3.1. Raw Materials
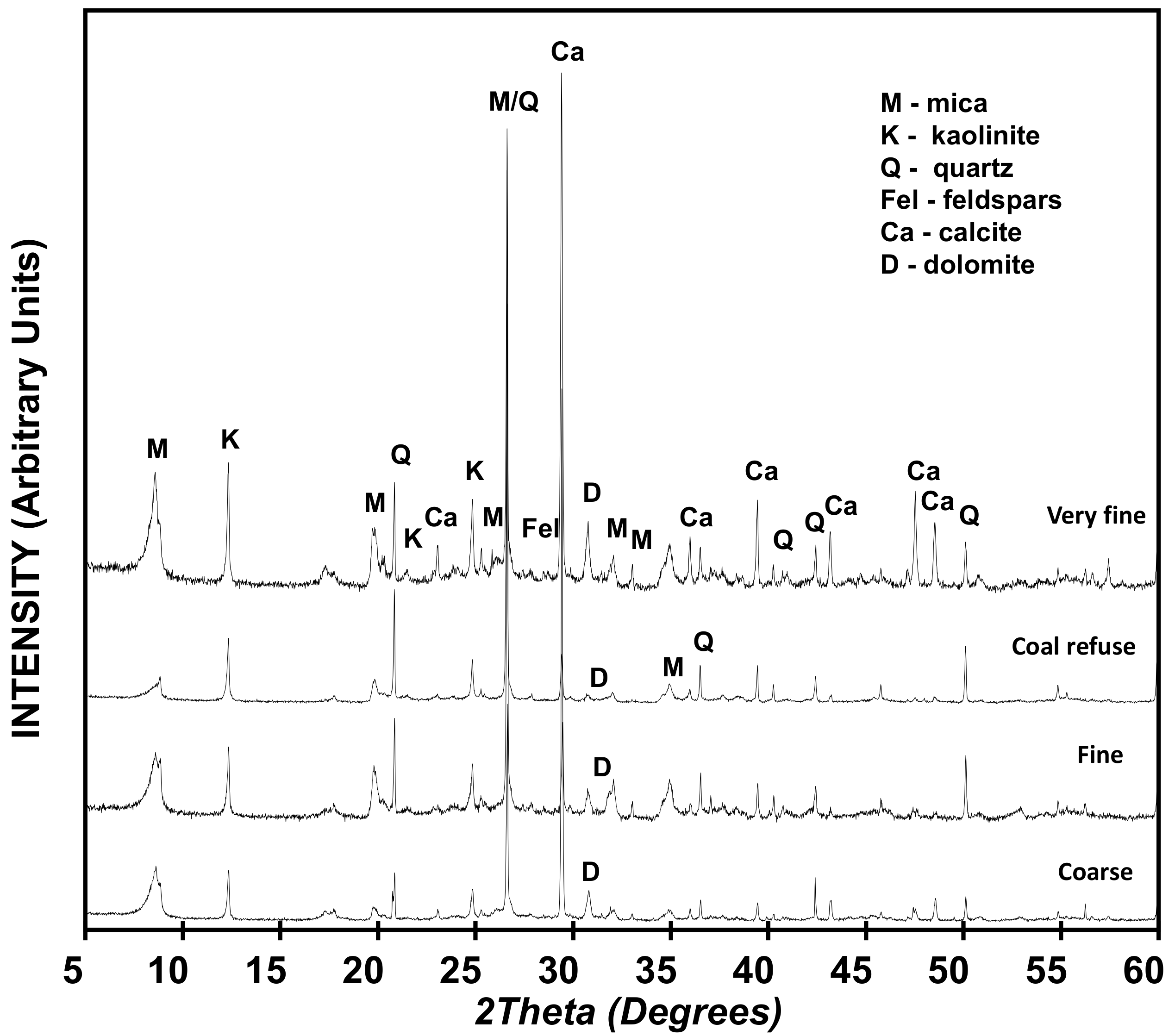
The mineralogical characterization of the four raw tailings samples by XRD (
Figure 3) identified a major presence of phyllosilicates (mica and kaolinite) accompanied by quartz, calcite, dolomite, and feldspars. Given that the phyllosilicates are responsible for the pozzolanic activity, a decisive property in the use of pozzolan materials is found in the very fine-grade samples with the highest content of micas and kaolinite (48%), as opposed to the coal refuse with a lower content of those minerals (24%). The other two tailing samples had intermediate values in relation to the rest of the mineral components. Trace concentrations of feldspars were found in all samples with variable quantities of calcite, dolomite, and quartz, depending on each particular one (
Table 1). Silicate and aluminium-silicate minerals were the dominant elements in all four samples. The granulometry of the samples is given in
Table 2, and it can be deduced that the coal refuse and very fine tailings samples are those with the finest granulometry including clay and silt.
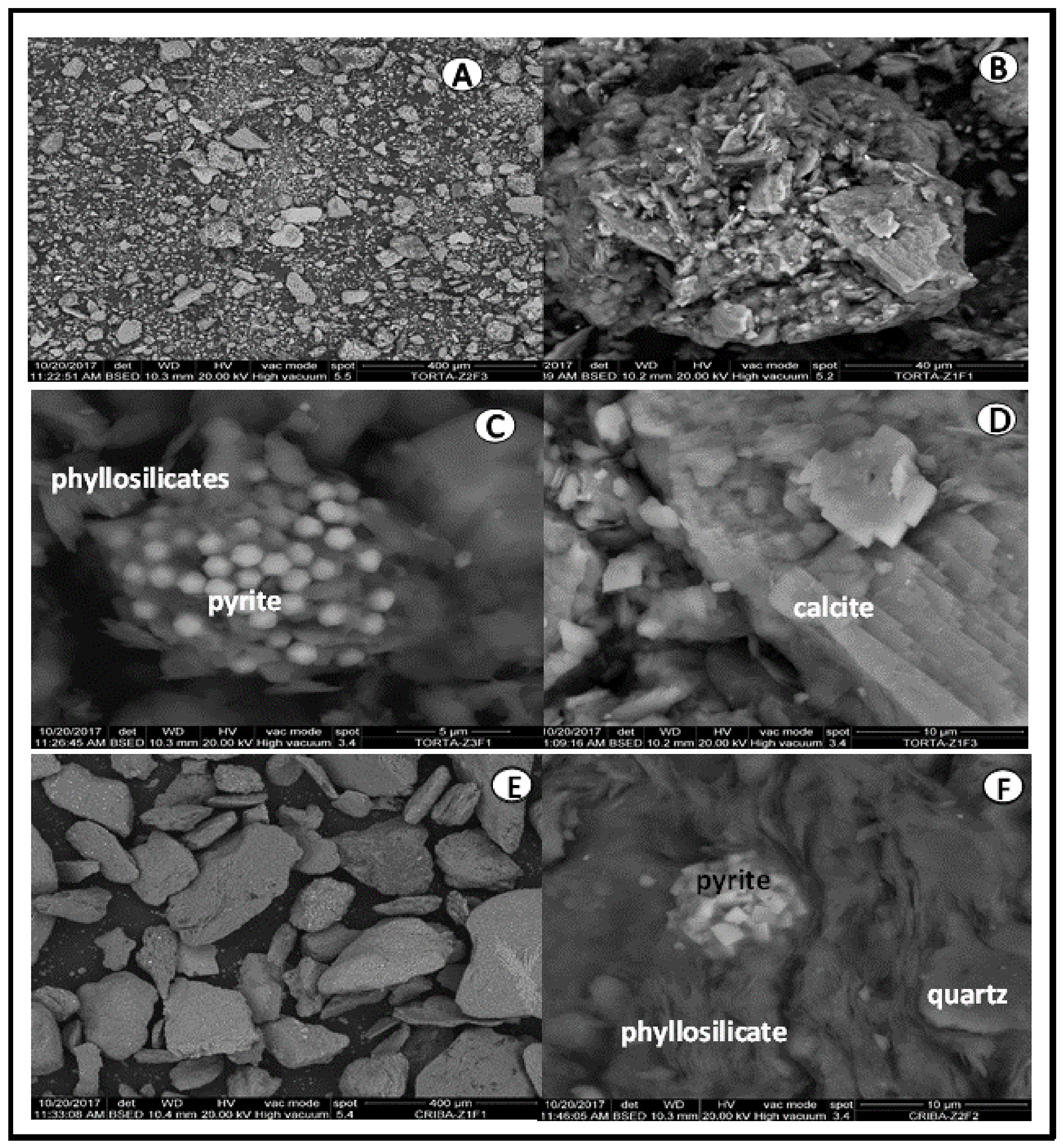
SEM/EDX analysis of the very fine-grade raw tailings sample indicated the presence of variable sizes of aggregates (
Figure 4A). The irregular aggregate surfaces (
Figure 4B) are filled with pores and cracks that assist their disaggregation. Idiomorphic pyrite crystals were identified in the gaps of the phyllosilicate layers, in dispersed and concentrated forms forming clusters (
Figure 4C). The aggregates consisted of calcite, quartz, and phyllosilicates (
Figure 4D and
Table 3) [
17,
34]. The grain sizes of the fine tailings were of greater uniformity (
Figure 4E) and of larger size than those of the very fine grade sample, with phyllosilicate and quartz aggregates and pyrite crystals in the gaps between the layers of phyllosilicate (
Figure 4F). The aggregates were, in general, of a compact appearance.
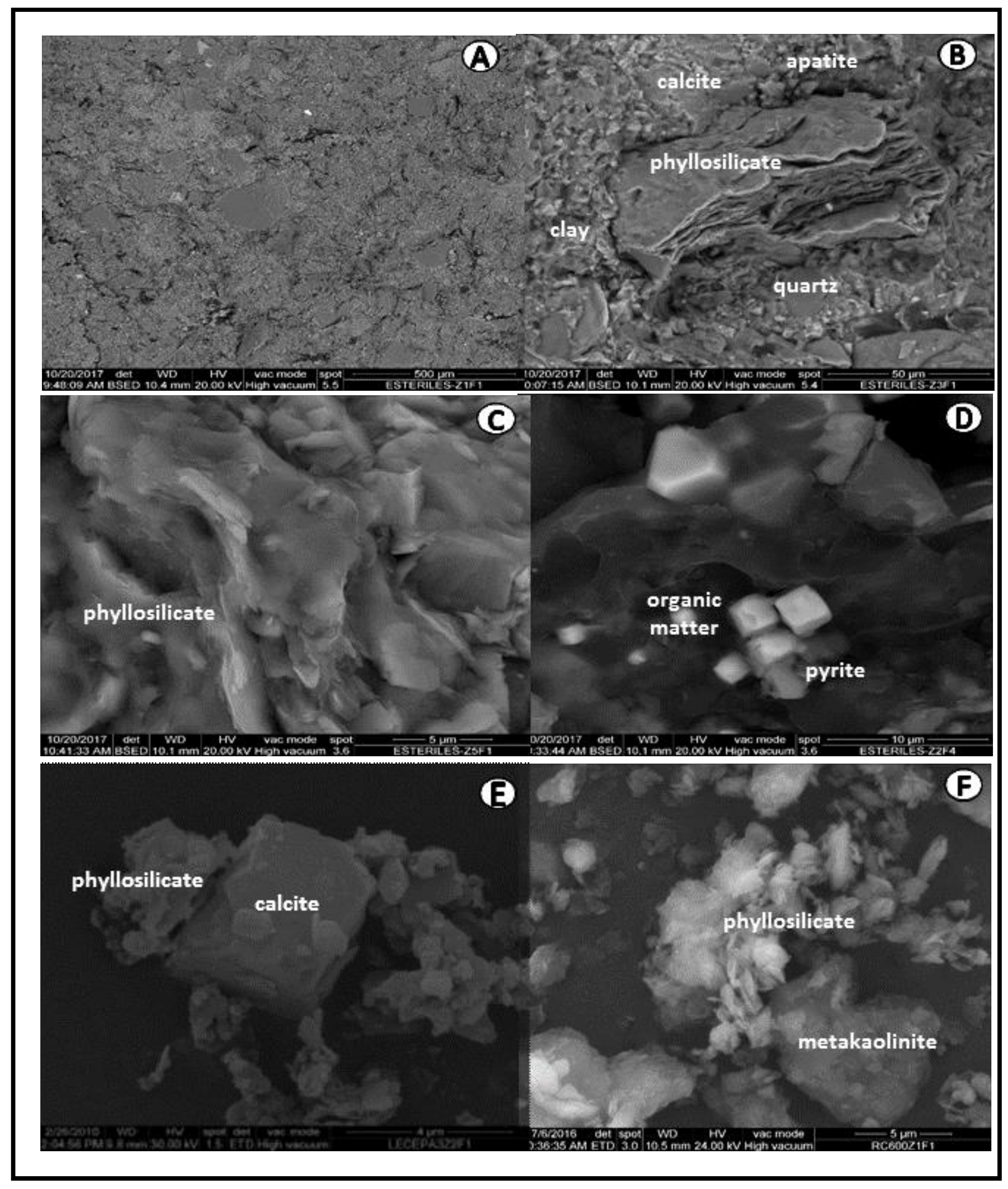
In turn, the presence of very compact aggregates in the coal-refuse samples were generally formed of phyllosilicates, calcite, and quartz (
Figure 5A). Phosphates, iron, calcium, and magnesium accumulated in disordered and random concentrations mainly in the clayey aggregates (
Figure 5B and
Table 4). These varied concentrations occur due to the undulatory surface forms of the phyllosilicates (
Figure 5C), where fractures and voids facilitate their deposition (
Figure 5D). The surface cavities of the aggregates were filled with compacted organic matter, and disorderly surface deposits of pyrite crystals were noted elsewhere [
15,
17].
The chemical composition of the four raw tailings samples (
Table 5) indicated that they were all of a silico-aluminous nature with percentages (SiO
2 + Al
2O
3) of between 50% and 70% of the total weight. There was a practically constant presence of iron oxide in all of them, and a very variable quantity of CaO (the fine-grade with the highest percentage—16.20%—and the coal refuse with the lowest—3.84%), as well as loss on ignition (very fine-grade with the highest weight loss—26.47%—and the mining refuse with the lowest—15.18%). MnO was practically constant in all the samples, fluctuating between 0.87% and 0.59%. The alkaline oxides (Na
2O + K
2O) were approximately 2.46% in the very fine–grade samples and 2.87% in the mining refuse. Higher concentrations of SO
3 were found in the fine-grade raw tailings (0.92%), and the lowest concentrations were once again found in the coal-refuse sample (0.27%). Finally, concentrations of P
2O
5 were at their highest in the very fine–grade samples (0.26%) and lowest in the coal refuse (0.13%). The very fine raw tailings had the highest content of organic carbon (24.27%), while the lowest content was found in the coal refuse (16.04%).
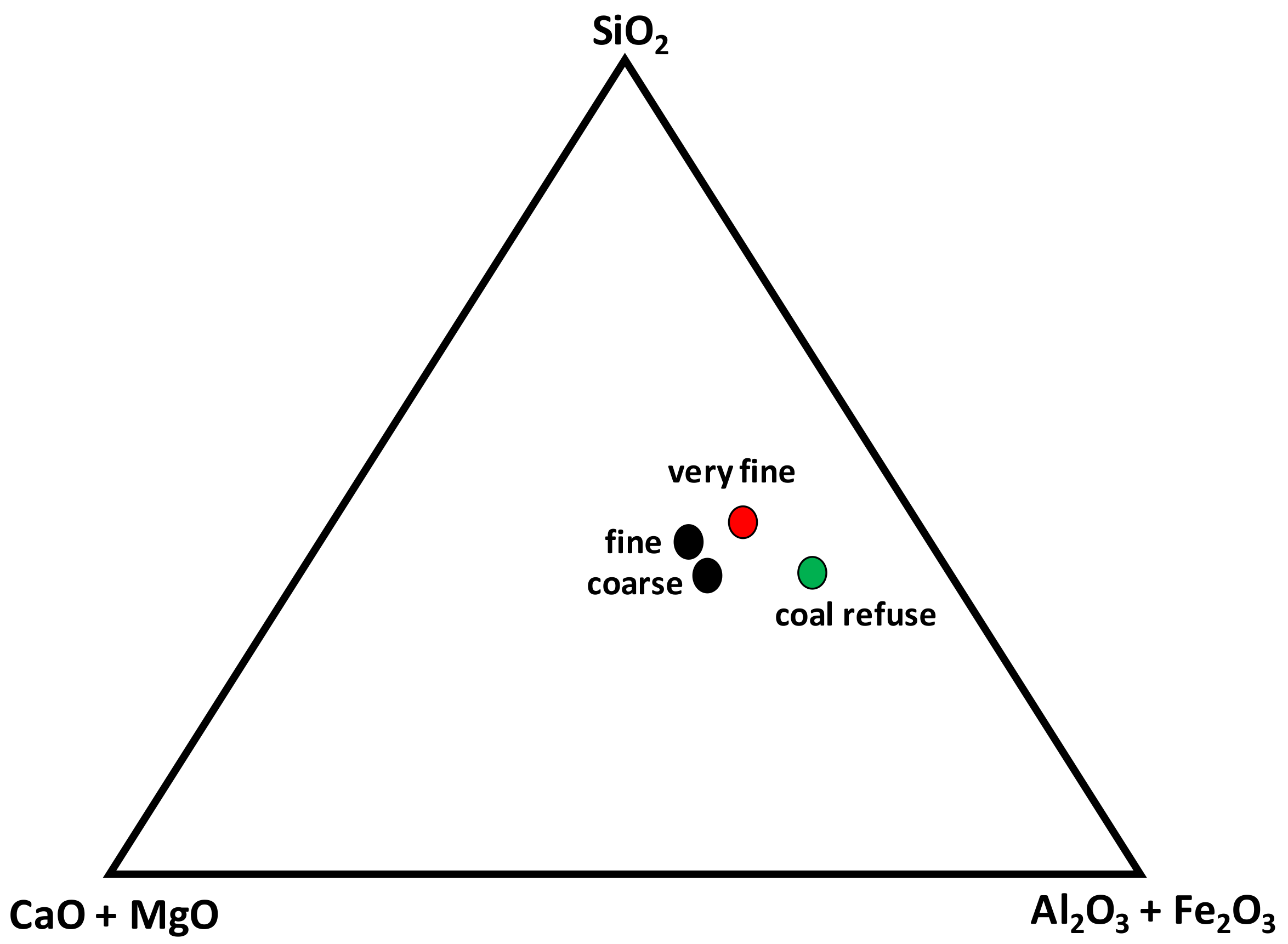
For a better understanding, and following Gorakhki and Bareither [
22], the triangular diagram is represented, showing the affinity of the coarse and fine tailings samples and the separation of the rest of the samples (very fine tailings and coal refuse) where the concentrations of silicon and aluminium are the major (
Figure 6).
Trace elements of Ni, Cr, V, Zn, Pb, As, Co, and Mo were found in concentrations lower than 200 mg/L (
Table 6) that reportedly [
36] have a direct influence on the rheological properties of the pozzolanic cements.
The chemical analyses indicated that the most appropriate sample for use as a coal-mining pozzolan waste would be the very fine-grade raw tailings samples with a granulometry of ≤0.05 mm, following flotation treatment of the tailings. Nevertheless, this product requires pre-flotation treatment after extraction, as previously mentioned, and low volumes are generated following the treatment process, in contrast with the amounts in the coal refuse.
3.2. Thermally Activated Materials
The raw tailings samples were calcinated within a thermal range of 500 °C to 900 °C after two hours in an electric furnace, which should transform the K into MK and simultaneously dehydroxylate the micas. The intention was to confirm this hypothesis, given that the most reactive state of the phyllosilicates occurs when the material is found in a dehydroxylated form due to loss of the hydroxyl groups by calcination [
37].
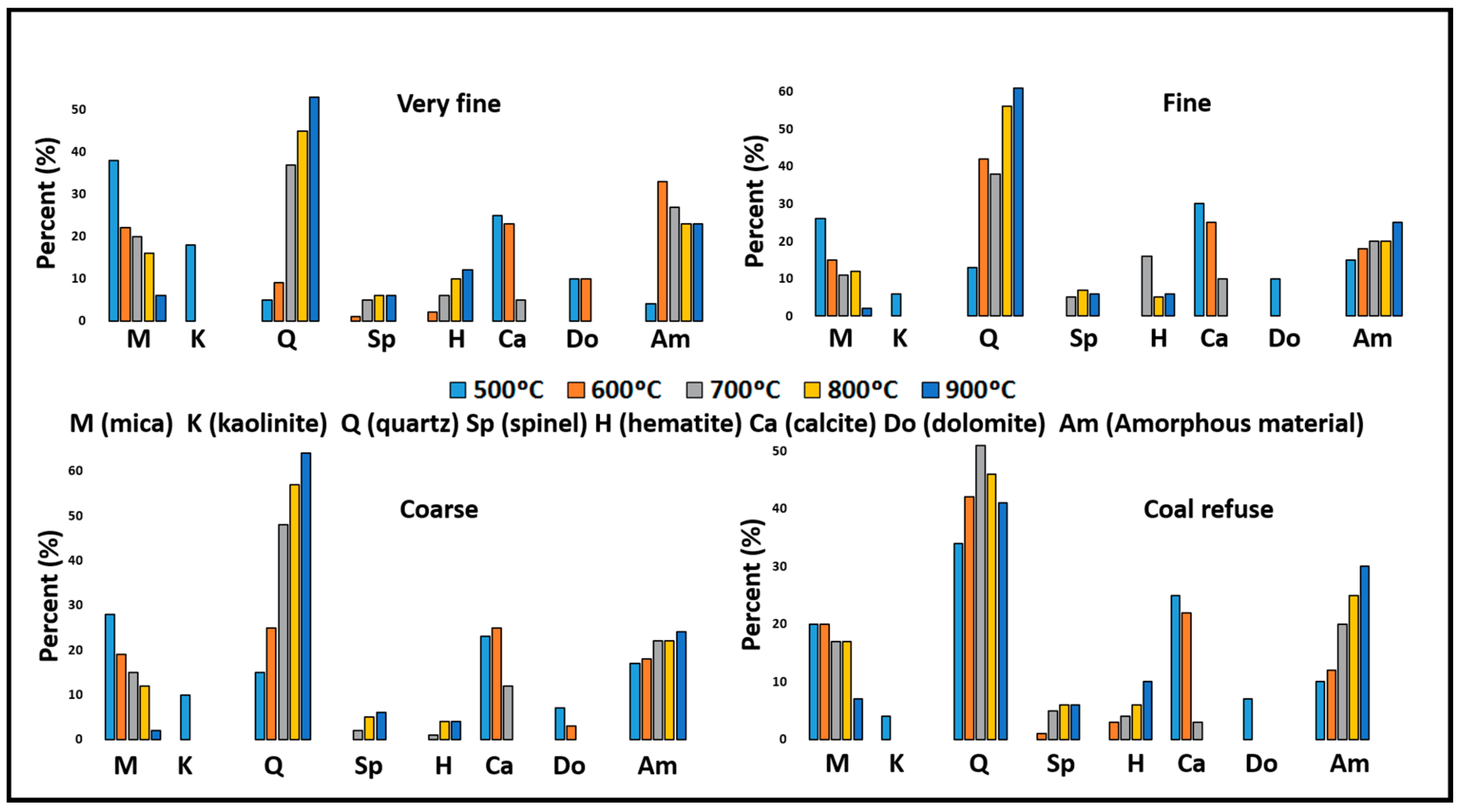
With the increase of temperature, the K loses crystallinity as well as the micas, which becomes an increase of amorphous materials. By XRD, the mineralogical composition of the calcinated samples a different temperatures are shown in
Figure 7. In all samples, the major material at 500 °C is the mica, except in the fine sample. The same happens with calcite, which decreases when the temperature is high, presenting a similar amount in the initial stages of calcination.
The highest concentration of K is identified in the very fine sample. The transformation both of the kaolinite at 600 °C (into MK unidentifiable by XRD due to its amorphousness) and of the dolomite was noted, as was the disappearance of the calcite at 800 °C.
On the other hand, the concentration of quartz increase in all samples with a decrease in coal refuse, compensated by the amorphous materials. Hematite increases in all raw tailing samples, except in fine sample. In addition, spinel is a new mineral that appears at 600 °C because of the thermal treatment of the aluminous minerals of the clay [
12,
38].
A temperature of 600 °C applied over 2 h was selected as the ideal temperature for thermal activation of the coal waste, thereby economizing on energy. At this temperature, all K is transformed into MK.
Given these results, and given the volume of waste for which no treatment is required, the coal refuse was chosen for its easy handling and provisioning, which saves the basic material.
For a better certainty in the election, the coal refuse was analyzed by SEM/EDX. These observation shows the disaggregation of the aggregates due to the destruction of the organic matter. The appearance was noted of MK laminar aggregates with surface deposits of sulphur, potassium, and calcium, as well as aggregates with frayed laminates from the partial dehydroxylation of the remaining phyllosilicates (
Figure 5E and
Table 7).
Pozzolanic properties are the fundamental property in industrial byproducts that are to be used as an active cement additive.
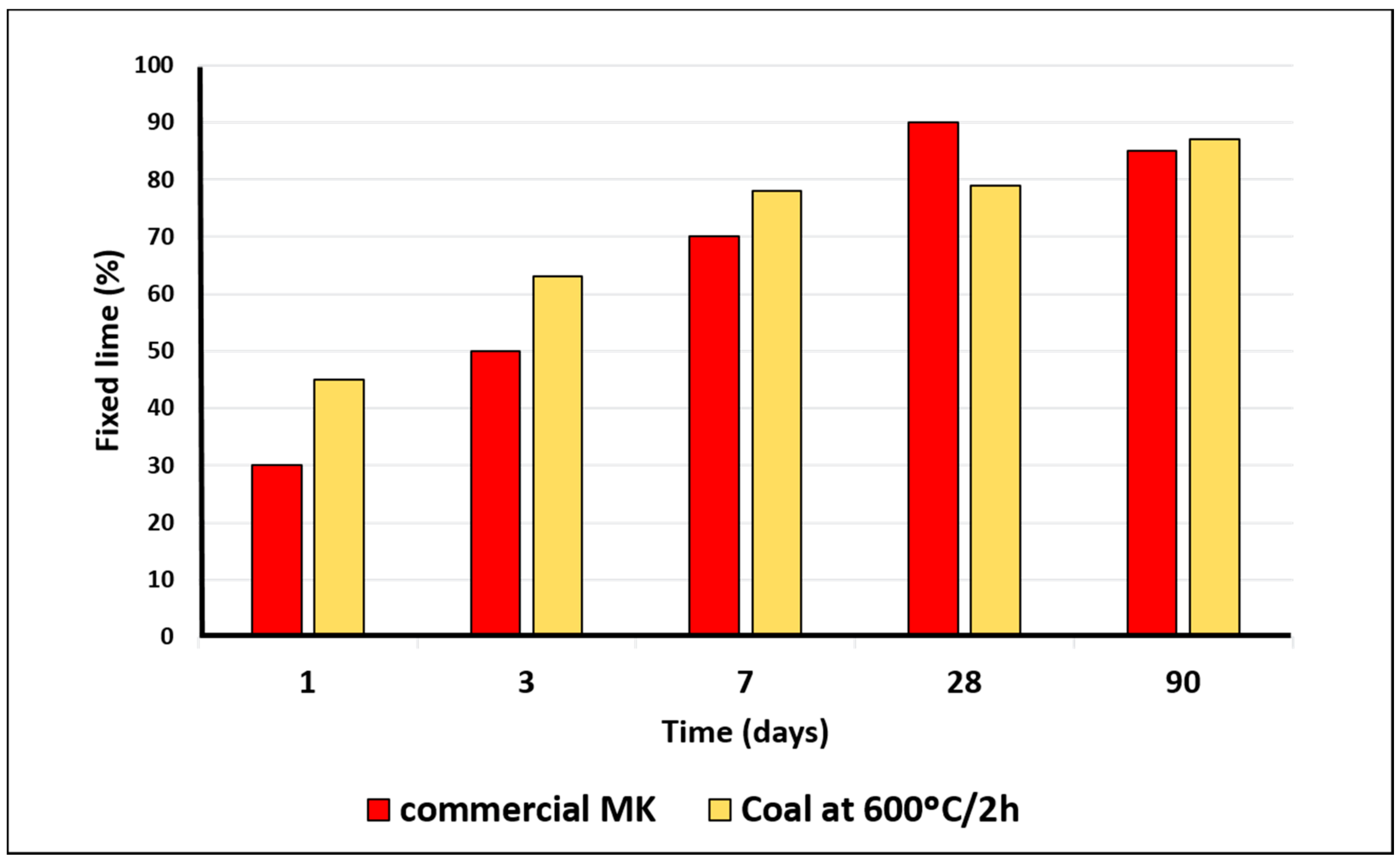
Figure 8 shows the graph of coal-refuse (overburden, waste rock, and occasional low-grade mineral waste) pozzolanic activity, activated at 600 °C/2 h over 90 days of reaction time. The analytic results, expressed as percentages of fixed lime, indicate the high pozzolanic activity of the coal refuse activated at 600 °C. This value is similar than the obtained with an available commercial MK in UK. The commercial MK is mayor oxides of SiO
2 (51.60%) and Al
2O
3 (41.30%), and a surface area of 15.0 m
2/g [
39] at all ages (except at 28 days).The mineralogical composition of commercial K was principally kaolinite and mica. Calcination at 600 °C/2 h produced total dehydroxylation of the K transforming it into MK, a highly pozzolanic product. Under these activation conditions, the mica showed an incipient state of dehydroxylation [
40].