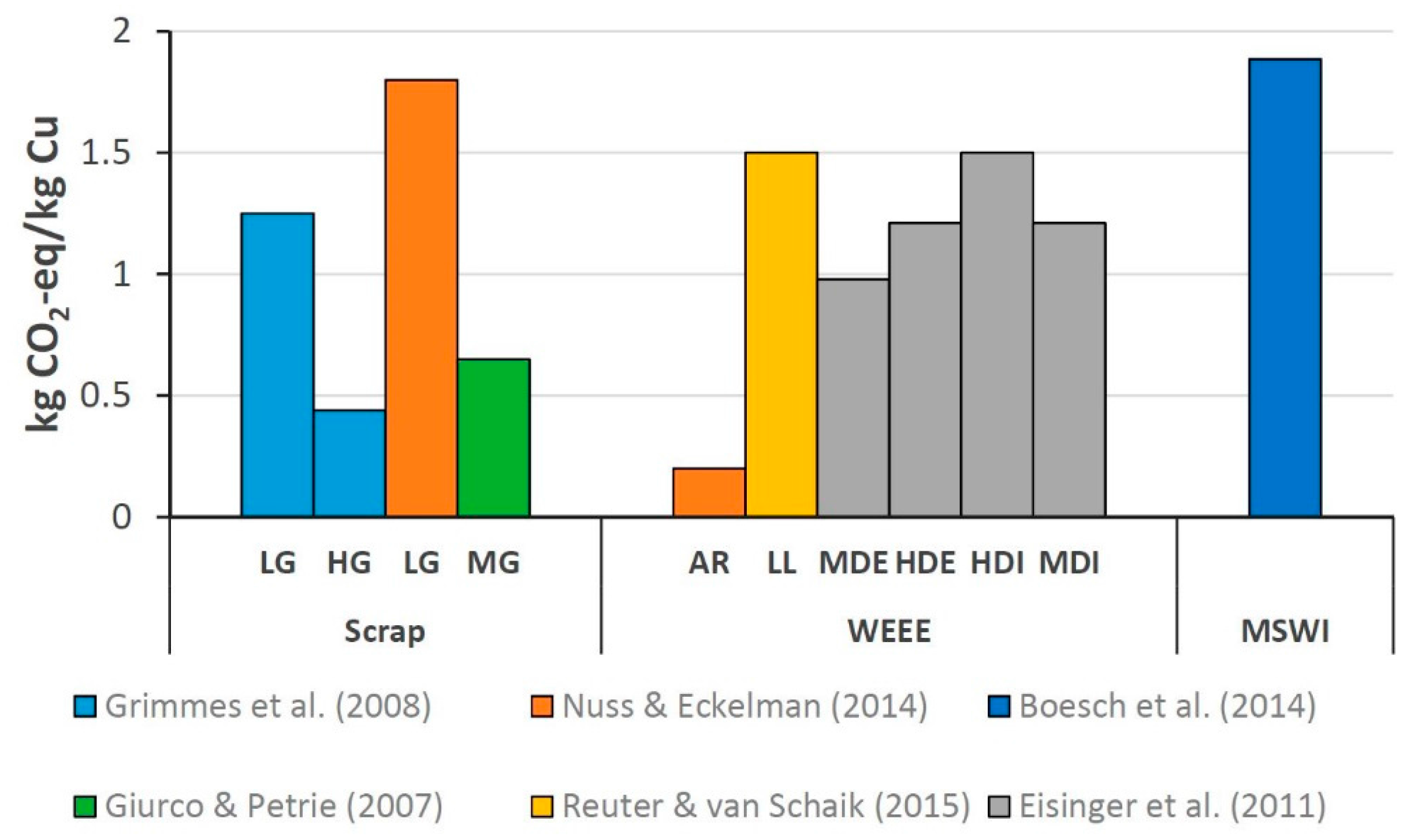
3.1. Copper
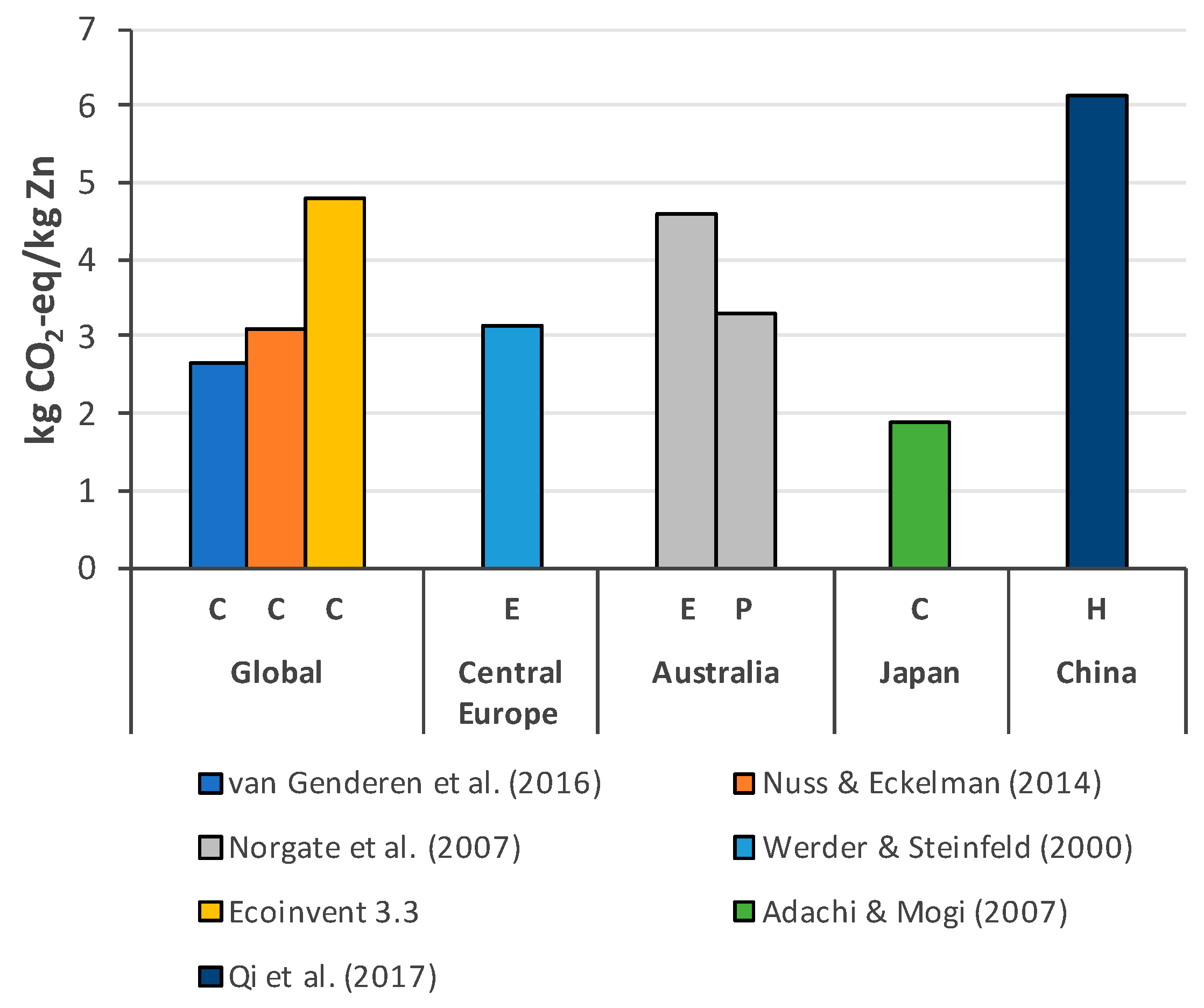
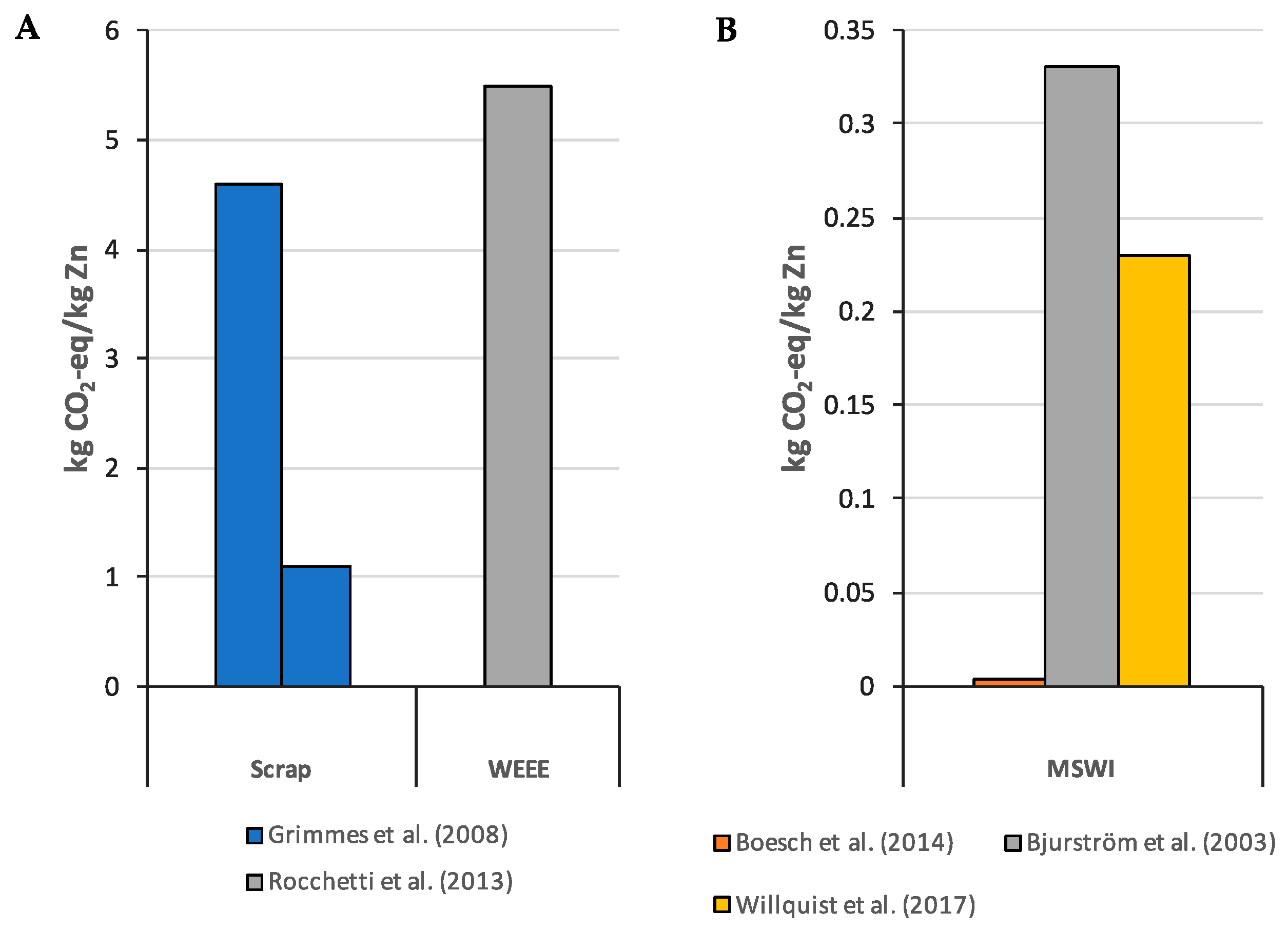
3.2. Zinc
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Northey, S.; Mohr, S.; Mudd, G.; Weng, Z.; Giurco, Y.D. Modelling future copper ore grade decline based on a detailed assessment of copper resources and mining. Resour. Conserv. Recycl. 2014, 83, 190–201. [Google Scholar] [CrossRef]
- Brown, L. Plan B. 2.0: Rescuing a Planet Under Stress and a Civilization in Trouble; W.W. Norton: New York, NY, USA, 2006. [Google Scholar]
- Vieira, M.D.; Goedkoop, M.J.; Storm, P.; Huijbregts, M.A. Ore grade Decrease as Life Cycle Impact Indicator for Metal Scarcity: The Case of Copper. Environ. Sci. Technol. 2012, 46, 12772–12778. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Head, I.; Premier, G.; Scott, K.; Yu, E.; Lloyd, J.; Sandhukhan, J. A multilevel sustainability analysis of Zn recovery from wastes. Resour. Conserv. Recycl. 2016, 113, 88–105. [Google Scholar] [CrossRef]
- Zinc Investing News. Available online: http://investingnews.com/category/daily/resource-investing/base-metals-investing/zinc-investing/ (accessed on 13 July 2017).
- Eugster, M. External Costs in the European Copper Value Chain. A Comparison of Copper Primary Production and Recycling. Master’s Thesis, ETH Zürich, Zürich, Switzerland, 2008. [Google Scholar]
- Boulamanti, A.; Moya, Y.J. Production costs of the non-ferrous metals in the EU and other countries: Copper and Zinc. Resour. Policy 2016, 49, 112–118. [Google Scholar] [CrossRef]
- ECORYS. 2011. Available online: http://www.ecorys.com (accessed on 13 July 2017).
- ASM Recycling. 2015. Available online: http://www.asm-recycling.co.uk/blog/the-world-of-metal-recycling-the-facts/ (accessed on 13 July 2017).
- Chao, D. BIR Annual Report 2016, Non-Ferrous Metal Division; Bureau of International Recycling BIR: Brussels, Belgium, 2016. [Google Scholar]
- E.C. Institute. 2017. Available online: http://copperalliance.eu/about-copper/recycling (accessed on 13 July 2017).
- Graedel, T.E. Recycling Rates of Metals—A Status Report, A Report of the Working Group on the Global Metal Flows to the International Resource Panel; UNEP: Athens, Greece, 2011. [Google Scholar]
- Song, X.; Pettersen, J.B.; Pedersen, K.B.; Roberg, S. Comparative life cycle assessment of tailings management and energy scenarios for a copper mine: A case study in Northern Norway. J. Clean. Prod. 2017, 164, 892–904. [Google Scholar] [CrossRef]
- Norgate, T.; Jahanshahi, S. Reducing the greenhouse gas footprint of primary metal production: Where should the focus be? Miner. Eng. 2011, 24, 1563–1570. [Google Scholar] [CrossRef]
- Eckelman, M. Facility-level energy and greenhouse gas life-cycle assessment of the global nickel industry. Resour. Conserv. Recycl. 2010, 54, 256–266. [Google Scholar] [CrossRef]
- Dudka, J.; Adriano, Y.P.; Dudka, S.; Adriano, D. Environmental Impacts of Metal Ore Mining and Processing: A Review. J. Environ. Qual. 1997, 26, 590–602. [Google Scholar] [CrossRef]
- CDAA (Copper Development Association Africa), Sources of Copper Ore. 2016. Available online: http://www.copper.co.za/copper-education/sources-of-copper-ore/ (accessed on 20 April 2017).
- Northey, S.; Haque, N.; Mudd, G. Using sustainability reporting to assess the environmental footprint of copper mining. J. Clean. Prod. 2013, 40, 118–128. [Google Scholar] [CrossRef]
- ICSG (International Copper Study Group). The World Copper Factbook. 2014. Available online: http://www.icsg.org (accessed on 13 July 2017).
- Muchova, L.; Eder, P.; Villanueva, A. End-of-Waste Criteria for Copper and Copper Alloy Scrap: Technical Proposals. Report EUR 24786 EN. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC64207/reqno_jrc64207_jrc64207.pdf (accessed on 20 August 2017).
- IZA. International Zinc Association Website. Available online: http://www.zinc.org/basics/ (accessed on 13 July 2017).
- Norgate, T.; Jahanshahi, S.; Rankin, W. Assessing the environmental impact of metal production processes. J. Clean. Prod. 2007, 15, 838–848. [Google Scholar] [CrossRef]
- Finnveden, G.; Hauschild, M.; Ekvall, T.; Guinée, J.; Heijungs, R.; Hellweg, S.; Koehler, A.; Pennington, D.; Suh, S. Recent developments in life cycle assessment. J. Environ. Manag. 2009, 91, 1–21. [Google Scholar] [CrossRef] [PubMed]
- ISO. Environmental Management—Life Cycle Assessment—Principles and Framework, ISO14040; The International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- ISO. Environmental Management—Life Cycle Assessment—Requirements and Guidelines, ISO 14044; The International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Raugei, M.; Ulgiati, S. A novel approach to the problem of geographic allocation of environmental impact in life cycle assessment and material flow analysis. Ecol. Indic. 2009, 9, 1257–1264. [Google Scholar] [CrossRef]
- ISO. Greenhouse Gases—Carbon Footprinting of Products-Requirements and Guidelines for Quantification and Communication; The International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- European Commission. 2017. Available online: http://ec.europa.eu/environment/eussd/smgp/ef_pilots.htm (accessed on 13 July 2017).
- Bartzas, G.; Komnitsas, K. Life cycle assessment of ferronickel production in Greece. Resour. Conserv. Recycl. 2015, 105A, 113–122. [Google Scholar] [CrossRef]
- Swiss Centre for Life Cycle Inventories. Ecoinvent Life Cycle Inventory Database v3.3; Ecoinvent: Zurich, Switzerland, 2016. [Google Scholar]
- Adachi, T.; Mogi, G. Life Cycle Inventory for Base Metal Ingots Production in Japan Including Mining and Mineral Processing Processes by Cost Estimating System Database. Trans. Nonferrous Met. Soc. China 2007, 17, 131–135. [Google Scholar]
- Barba-Gutierrez, Y.; Adenso-Diaz, B.; Hopp, M. An analysis of some environmental consequences of European electrical and electronic waste regulation. Resour. Conserv. Recycl. 2008, 52, 481–495. [Google Scholar] [CrossRef]
- Grimes, S.; Donaldson, J.; Cebrian Gomez, G. Report on the Environmental Benefits of Recycling; Bureau of International Recycling: Brussels, Belgium, 2008. [Google Scholar]
- Nuss, P.; Eckelman, M.J. Life Cycle Assessment of Metals: A Scientific Synthesis. PLoS ONE 2014, 9, e101298. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.; Barr, R.; Browning, M.; Diao, Z.; Friedlander, E. Criticality of the Geological Copper Family. Environ. Sci. Technol. 2011, 46, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Giurco, D.; Petrie, J. Strategies for reducing the carbon footprint of copper: New technologies, more recycling or demand management? Miner. Eng. 2007, 20, 842–853. [Google Scholar] [CrossRef]
- Eisinger, F.; Chakrabarti, R.; Krüger, C.; Alexeew, J. Carbon Footprint of E-Waste Recycling-Scenarios in India, Version 1.0; Adelphi: Berlin, Germany, 2011. [Google Scholar]
- Reuter, M.; van Schaik, A.; Gediga, J. Simulation-based design for resource efficiency of metal production and recycling systems: Cases-copper production and recycling, e-waste (LED lamps) and nickel pig iron. Int. J. Life Cycle Assess. 2015, 20, 671–693. [Google Scholar] [CrossRef]
- Boesch, M.; Vadenbo, C.; Saner, D.; Huter, C.; Hellweg, S. An LCA model for waste incineration enhanced with new technologies for metal recovery and application to the case of Switzerland. Waste Manag. 2014, 34, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Soares Rubin, R.; Soares de Castro, M.; Brandão, D.; Schalch, V.; Ometto, A. Utilization of Life Cycle Assessment methodology to compare two strategies for recovery of copper from printed circuit board scrap. J. Clean. Prod. 2014, 64, 297–305. [Google Scholar] [CrossRef]
- Fogarasi, S.; Imre-Lucaci, F.; Ilea, P.; Imre-Lucaci, A. The environmental assessment of two new copper recovery processes from Waste Printed Circuit Boards. J. Clean. Prod. 2013, 54, 264–269. [Google Scholar] [CrossRef]
- Van Genderen, E.; Wildnauer, M.; Santero, N.; Sidi, N. A Global Life Cycle Assessment for Primary Zinc Production. Int. J. Life Cycle Assess. 2016, 21, 1580–1593. [Google Scholar] [CrossRef]
- Qi, C.; Ye, L.; Ma, X.; Yang, D.; Hong, J. Life cycle assessment of the hydrometallurgical zinc production chain in China. J. Clean. Prod. 2017, 156, 451–458. [Google Scholar] [CrossRef]
- Werder, M.; Steinfeld, A. Life cycle assessment of the conventional and solar thermal production of zinc and synthesis gas. Energy 2000, 25, 395–409. [Google Scholar] [CrossRef]
- Rocchetti, L.; Veglio, E.; Kopacek, B.; Beolchini, F. Environmental Impact Assessment of hydrometallurgical Processes for Metal Recovery from WEEE Residues Using a Portable Prototype Plant. Environ. Sci. Technol. 2013, 47, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Morf, L.; Gloor, R.; Haag, O.; Haupt, M.; Skutan, S.; Lorenzo, F.; Böni, D. Precious metals and rare earth elements in municipal solid waste–sources and rate in a Swiss incineration plant. Waste Manag. 2013, 33, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Bühler, A.; Schlumberger, S. Schwermetalle aus der Flugasche Zurückgewinnen: Saure Flugaschenwäsche—FLUWA Verfahren, ein Zukunftsweisendes Verfahren in der Abfallverbrennung (Recovering Heavy Metals from Fly Ash: Acidic Fly Ash Scrubbing ‘FLUWA’, a Trendsetting Procedure in Waste I; Swiss Federal Office for the Environment: Bern, Switzerland, 2010. [Google Scholar]
- Karlfeldt Fedje, K.; Andersson, O.; Modin, P.; Frändegard, P.; Pettersson, A. Opportunities for Zn recovery from Swedish MSWI fly ashes. In Proceedings of the Second Symposium on Urban Mining, Bergamo, Italy, 19–21 May 2014. [Google Scholar]
- Willquist, K.; Johansson, I.; Andersson, S.; Angel, H.; Harfeldt, L.; Karfeldt Fedje, K. Critical metal extraction from municipal solid waste incineration ashes and the impact on a circular economy. In Proceedings of the 5th International Slag Valorisation Symposium, Leuven, Belgium, 3–5 April 2017. [Google Scholar]
- Bjurström, H.; Steenari, B.M. Wet Treatment of Ashes, a Survey of Methods; Värmeforsk Service AB: Stockholm, Sweden, 2003. [Google Scholar]
- HYDROWEEE Project—Innovative Hydrometallurgical Processes to Recover Metals from WEEE including Lamps and Batteries. Available online: http://cordis.europa.eu/result/rcn/45388_en.html (accessed on 13 July 2017).
- Salmi, O.; Wierink, M. Effects of waste recovery on carbon footprint: A case study of the Gulf of Bothnia steel and zinc industries. J. Clean. Prod. 2011, 19, 1857–1864. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekman Nilsson, A.; Macias Aragonés, M.; Arroyo Torralvo, F.; Dunon, V.; Angel, H.; Komnitsas, K.; Willquist, K. A Review of the Carbon Footprint of Cu and Zn Production from Primary and Secondary Sources. Minerals 2017, 7, 168. https://doi.org/10.3390/min7090168
Ekman Nilsson A, Macias Aragonés M, Arroyo Torralvo F, Dunon V, Angel H, Komnitsas K, Willquist K. A Review of the Carbon Footprint of Cu and Zn Production from Primary and Secondary Sources. Minerals. 2017; 7(9):168. https://doi.org/10.3390/min7090168
Chicago/Turabian StyleEkman Nilsson, Anna, Marta Macias Aragonés, Fatima Arroyo Torralvo, Vincent Dunon, Hanna Angel, Konstantinos Komnitsas, and Karin Willquist. 2017. "A Review of the Carbon Footprint of Cu and Zn Production from Primary and Secondary Sources" Minerals 7, no. 9: 168. https://doi.org/10.3390/min7090168





