Effect of Mica and Hematite (001) Surfaces on the Precipitation of Calcite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Calcite on (001) Surfaces of Mica and Hematite
2.2. X-ray Diffraction Analyses
2.3. SEM and EBSD
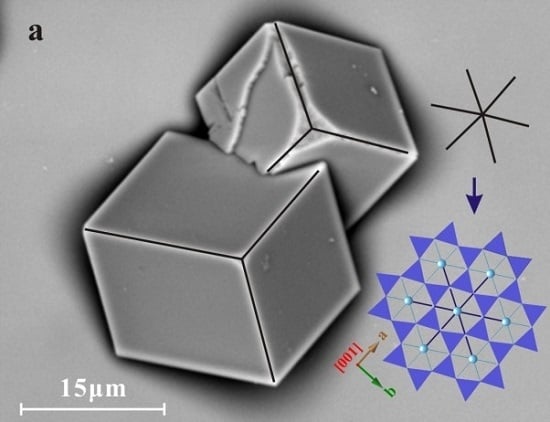
3. Results
3.1. Substrate Effect in Pure Ca2+ Solution
3.2. Influence of Mg2+ on Epitaxial Growth of Calcite
4. Discussion
4.1. Calcite Growth in Mg2+-Free System
4.2. Calcite Growth in Ca2+-Mg2+ System
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Conti, J.J.; Holtberg, P.D.; Beamon, J.A.; Schaal, A.M.; Ayoub, J.C.; Turnure, J.T. Annual energy outlook 2011: With Projections to 2035. 2011. Available online: https://www.eia.gov/outlooks/archive/aeo11/pdf/0383(2011).pdf (accessed on 10 September 2012).
- Mani, D.; Charan, S.N.; Kumar, B. Assessment of carbon dioxide sequestration potential of ultramafic rocks in the greenstone belts of southern India. Curr. Sci. 2008, 94, 53–61. [Google Scholar]
- Conwa, T.; Tans, P. Trends in Atmospheric Carbon Dioxide. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/ (accessed on 1 September 2017).
- Stern, N.H. Executive review. In The Economics of Climate Change: The Stern Review; Cambridge University Press: Cambridge, UK, 2007; ISBN 0521700809. [Google Scholar]
- Benson, L. Carbonate deposition, Pyramid Lake subbasin, Nevada: 1. Sequence of formation and elevational distribution of carbonate deposits (Tufas). Palaeogeogr. Palaeoclimatol. Palaeoecol. 1994, 109, 55–87. [Google Scholar] [CrossRef]
- Ryu, K.W.; Lee, M.G.; Jang, Y.N. Mechanism of tremolite carbonation. Appl. Geochem. 2011, 26, 1215–1221. [Google Scholar] [CrossRef]
- Goff, F.; Lackner, K.S. Carbon dioxide sequestering using ultramafic rocks. Environ. Geosci. 1998, 5, 89–101. [Google Scholar]
- Koustoukos, P.G.; Nancollas, G.H. Crystal growth of calcium phosphates - epitaxial considerations. J. Cryst. Growth 1981, 53, 10–19. [Google Scholar]
- Belcher, A.M.; Wu, X.H.; Christensen, R.J.; Hansma, P.K.; Stucky, G.D.; Morse, D.E. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature 1996, 381, 56–58. [Google Scholar] [CrossRef]
- DeOliveira, D.B.; Laursen, R.A. Control of Calcite Crystal Morphology by a Peptide Designed To Bind to a Specific Surface. J. Am. Chem. Soc. 1997, 119, 10627–10631. [Google Scholar] [CrossRef]
- Aizenberg, J.; Black, A.J.; Whitesides, G.M. Control of crystal nucleation by patterned self-assembled monolayers. Nature 1999, 398, 495–498. [Google Scholar]
- Travaille, A.M.; Donners, J.J.J.M.; Gerritsen, J.W.; Sommerdijk, N.A.J.M.; Nolte, R.J.M.; van Kempen, H. Aligned Growth of Calcite Crystals on a Self-Assembled Monolayer. Adv. Mater. 2002, 14, 492–495. [Google Scholar]
- Han, Y.; Aizenberg, J. Effect of Magnesium Ions on Oriented Growth of Calcite on Carboxylic Acid Functionalized Self-Assembled Monolayer. J. Am. Chem. Soc. 2003, 125, 4032–4033. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.J.; Mouhamad, Y.; Meldrum, F.C.; Christenson, H.K. Epitaxy of Calcite on Mica. Cryst. Growth Des. 2010, 10, 734–738. [Google Scholar] [CrossRef]
- Yamanaka, S.; Ito, N.; Shimosaka, A.; Shirakawa, Y.; Hidaka, J. AFM Investigation for the initial growth processes of calcium carbonate on hydrophilic and hhydrophobic substrate. Cryst. Growth Des. 2009, 9, 3245–3250. [Google Scholar] [CrossRef]
- Shelobolina, E.; Xu, H.; Konishi, H.; Kukkadapu, R.; Wu, T.; Blöthe, M.; Roden, E. Microbial Lithotrophic Oxidation of Structural Fe(II) in Biotite. Appl. Environ. Microbiol. 2012, 78, 5746–5752. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Moradian, J.; Shay, E.; Maroudas, N.G.; Weiner, S. A chemical model for the cooperation of sulfates and carboxylates in calcite crystal nucleation: Relevance to biomineralization. Proc. Natl. Acad. Sci. USA 1987, 84, 2732–2736. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg, J.; Albeck, S.; Weiner, S.; Addadi, L. Crystal—Protein interactions studied by overgrowth of calcite on biogenic skeletal elements. J. Cryst. Growth 1994, 142, 156–164. [Google Scholar] [CrossRef]
- Paquette, J.; Reeder, R.J. Relationship between surface structure, growth mechanism, and trace element incorporation in calcite. Geochim. Cosmochim. Acta 1995, 59, 735–749. [Google Scholar] [CrossRef]
- Jiménez-López, C.; Caballero, E.; Huertas, F.J.; Romanek, C.S. Chemical, mineralogical and isotope behavior, and phase transformation during the precipitation of calcium carbonate minerals from intermediate ionic solution at 25 °C. Geochim. Cosmochim. Acta 2001, 65, 3219–3231. [Google Scholar] [CrossRef]
- Becker, A.; Becker, W.; Marxen, J.C.; Epple, M. In-vitro Crystallization of Calcium Carbonate in the Presence of Biological Additives—Comparison of the Ammonium Carbonate Method with Double-Diffusion Techniques. Z. Anorg. Allg. Chem. 2003, 629, 2305–2311. [Google Scholar] [CrossRef]
- Gruzensky, P.M. Growth of calcite crystals. J. Phys. Chem. Solids 1967, 365–367. [Google Scholar]
- Zhang, F.; Xu, H.; Konishi, H.; Roden, E.E. A relationship between d104 value and composition in the calcite-disordered dolomite solid-solution series. Am. Mineral. 2010, 95, 1650–1656. [Google Scholar] [CrossRef]
- Chave, K.E.; Deffeyes, K.S.; Weyl, P.K.; Garrels, R.M.; Thompson, M.E. Observations on the solubility of skeletal carbonates in aqueous solutions. Science 1962, 137, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Berner, R.A. The role of magnesium in the crystal growth of calcite and aragonite from sea water. Geochim. Cosmochim. Acta 1975, 39, 489–494. [Google Scholar] [CrossRef]
- Walter, L.M.; Hanor, S. Effect of orthophosphate on the dissolution kinetics of biogenic magnesian calcites. Geochim. Cosmochim. Acta 1979, 43, 1377–1385. [Google Scholar] [CrossRef]
- Christenson, H.K. Adhesion and surface energy of mica in air and water. J. Phys. Chem. 1993, 97, 12034–12041. [Google Scholar] [CrossRef]
- Xu, L.; Salmeron, M. Effects of surface ions on the friction and adhesion properties of mica. Langmuir 1998, 14, 2187–2190. [Google Scholar] [CrossRef]
- Shtukenberg, A.G.; Popov, D.Y.; Punin, Y.O. Growth ordering and anomalous birefringence in ugrandite garnets. Mineral. Mag. 2005, 69, 537–550. [Google Scholar] [CrossRef]
- Morse, J.W.; Wang, Q.; Tsio, M.Y. Influences of temperature and Mg:Ca ratio on CaCO3 precipitates from seawater. Geology 1997, 25, 85–87. [Google Scholar] [CrossRef]
- Reddy, M.M.; Nancollas, G.H. The crystallization of calcium carbonate. IV. The effect of magnesium, strontium and sulfate ions. J. Cryst. Growth 1976, 35, 33–38. [Google Scholar] [CrossRef]
- Reddy, M.M.; Wang, K.K. Crystallization of calcium carbonate in the presence of metal ions. I. Inhibition by magnesium ions at pH 8.8 and 25 °C. J. Cryst. Growth 1980, 50, 470–480. [Google Scholar] [CrossRef]
- Falini, G.; Gazzano, M.; Ripamonti, A. Crystallization of calcium carbonate in presence of magnesium and polyelectrolytes. J. Cryst. Growth 1994, 137, 577–584. [Google Scholar] [CrossRef]
- Fernandez-Diaz, L.; Putnis, A.; Prieto, M.; Putnis, C.V. The role of magnesium in the crystallization of calcite and aragonite in a porous medium. J. Sediment. Res. 1996, 66, 482–491. [Google Scholar]
- Chen, T.; Neville, A.; Yuan, M. Influence of Mg2+ on CaCO3 formation—Bulk precipitation and surface deposition. Chem. Eng. Sci. 2006, 61, 5318–5327. [Google Scholar] [CrossRef]
- Astilleros, J.M.; Fernandez-Diaz, L.; Putnis, A. The role of magnesium in the growth of calcite: An AFM study. Chem. Geol. 2010, 271, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.H.; Dove, P.M.; De Yoreo, J.J. Kinetics of calcite growth: Surface processes and relationships to macroscopic rate laws. Geochim. Cosmochim. Acta 2000, 64, 2255–2266. [Google Scholar] [CrossRef]
- Hong, M.; Teng, H.H. Implications of solution chemistry effects: Direction-specific restraints on the step kinetics of calcite growth. Geochim. Cosmochim. Acta 2014, 141, 228–239. [Google Scholar] [CrossRef]
- Van Der Weijden, C.H.; Van Der Weijden, R.D. Calcite growth: Rate dependence on saturation, on ratios of dissolved calcium and (bi)carbonate and on their complexes. J. Cryst. Growth 2014, 394, 137–144. [Google Scholar] [CrossRef]
- Sand, K.K.; Tobler, D.J.; Dobberschütz, S.; Larsen, K.K.; Makovicky, E.; Andersson, M.P.; Wolthers, M.; Stipp, S.L.S. Calcite Growth Kinetics: Dependence on Saturation Index, Ca2+:CO32− Activity Ratio, and Surface Atomic Structure. Cryst. Growth Des. 2016, 16, 3602–3612. [Google Scholar] [CrossRef]
- Raz, S.; Weiner, S.; Addadi, L. Formation of high-magnesian calcites via an amorphous precursor phase: Possible biological implications. Adv. Mater. 2000, 12, 38–42. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, C.; Teng, H.H.; Roden, E.E.; Xu, H. In situ AFM observations of Ca-Mg carbonate crystallization catalyzed by dissolved sulfide: Implications for sedimentary dolomite formation. Geochim. Cosmochim. Acta 2013, 105, 44–55. [Google Scholar] [CrossRef]













| Difference Values for Biotite and Muscovite | |||||
|---|---|---|---|---|---|
| Substrate | a-Axis (Å) | b-Axis (Å) | Angle (°) | Δ Area (Å2) | Overall |
| Biotite | 0.348 | 0.57 | 0 | 6.3% | 14% |
| Muscovite | 0.201 | 0.40 | 0 | 4.0% | 9% |
| Hematite | 0.050 | 0.07 | 0 | 1.0% | 2% |
| Mg/Ca Ratio | Calcite % (±1%) | MgCO3 % (±2%) |
|---|---|---|
| 0 | 95.0% | 0% |
| 1 | 45.1% | 5.2% |
| 2 | 10.9% | 8.4% |
| 5 | 2.4% | 18.7% |
| 6 | 0.0% | N/A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Zhou, M.; Fang, Y.; Teng, H.H. Effect of Mica and Hematite (001) Surfaces on the Precipitation of Calcite. Minerals 2018, 8, 17. https://doi.org/10.3390/min8010017
Xu H, Zhou M, Fang Y, Teng HH. Effect of Mica and Hematite (001) Surfaces on the Precipitation of Calcite. Minerals. 2018; 8(1):17. https://doi.org/10.3390/min8010017
Chicago/Turabian StyleXu, Huifang, Mo Zhou, Yihang Fang, and H. Henry Teng. 2018. "Effect of Mica and Hematite (001) Surfaces on the Precipitation of Calcite" Minerals 8, no. 1: 17. https://doi.org/10.3390/min8010017