Vanadium Transitions during Roasting-Leaching Process of Vanadium Extraction from Stone Coal
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Procedure
2.3. Measurement Methods
3. Results
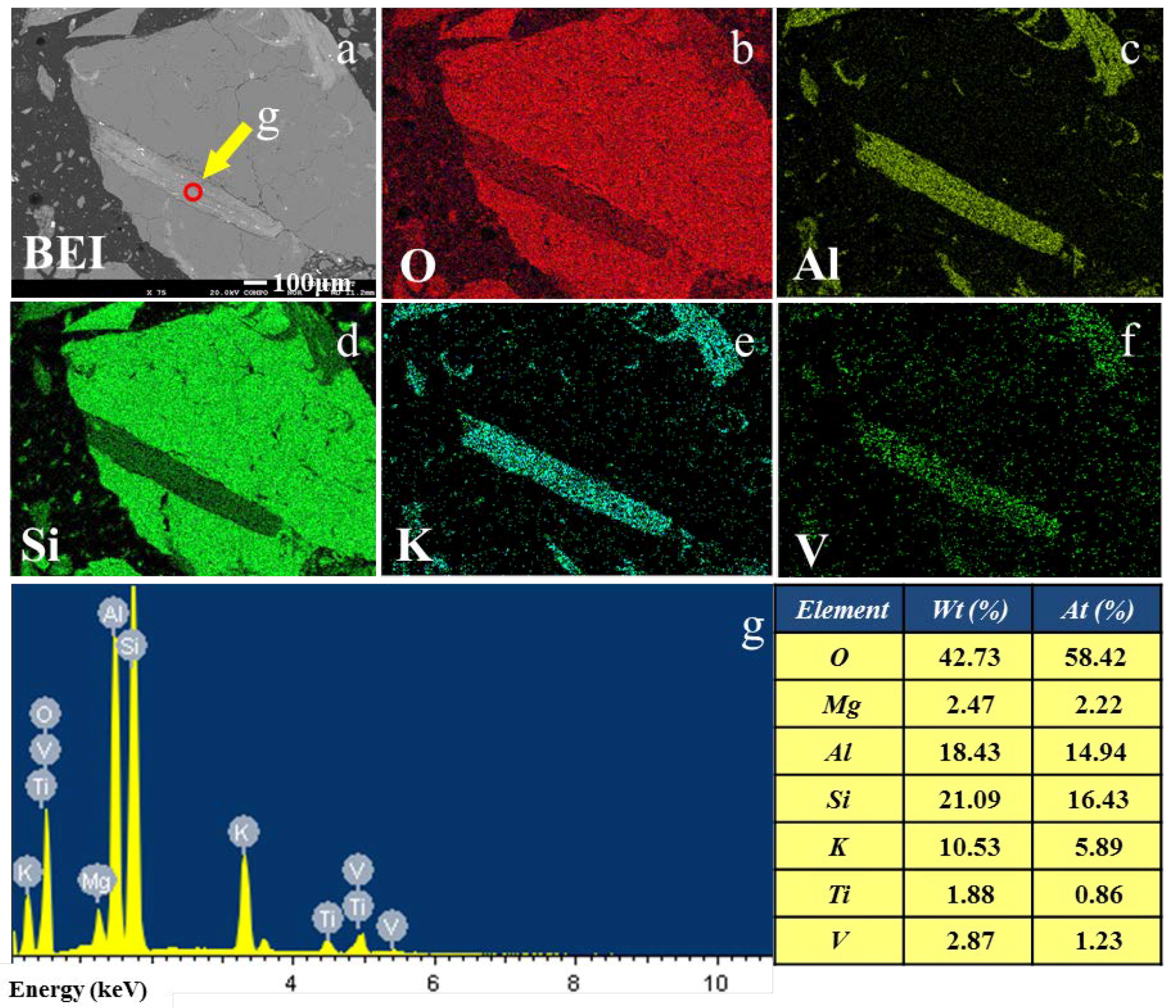
3.1. Vanadium Occurrence in Decarbonized Stone Coal
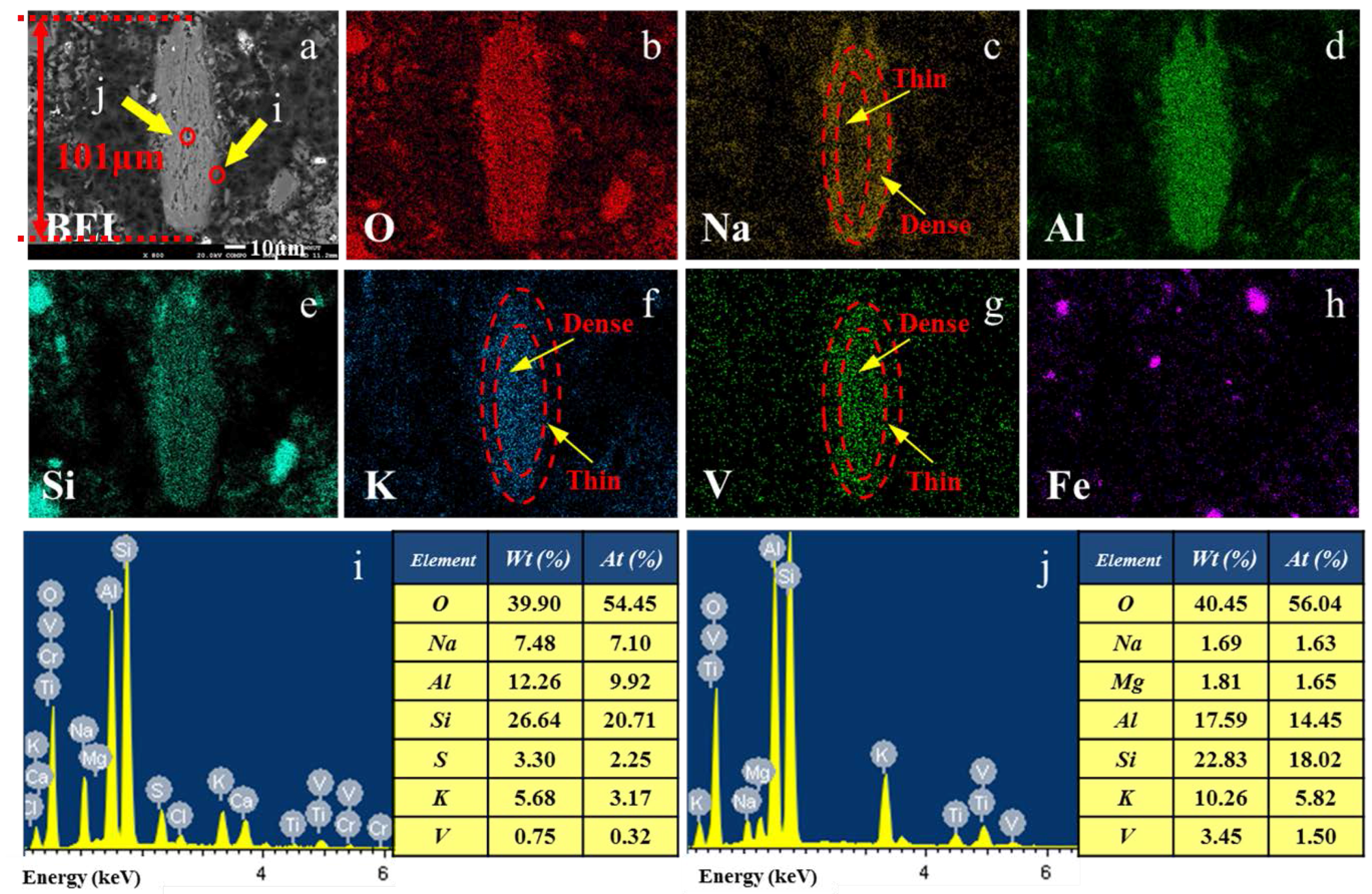
3.2. Vanadium Occurrence in Water Leaching Residue
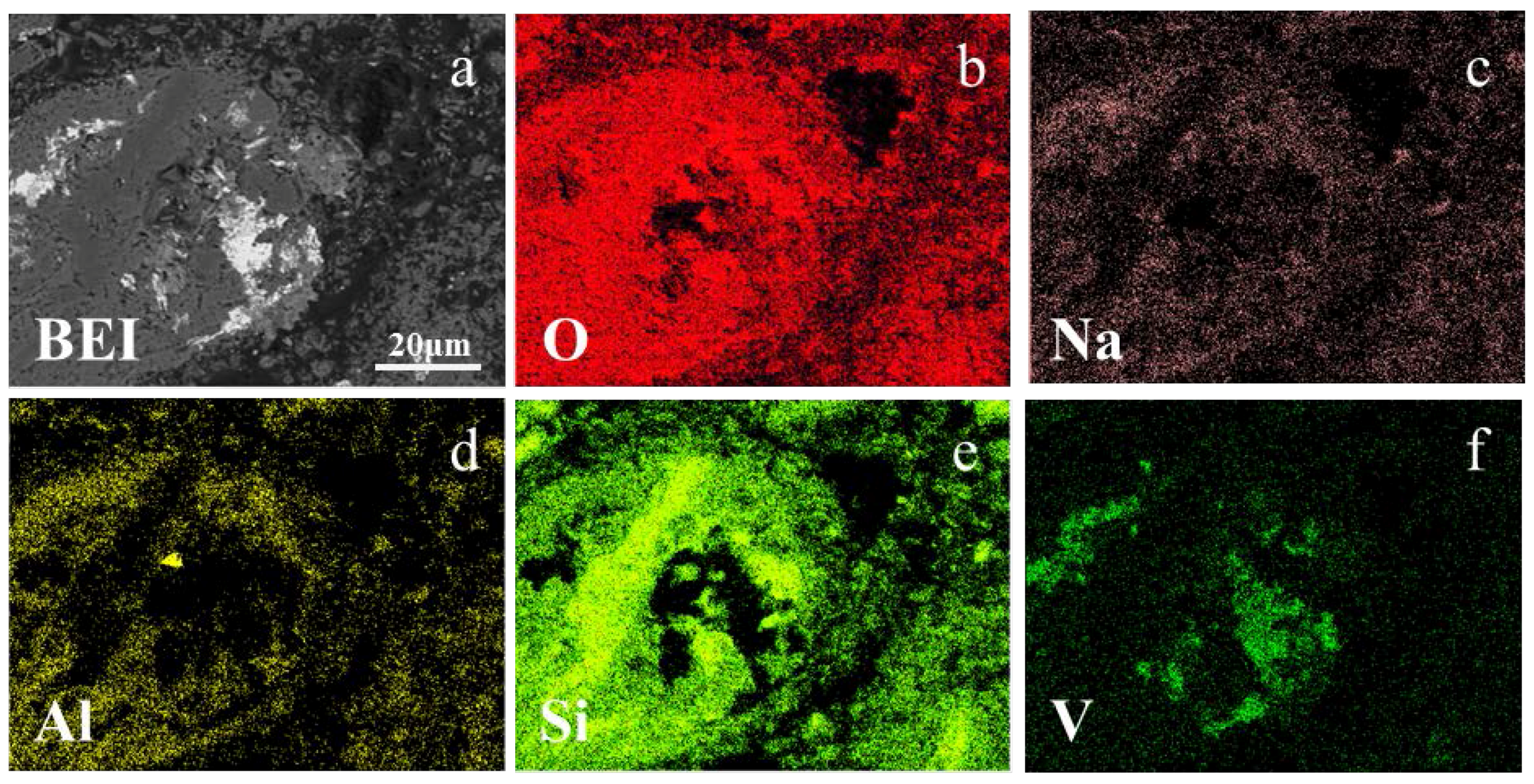
3.3. Vanadium Occurrence in Acid Leaching Residue
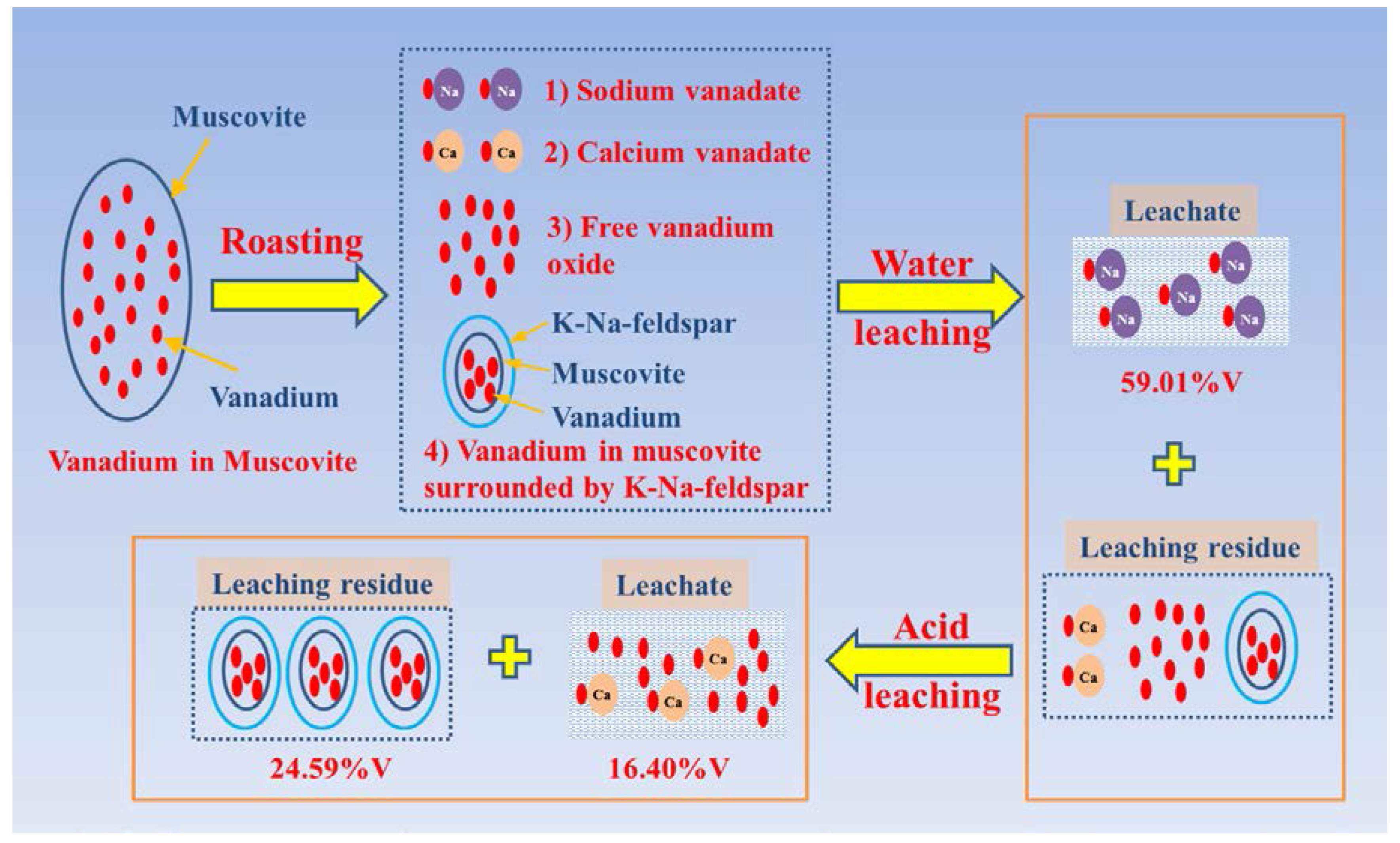
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mirazimi, S.M.J.; Rashchi, F.; Saba, M. A new approach for direct leaching of vanadium from LD converter slag. Chem. Eng. Res. Des. 2015, 94, 131–140. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Bao, S.; Chen, T.; Xiang, L. Effect of Stone Coal Chemical Composition on Sintering Behavior during Roasting. Ind. Eng. Chem. Res. 2014, 53, 157–163. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Bao, S.; Chen, T.; Han, J. Calculation of mineral phase and liquid phase formation temperature during roasting of vanadium-bearing stone coal using FactSage software. Int. J. Miner. Process. 2013, 124, 150–153. [Google Scholar] [CrossRef]
- Cai, Z.; Feng, Y.; Li, H.; Du, Z.; Liu, X. Co-recovery of manganese from low-grade pyrolusite and vanadium from stone coal using fluidized roasting coupling technology. Hydrometallurgy 2013, 131–132, 40–45. [Google Scholar] [CrossRef]
- Cai, Z.; Feng, Y.; Li, H.; Zhou, Y. Selective separation and extraction of Vanadium(IV) and Manganese(II) from co-leaching solution of roasted stone coal and pyrolusite via solvent extraction. Ind. Eng. Chem. Res. 2013, 52, 13768–13776. [Google Scholar] [CrossRef]
- Liang, L.; Bao, S.; Zhang, Y.; Tang, Y. Separation and recovery of V(IV) from sulfuric acid solutions containing Fe(III) and Al(III) using bis(2-ethylhexyl)phosphoric acid impregnated resin. Chem. Eng. Res. Des. 2016, 111, 109–116. [Google Scholar] [CrossRef]
- Ye, P.; Wang, X.; Wang, M.; Fan, Y.; Xiang, X. Recovery of vanadium from stone coal acid leaching solution by coprecipitation, alkaline roasting and water leaching. Hydrometallurgy 2012, 117–118, 108–115. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Deng, Z.; Li, M.; Li, C.; Fan, G. Selective solvent extraction of vanadium over iron from a stone coal/black shale acid leach solution by D2EHPA/TBP. Hydrometallurgy 2011, 105, 359–363. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Liu, T.; Huang, J.; Wang, Y. Comparison of ion exchange and solvent extraction in recovering vanadium from sulfuric acid leach solutions of stone coal. Hydrometallurgy 2013, 131–132, 1–7. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Song, S.; Chen, T.; Bao, S. Behaviors of impurity elements Ca and Fe in vanadium-bearing stone coal during roasting and its control measure. Int. J. Miner. Process. 2016, 148, 100–104. [Google Scholar] [CrossRef]
- Li, M.; Wei, C.; Fan, G.; Li, C.; Deng, Z.; Li, X. Extraction of vanadium from black shale using pressure acid leaching. Hydrometallurgy 2009, 98, 308–313. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Jing, H.; Tao, L.; Yi, W. A kinetics study of multi-stage counter-current circulation acid leaching of vanadium from stone coal. Int. J. Miner. Process. 2012, 114–117, 1–6. [Google Scholar] [CrossRef]
- Hu, Y.J.; Zhang, Y.M.; Bao, S.X.; Liu, T. Effects of the mineral phase and valence of vanadium on vanadium extraction from stone coal. Int. J. Miner. Mater. 2012, 19, 893–898. [Google Scholar] [CrossRef]
- Wang, M.; Xiang, X.; Zhang, L.; Xiao, L. Effect of vanadium occurrence state on the choice of extracting vanadium technology from stone coal. Rare Met. 2008, 27, 112–115. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Liu, T.; Chen, T.; Bian, Y.; Bao, S. Pre-concentration of vanadium from stone coal by gravity separation. Int. J. Miner. Process. 2013, 121, 1–5. [Google Scholar] [CrossRef]
- Ferrovanadium-Determination of vanadium content-The ammonium ferrous sulfate titrimetric method; GBT 8704.5–2007; Bureau of Quality and Technical Supervision of China: Beijing, China, 2007.
- Determination of vanadium in coal; GBT19226–2003; Bureau of Quality and Technical Supervision of China: Beijing, China, 2003.
- Lin, H.; Fan, B. Study on mechanism of phase transformation during roasting and extracting vanadium from fangshankou bone coal. Chin. J. Rare Met. 2001, 25, 273–277. [Google Scholar]
- Shu, B.; Wang, Z.; Li, Q.; Zhang, Y. Review of vanadium extraction from stone coal by roasting technique with sodium chloride and calcium oxide. Chin. J. Rare Met. 2010, 34, 291–297. [Google Scholar]
- Chen, T.J.; Qiu, G.Z.; Zhu, D.Q. Experiment study on new technology of extracting vanadium from stone coal with cyclic oxidation. J. China Coal Soc. 2008, 33, 454–458. [Google Scholar]
- Zhao, Y.; Wang, W.; Zhang, Y.; Song, S.; Bao, S. In-situ investigation on mineral phase transition during roasting of vanadium-bearing stone coal. Adv. Powder Technol. 2017, 28, 1103–1107. [Google Scholar] [CrossRef]
- Li, M.; Wei, C.; Qiu, S.; Zhou, X.; Li, C.; Deng, Z. Kinetics of vanadium dissolution from black shale in pressure acid leaching. Hydrometallurgy 2010, 104, 193–200. [Google Scholar] [CrossRef]
- Song, S.; Campos-Toro, E.F.; López-Valdivieso, A. Formation of micro-fractures on an oolitic iron ore under microwave treatment and its effect on selective fragmentation. Powder Technol. 2013, 243, 155–160. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Chen, L.; Yi, H.; Zhang, Y.; Song, S.; Bao, S. Vanadium Transitions during Roasting-Leaching Process of Vanadium Extraction from Stone Coal. Minerals 2018, 8, 63. https://doi.org/10.3390/min8020063
Zhao Y, Chen L, Yi H, Zhang Y, Song S, Bao S. Vanadium Transitions during Roasting-Leaching Process of Vanadium Extraction from Stone Coal. Minerals. 2018; 8(2):63. https://doi.org/10.3390/min8020063
Chicago/Turabian StyleZhao, Yunliang, Licai Chen, Hao Yi, Yimin Zhang, Shaoxian Song, and Shenxu Bao. 2018. "Vanadium Transitions during Roasting-Leaching Process of Vanadium Extraction from Stone Coal" Minerals 8, no. 2: 63. https://doi.org/10.3390/min8020063




