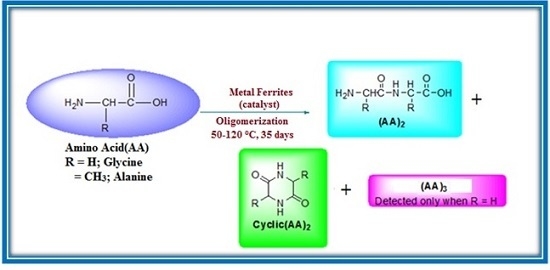
Thermal Condensation of Glycine and Alanine on Metal Ferrite Surface: Primitive Peptide Bond Formation Scenario
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.1.1. Materials
2.1.2. Preparation of Metal Ferrites
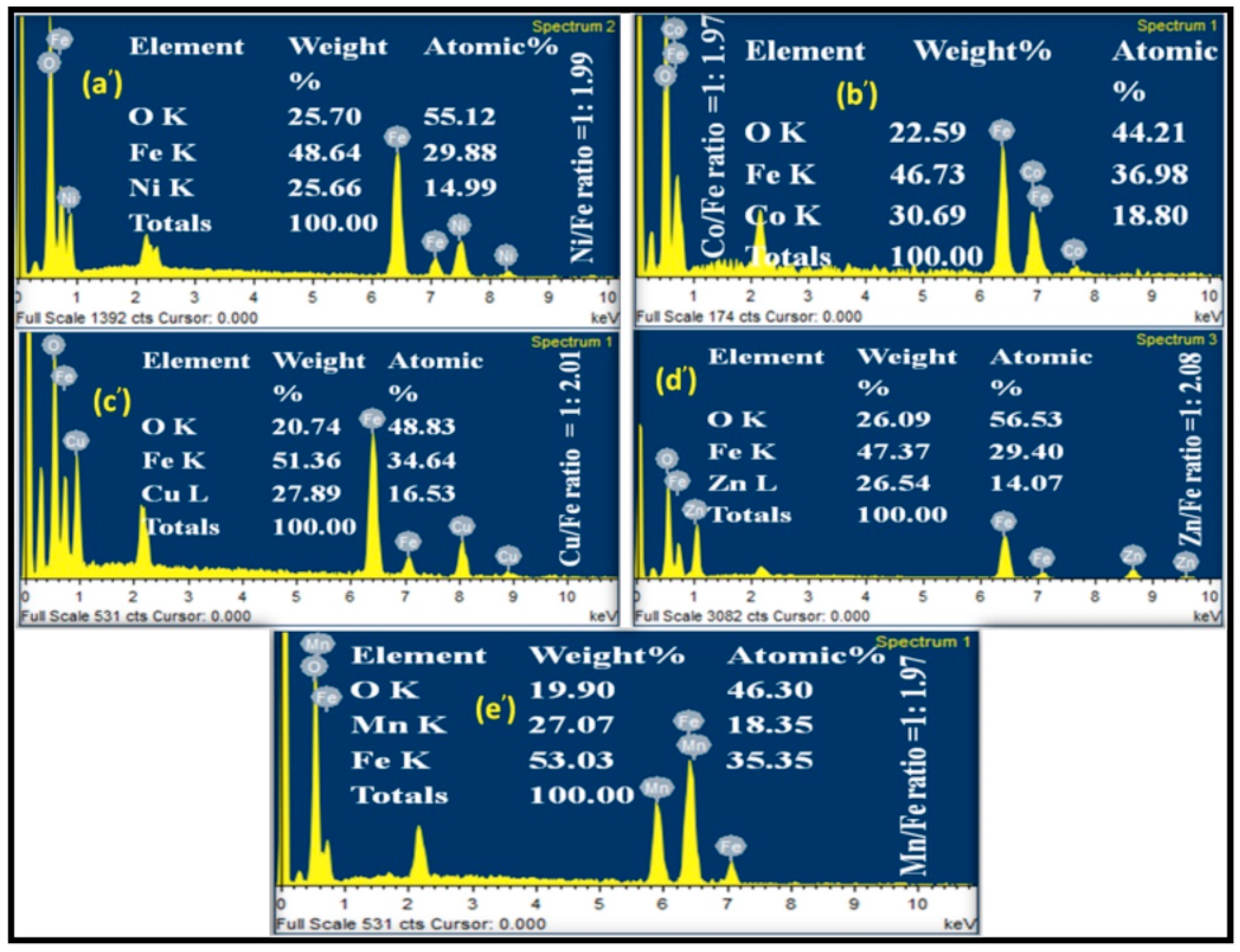
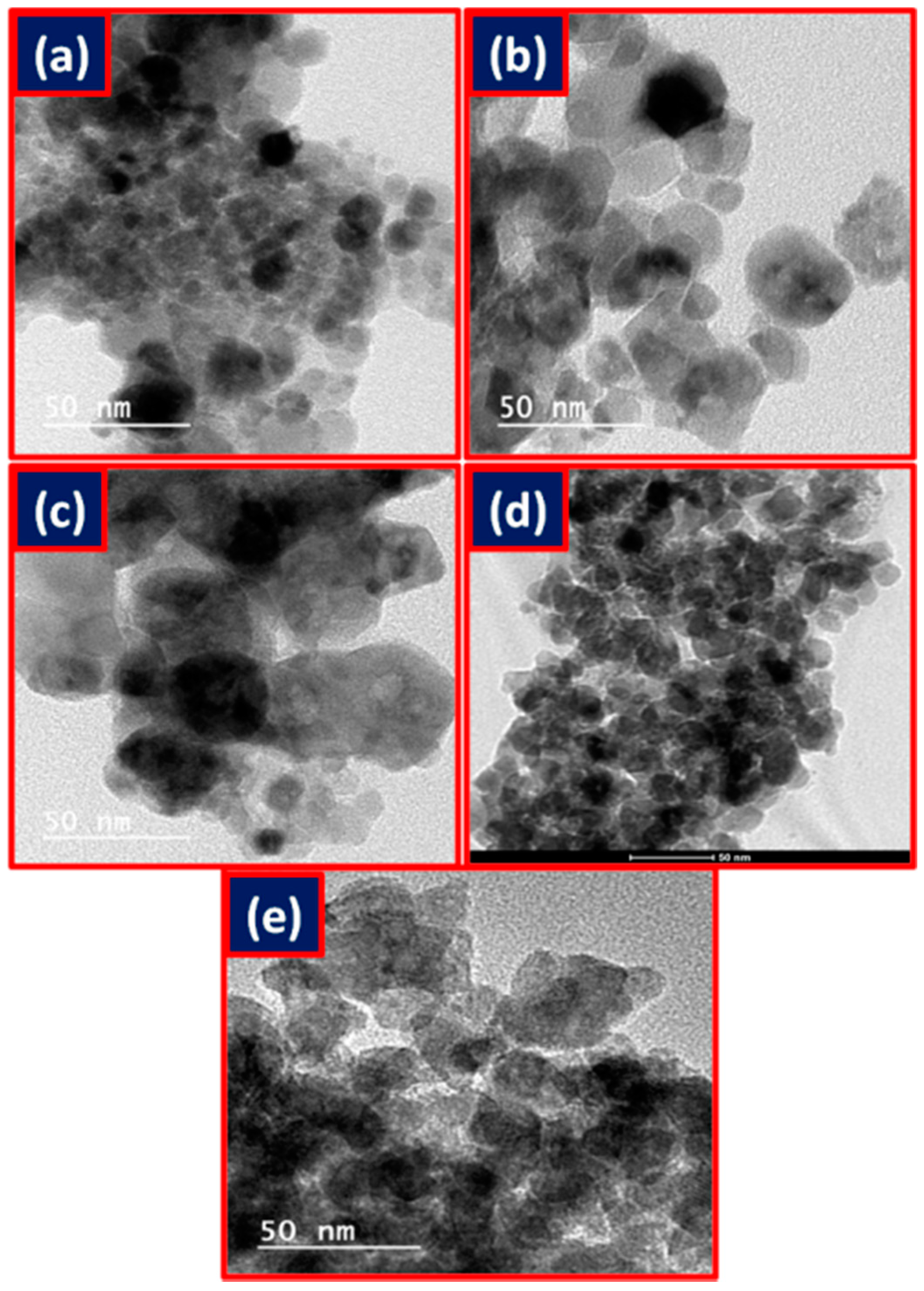
2.2. Characterization of Metal Ferrites
2.3. Oligopeptide Synthesis Protocol from Amino Acid Monomer
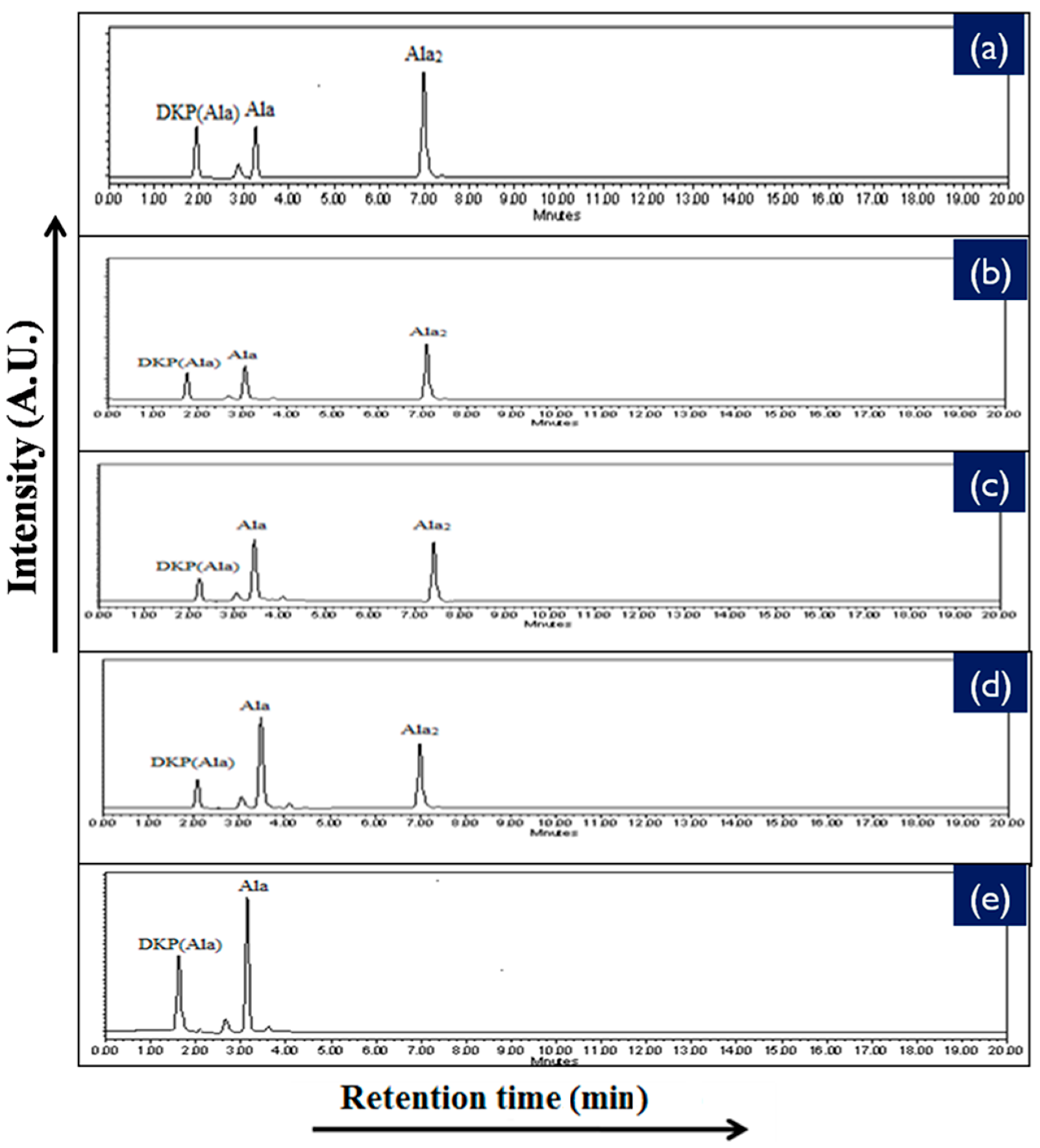
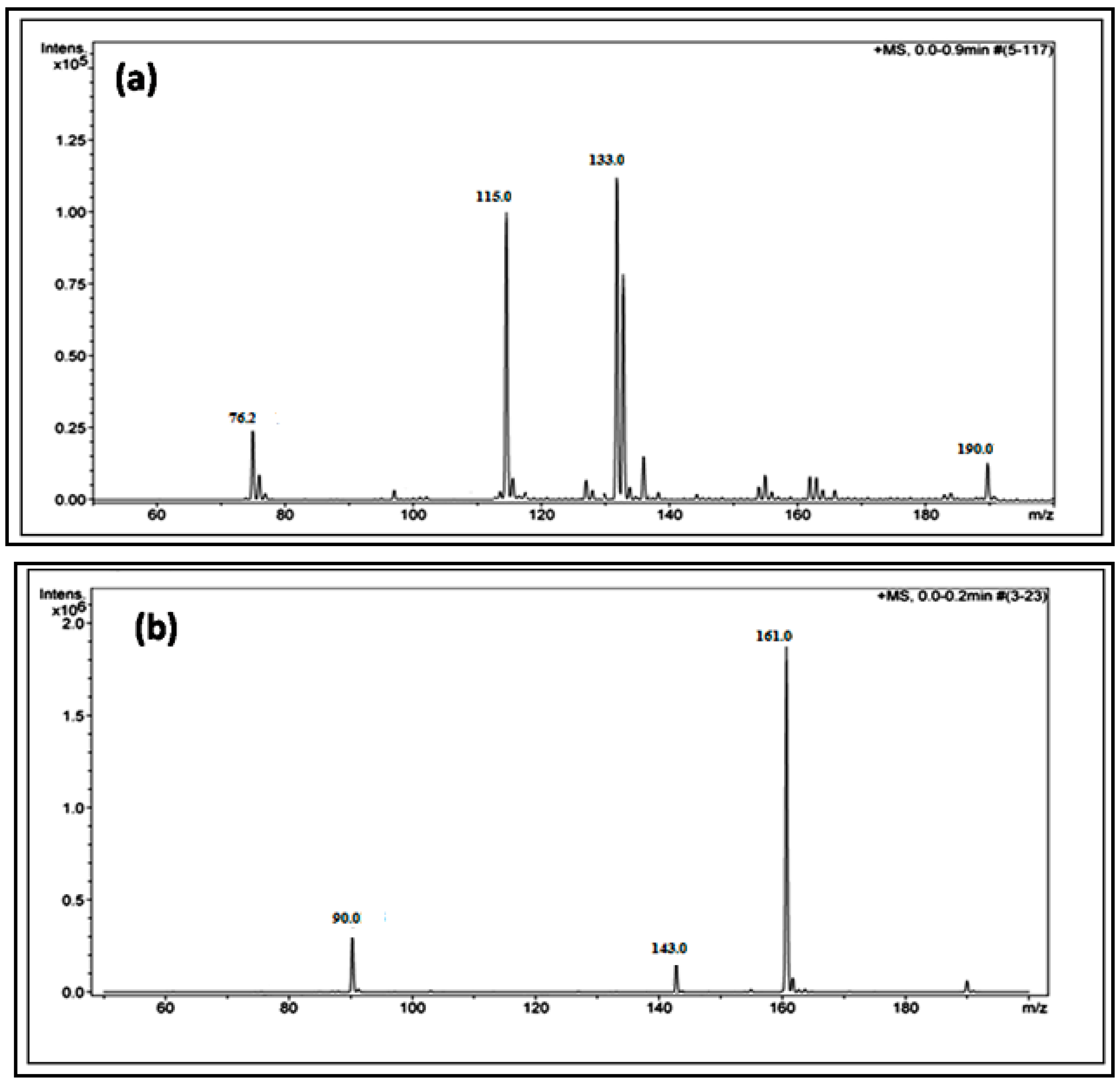
2.4. High Performance Liquid Chromatography Analysis (HPLC)
2.5. Electrospray Ionization-Mass Spectrometry Analysis (ESI-MS)
3. Results and Discussion
4. Implications for Chemical Evolution
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Altman, S. Enzymatic cleavage of RNA by RNA (Nobel lecture). Angew. Chem. Int. Ed. 1990, 29, 749–758. [Google Scholar] [CrossRef]
- Cech, T.R. Self-splicing and Enzymatic Activity of an Intervening Sequence RNA from Tetrahymena (Nobel Lecture). Angew. Chem. Int. Ed. 1990, 29, 759–768. [Google Scholar] [CrossRef]
- Bernhardt, H.S. The RNA world hypothesis: The worst theory of the early evolution of life (except for all the others). Biol. Direct. 2012, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Francklyn, C.; Carter, C.W. Aminoacylatingurzymes challenge the RNA world hypothesis. J. Biol. Chem. 2013, 288, 26856–26863. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.C.; Hud, N.V.; Williams, L.D. The ribosome challenge to the RNA world. J. Mol. Evolut. 2015, 80, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. A Hypothesis: Life Initiated from Two Genes, as Deduced from the RNA World Hypothesis and the Characteristics of Life-Like Systems. Life 2016, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Drawbacks of the ancient RNA-based life-like system under primitive earth conditions. Biochimie 2012, 94, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Degens, E.T.; Matheja, J.; Jackson, T.A. Template catalysis: Asymmetric polymerization of amino-acids on clay minerals. Nature 1970, 227, 492–493. [Google Scholar] [CrossRef] [PubMed]
- Paecht-Horowitz, M. Inorganic clays as possible prebiotic peptide templates. Isr. J. Chem. 1973, 11, 369–378. [Google Scholar] [CrossRef]
- Warden, J.T.; McCullough, J.J.; Lemmon, R.M.; Calvin, M. A re-examination of the zeolite-promoted, clay-mediated peptide synthesis. J. Mol. Evolut. 1974, 4, 189–194. [Google Scholar] [CrossRef]
- Paecht-Horowitz, M. Clays as possible catalysts for peptide formation in the prebiotic era. Orig. Life Evolut. Biosph. 1976, 7, 369–381. [Google Scholar] [CrossRef]
- Paecht-Horowitz, M.; Lahav, N. Polymerization of alanine in the presence of a non-swelling montmorillonite. J. Mol. Evolut. 1977, 10, 73–76. [Google Scholar] [CrossRef]
- Flegmann, A.W.; Scholefield, D. Thermodynamics of peptide bond formation at clay mineral surfaces. J. Mol. Evolut. 1978, 12, 101–112. [Google Scholar] [CrossRef]
- Lahav, N.; White, D.; Chang, S. Peptide formation in the Prebiotic era: Thermal condensation of glycine in fluctuating clay environments. Science 1978, 201, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Lawless, J.G.; Levi, N. The role of metal ions in chemical evolution: Polymerization of alanine and glycine in a cation-exchanged clay environment. J. Mol. Evolut. 1979, 13, 281–286. [Google Scholar] [CrossRef]
- Lahav, N.; White, D.H. A possible role of fluctuating clay-water systems in the production of ordered prebiotic oligomers. J. Mol. Evolut. 1980, 16, 11–21. [Google Scholar] [CrossRef]
- Luke, B.T.; Gupta, A.G.; Loew, G.H.; Lawless, J.G.; White, D.H. Theoretical investigation of the role of clay edges in prebiotic peptide bond formation. I. Structures of acetic acid, glycine, H2SO4, H3PO4, Si(OH)4, and Al(OH)4. Int. J. Quantum Chem. 1984, 26, 117–135. [Google Scholar] [CrossRef]
- White, D.H.; Kennedy, R.M.; Macklin, J. Acyl silicates and acyl aluminates as activated intermediates in peptide formation on clays. Orig. Life Evolut. Biosph. 1984, 14, 273–278. [Google Scholar] [CrossRef]
- Collins, J.R.; Loew, G.H.; Luke, B.T.; White, D.H. Theoretical investigation of the role of clay edges in prebiotic peptide bond formation. Orig. Life Evolut. Biosph. 1988, 18, 107–119. [Google Scholar] [CrossRef]
- Bujdak, J.; Slosiarikova, H.; Texler, N.; Schwendinger, M.; Rode, B.M. On the possible role of montmorillonites in prebiotic peptide formation. Monatsh. Für. Chem. 1994, 125, 1033–1039. [Google Scholar] [CrossRef]
- Bujdák, J.; Faybíková, K.; Eder, A.; Yongyai, Y.; Rode, B.M. Peptide chain elongation: A possible role of montmorillonite in prebiotic synthesis of protein precursors. Orig. Life Evol. Biosph. 1995, 25, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Bujdák, J.; Le Son, H.; Yongyai, Y.; Rode, B.M. The effect of reaction conditions on montmorillonite-catalysed peptide formation. Catal. Lett. 1996, 37, 267–272. [Google Scholar] [CrossRef]
- Bujdak, J.; Rode, B.M. The effect of smectite composition on the catalysis of peptide bond formation. J. Mol. Evolut. 1996, 43, 326–333. [Google Scholar] [CrossRef]
- Zamaraev, K.I.; Romannikov, V.N.; Salganik, R.I.; Wlassoff, W.A.; Khramtsov, V.V. Modelling of the prebiotic synthesis of oligopeptides: Silicate catalysts help to overcome the critical stage. Orig. Life Evolut. Biosph. 1997, 27, 325–337. [Google Scholar] [CrossRef]
- Porter, T.L.; Eastman, M.P.; Hagerman, M.E.; Price, L.; Shand, R.F. Site-specific Prebiotic Oligomerization reactions of Glycine on the surface of Hectorite. J. Mol. Evolut. 1998, 47, 373–377. [Google Scholar] [CrossRef]
- Bujdak, J.; Rode, B.M. The effect of clay structure on peptide bond formation catalysis. J. Mol. Catal. A 1999, 144, 129–136. [Google Scholar] [CrossRef]
- Porter, T.L.; Eastman, M.P.; Bain, E.; Begay, S. Analysis of peptides synthesized in the presence of SAz-1 montmorillonite and Cu 2+ exchanged hectorite. Biophys. Chem. 2001, 91, 115–124. [Google Scholar] [CrossRef]
- Aquino, A.J.; Tunega, D.; Gerzabek, M.H.; Lischka, H. Modeling catalytic effects of clay mineral surfaces on peptide bond formation. J. Phys. Chem. B 2004, 108, 10120–10130. [Google Scholar] [CrossRef]
- Bujdak, J.; Rode, B.M. On the mechanisms of oligopeptide reactions in solution and clay dispersion. J. Pept. Sci. 2004, 10, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Rimola, A.; Sodupe, M.; Ugliengo, P. Aluminosilicate surfaces as promoters for peptide bond formation: An assessment of Bernal’s hypothesis by AB Initio methods. J. Am. Chem. Soc. 2007, 129, 8333–8344. [Google Scholar] [CrossRef] [PubMed]
- Pant, C.K.; Lata, H.; Pathak, H.D.; Mehata, M.S. Heat-initiated prebiotic formation of peptides from glycine/aspartic acid and glycine/valine in aqueous environment and clay suspension. Int. J. Astrobiol. 2009, 8, 107–115. [Google Scholar] [CrossRef]
- Jaber, M.; Georgelin, T.; Bazzi, H.; Costa-Torro, F.; Lambert, J.F.; Bolbach, G.; Clodic, G. Selectivities in adsorption and peptidic condensation in the (arginine and glutamic acid)/montmorillonite clay system. J. Phys. Chem. C 2014, 118, 25447–25455. [Google Scholar] [CrossRef]
- Fuchida, S.; Masuda, H.; Shinoda, K. Peptide formation mechanism on montmorillonite under thermal conditions. Orig. Life Evolut. Biosph. 2014, 44, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Bujdak, J.; Rode, B.M. Silica, alumina, and clay-catalyzed alanine peptide bond formation. J. Mol. Evolut. 1997, 45, 457–466. [Google Scholar] [CrossRef]
- Bujdak, J.; Rode, B.M. Glycine oligomerization on silica and alumina. React. Kinet. Catal. Lett. 1997, 62, 281–286. [Google Scholar] [CrossRef]
- Smith, J.V. Biochemical evolution. I. Polymerization on internal, Organophilic silica surfaces of Dealuminated Zeolites and Feldspars. Proc. Natl. Acad. Sci. USA 1998, 95, 3370–3375. [Google Scholar] [CrossRef] [PubMed]
- Bujdak, J.; Rode, B.M. Silica, alumina and clay catalyzed peptide bond formation: Enhanced efficiency of alumina catalyst. Orig. Life Evolut. Biosph. 1999, 29, 451–461. [Google Scholar] [CrossRef]
- Bujdak, J.; Rode, B.M. Activated alumina as an energy source for peptide bond formation: Consequences for mineral-mediated prebiotic processes. Amino Acids 2001, 21, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Basiuk, V.A.; Sainz-Rojas, J. Catalysis of peptide formation by inorganic oxides: High efficiency of alumina under mild conditions on the earth-like planets. Adv. Space Res. 2001, 27, 225–230. [Google Scholar] [CrossRef]
- Bujdak, J.; Rode, B.M. Preferential amino acid sequences in alumina-catalyzed peptide bond formation. J. Inorg. Biochem. 2002, 90, 1–7. [Google Scholar] [CrossRef]
- Matrajt, G.; Blanot, D. Properties of synthetic ferrihydrite as an amino acid adsorbent and a promoter of peptide bond formation. Amino Acids 2004, 26, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Shanker, U.; Bhushan, B.; Bhattacharjee, G.; Kamaluddin. Oligomerization of glycine and alanine catalyzed by iron oxides: Implications for prebiotic chemistry. Orig. Life Evolut. Biosph. 2012, 42, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Holland, G.P. Alanine Adsorption and Thermal Condensation at the Interface of Fumed Silica Nanoparticles: A Solid-State NMR Investigation. J. Phys. Chem. C 2015, 119, 25663–25672. [Google Scholar] [CrossRef]
- Kumar, A.; Kamaluddin. Oligomerization of glycine and alanine on metal (II) octacynaomolybdate (IV): Role of double metal cyanides in prebiotic chemistry. Amino Acids 2012, 43, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Takeya, H.; Kushibe, T.; Koizumi, Y. Mineral-enhanced hydrothermal oligopeptide formation at the second time scale. Astrobiology 2011, 11, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, M.A.; Sharma, R.; Kamaluddin. Studies on interaction of ribonucleotides with zinc ferrite nanoparticles using spectroscopic and microscopic techniques. Karbala Int. J. Mod. Sci. 2015, 1, 49–59. [Google Scholar] [CrossRef]
- Iqubal, M.A.; Sharma, R.; Kamaluddin. Surface Interaction of Ribonucleic Acid Constituents with Spinel Ferrite Nanoparticles: A Prebiotic Chemistry Experiment. RSC Adv. 2016, 6, 68574–68583. [Google Scholar] [CrossRef]
- Sharma, R.; Bansal, S.; Singhal, S. Tailoring the photo-Fenton activity of spinel ferrites (MFe2O4) by incorporating different cations (M = Cu, Zn, Ni and Co) in the structure. RSC Adv. 2015, 5, 6006–6018. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Greenstein, J.P.; Winitz, M. Chemistry of the Amino Acids; John Wiley & Sons Ltd.: New York, NY, USA; London, UK, 1961; Volume 1. [Google Scholar]
- Orgel, L.E. The origin of polynucleotide-directed protein synthesis. J. Mol. Evolut. 1989, 29, 465–474. [Google Scholar] [CrossRef]
- Nagayama, M.; Takaoka, O.; Inomata, K.; Yamagata, Y. Diketopiperazine-mediated peptide formation in aqueous solution. Orig. Life Evolut. Biosph. 1990, 20, 249–257. [Google Scholar] [CrossRef]
- Rode, B.M.; Schwendinger, M.G. Copper-catalyzed amino acid condensation in water—A simple possible way of prebiotic peptide formation. Orig. Life Evolut. Biosph. 1990, 20, 401–410. [Google Scholar] [CrossRef]
- Cox, J.S.; Seward, T.M. The reaction kinetics of alanine and glycine under hydrothermal conditions. Geochim. Cosmochim. Acta 2007, 71, 2264–2284. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Yu, X.; Pan, H.; Jiang, W.; Xu, X.; Tang, R. Mechanism of promoted dipeptide formation on hydroxyapatite crystal surfaces. Chin. Sci. Bull. 2011, 56, 633–639. [Google Scholar] [CrossRef]
- Kawamura, K.; Nishi, T.; Sakiyama, T. Consecutive elongation of alanine oligopeptides at the second time range under hydrothermal conditions using a microflow reactor system. J. Am. Chem. Soc. 2005, 127, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Yukioka, M. Kinetics of the racemization of amino acids at 225–275 °C using a real-time monitoring method of hydrothermal reactions. Thermochimicaacta 2001, 375, 9–16. [Google Scholar] [CrossRef]
- Baudisch, O.S. The importance of trace elements in biologic activity. Am. Sci. 1943, 31, 211. [Google Scholar] [CrossRef]
- Clarke, F.W.; Washington, H.S. The Composition of the Earth’s Crust; US Government Printing Office: Washington, DC, USA, 1924; Volume 127.
- Reichmann, H.J.; Jacobsen, S.D. Sound velocities and elastic constants of ZnAl2O4 spinel and implication for spinel-elasticity systematics. Am. Miner. 2006, 91, 1049–1054. [Google Scholar] [CrossRef]
- Hoover, R.B. Microfossils of cyanobacteria in carbonaceous meteorites. In Proceedings of the SPIE 6694, Instruments, Methods, and Missions for Astrobiology X, 669408, San Diego, CA, USA, 26 August 2007. [Google Scholar]
- Karwowski, L. Sołtmany meteorite. Meteorites 2012, 2, 15–30. [Google Scholar]
- Bindi, L.; Yao, N.; Lin, C.; Hollister, L.S.; Andronicos, C.L.; Distler, V.V.; Eddy, M.P.; Kostin, A.; Kryachko, V.; MacPherson, G.J.; et al. Natural quasicrystal with decagonal symmetry. Sci. Rep. 2015, 5, 9111. [Google Scholar] [CrossRef] [PubMed]
- Rychagov, S.N.; Glavatskikh, S.F.; Sandimirova, E.I. Ore and silicate magnetic pellets as indicators of structure and fluid regime, as well as mineral and ore formation in the present-day Baranskii hydrothermal system, Iturup Island. Geol. Ore Deposits 1996, 38, 26–34. [Google Scholar]
- Essene, E.J.; Peacor, D.R. Crystal chemistry and petrology of coexisting galaxite and jacobsite and other spinel solutions and solvi. Am. Miner. 1983, 68, 449–455. [Google Scholar]
- Stalder, M.; Rozendaal, A. Calderite-rich garnet and franklinite-rich spinel in amphibolite-facies hydrothermal sediments, Gamsberg Zn-Pb deposit, Namaqua Province, South Africa. Can. Miner. 2005, 43, 589–599. [Google Scholar] [CrossRef]
- Exon, N.F.; Raven, M.D.; Carlo, E.D. Ferromanganese nodules and crusts from the Christmas Island region, Indian Ocean. Mar. Georesour. Geotechnol. 2002, 20, 275–297. [Google Scholar] [CrossRef]
- Timofeeva, Y.O.; Karabtsov, A.A.; Semal, V.A.; Burdukovskii, M.L.; Bondarchuk, N. Iron-Manganese Nodules in Updates: The Dependences of the accumulation of trace elements on nodule size. Soil Sci. Soc. Am. J. 2014, 78, 795–813. [Google Scholar] [CrossRef]
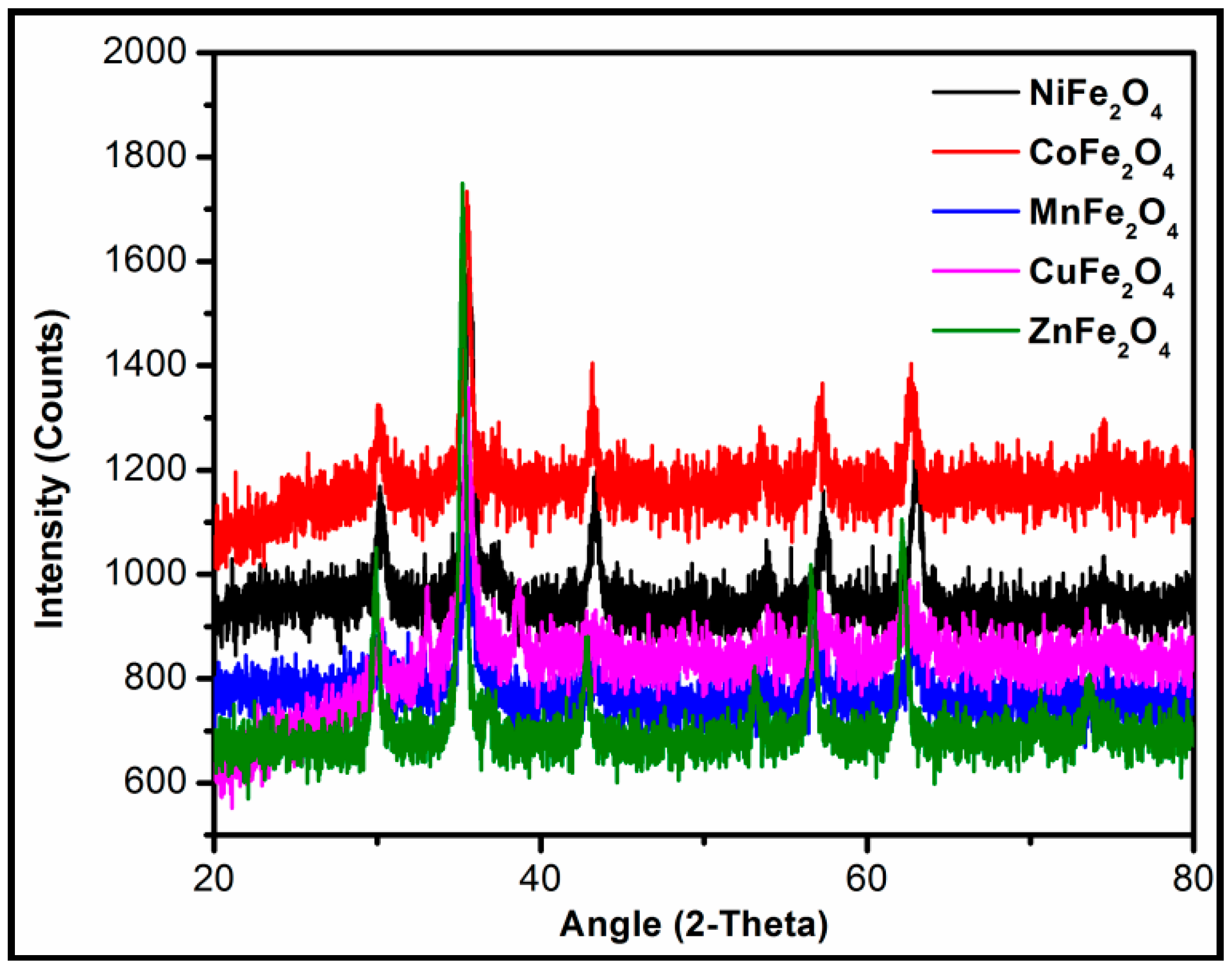
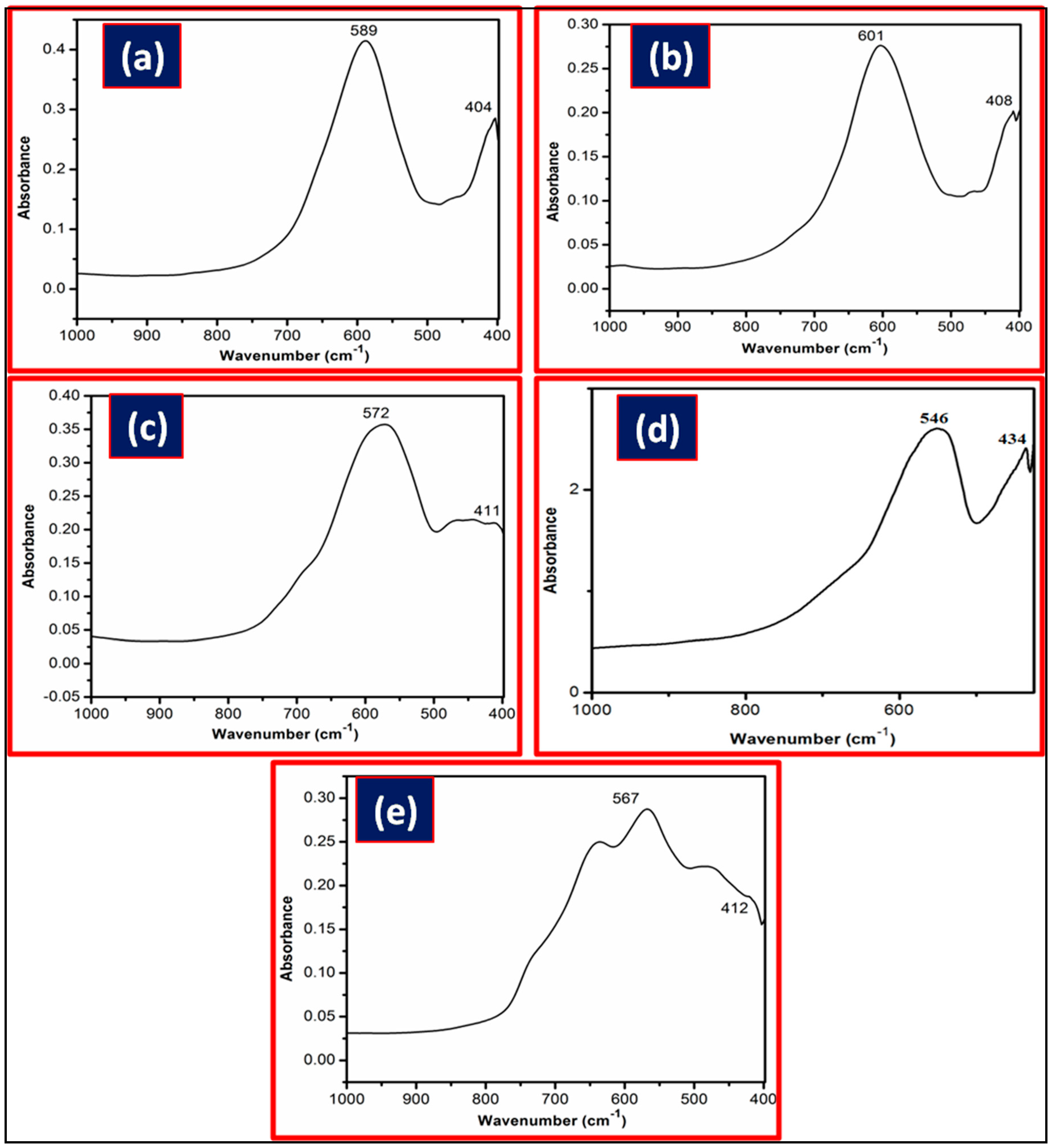
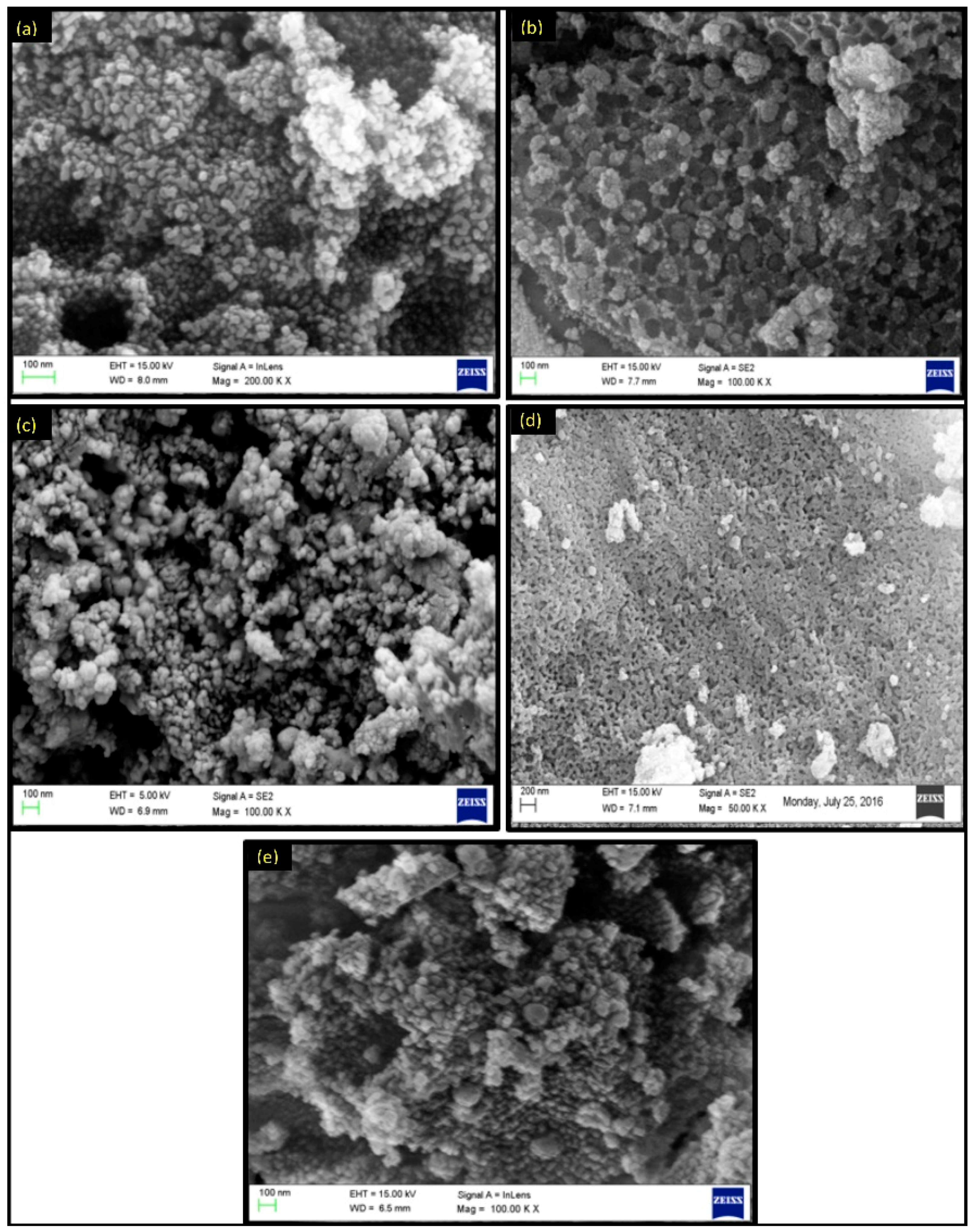
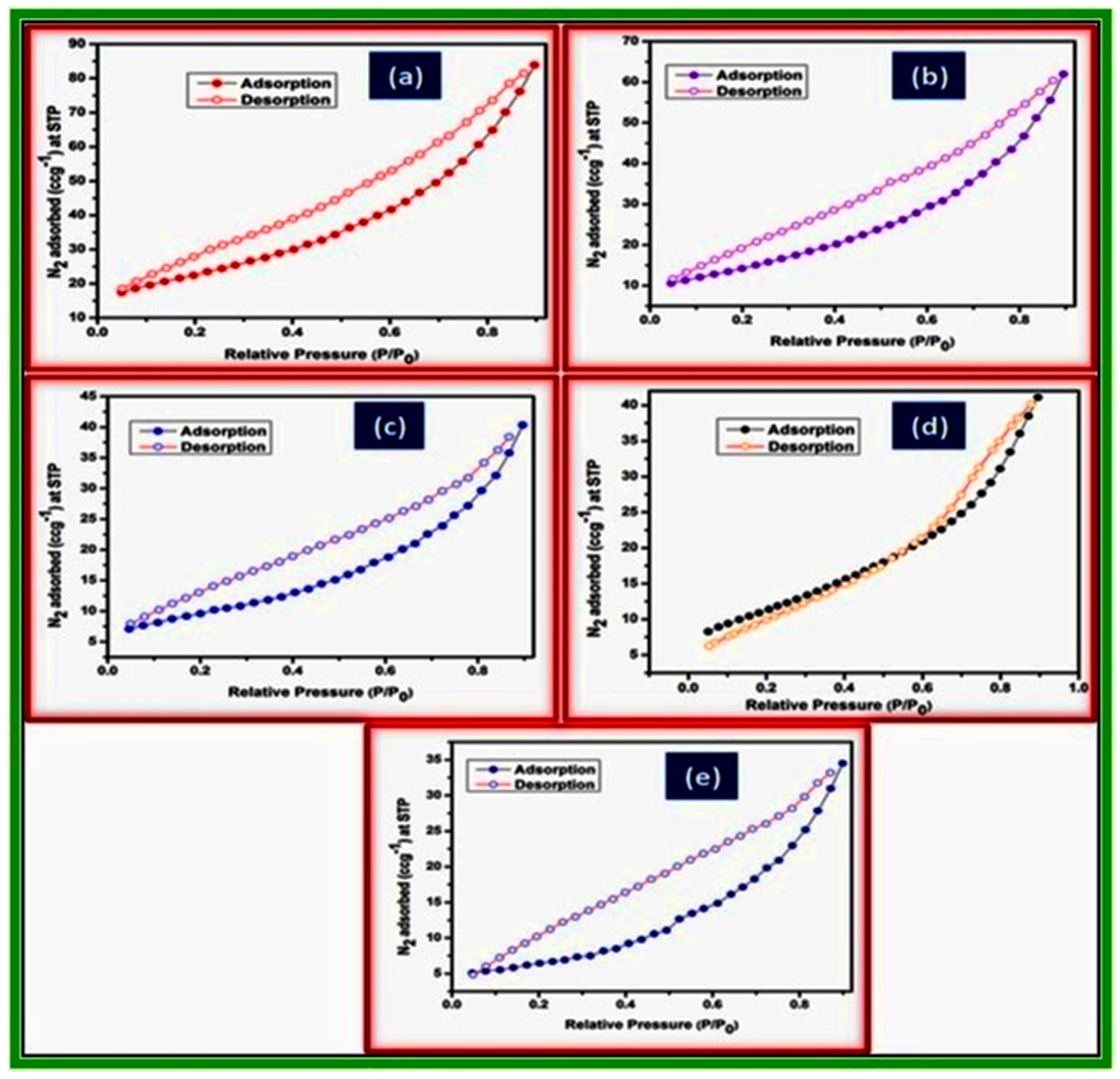

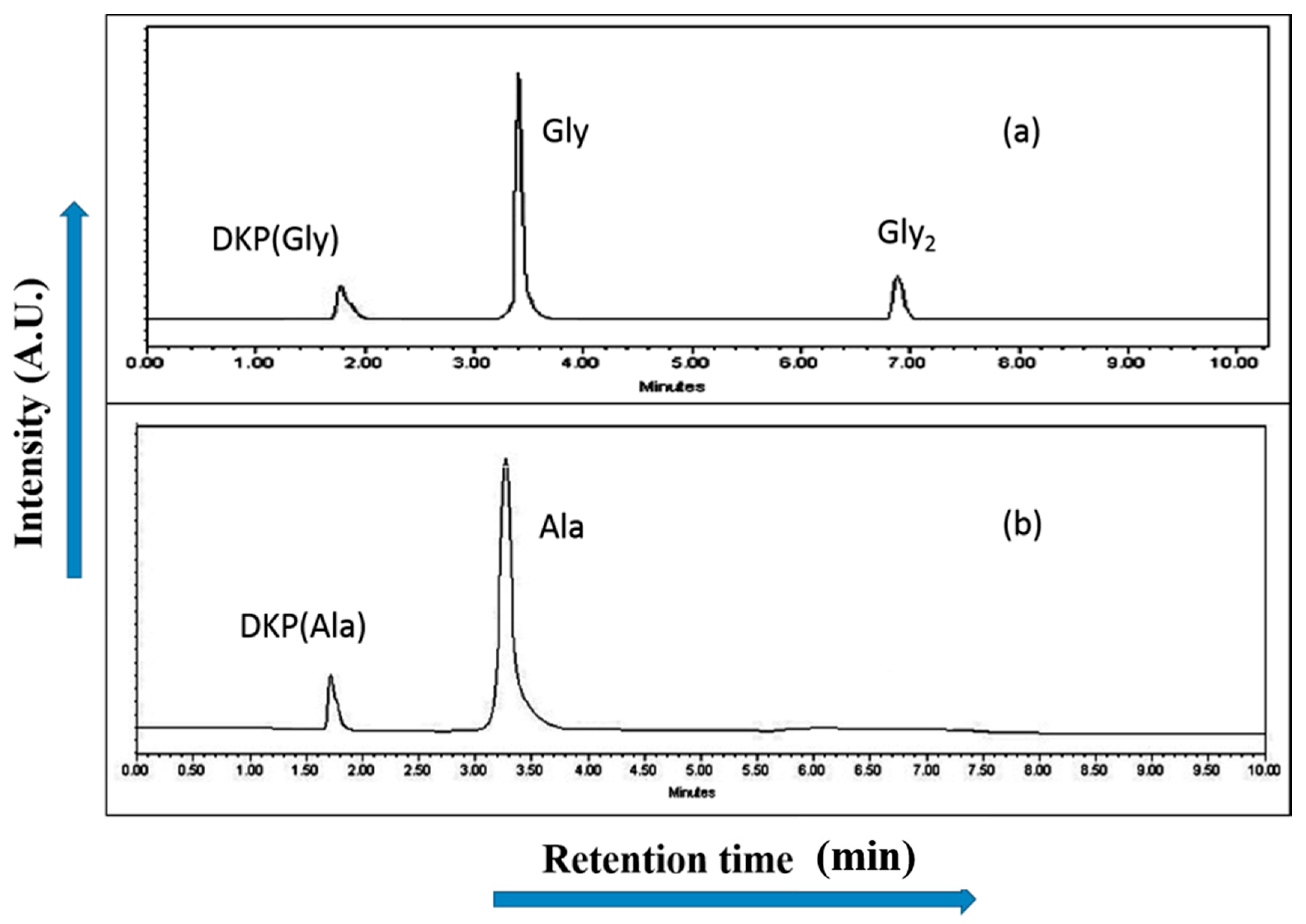
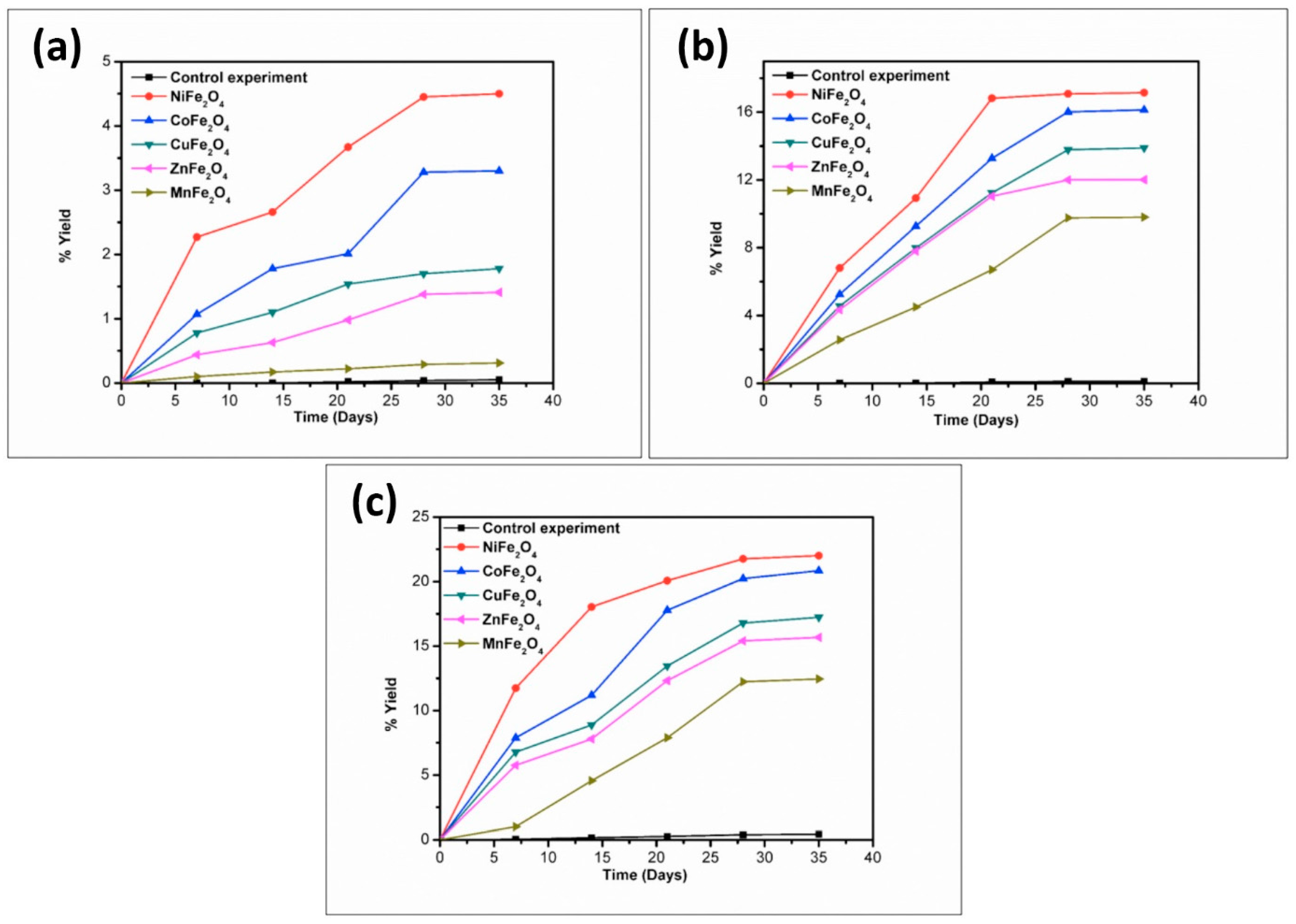
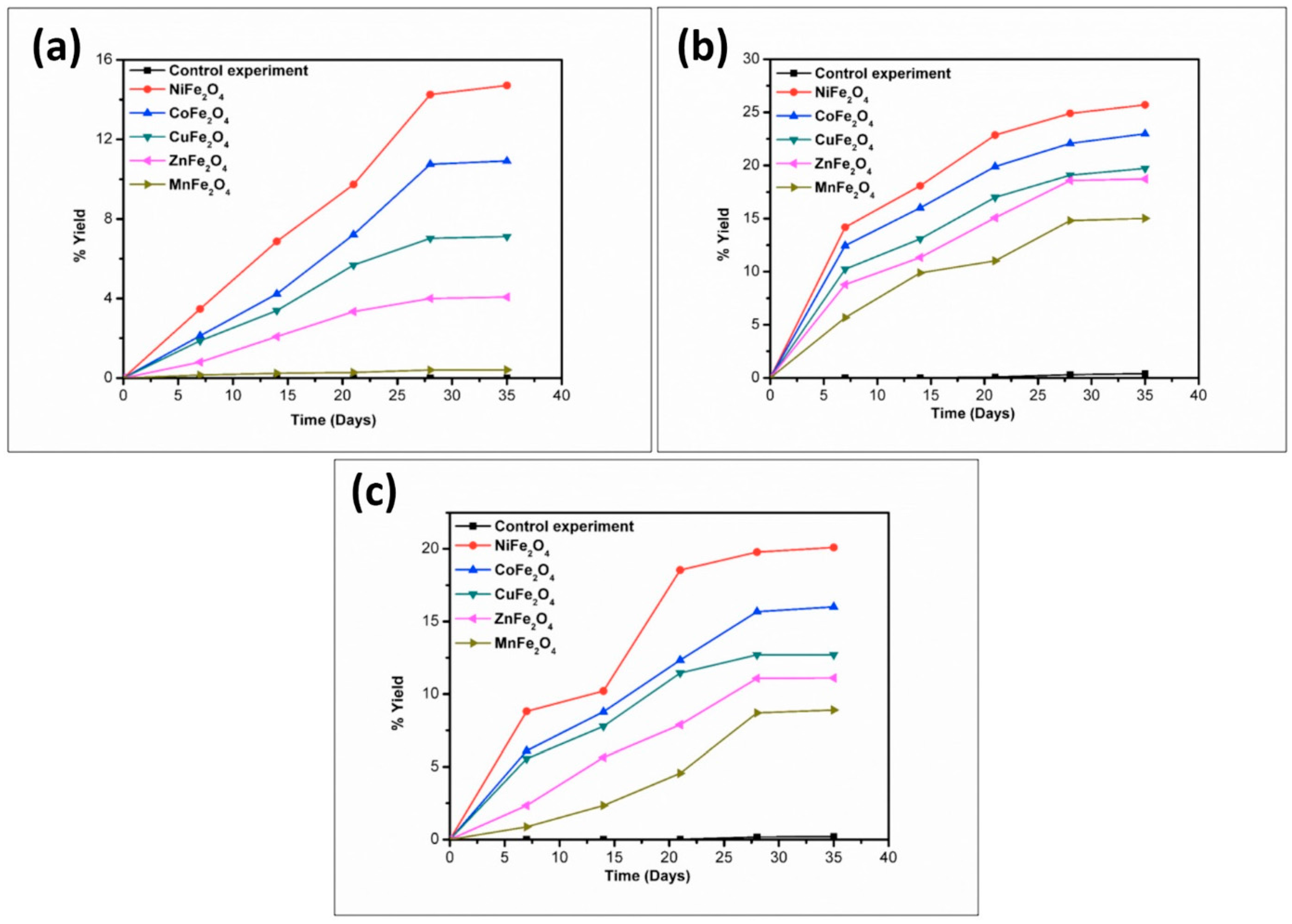
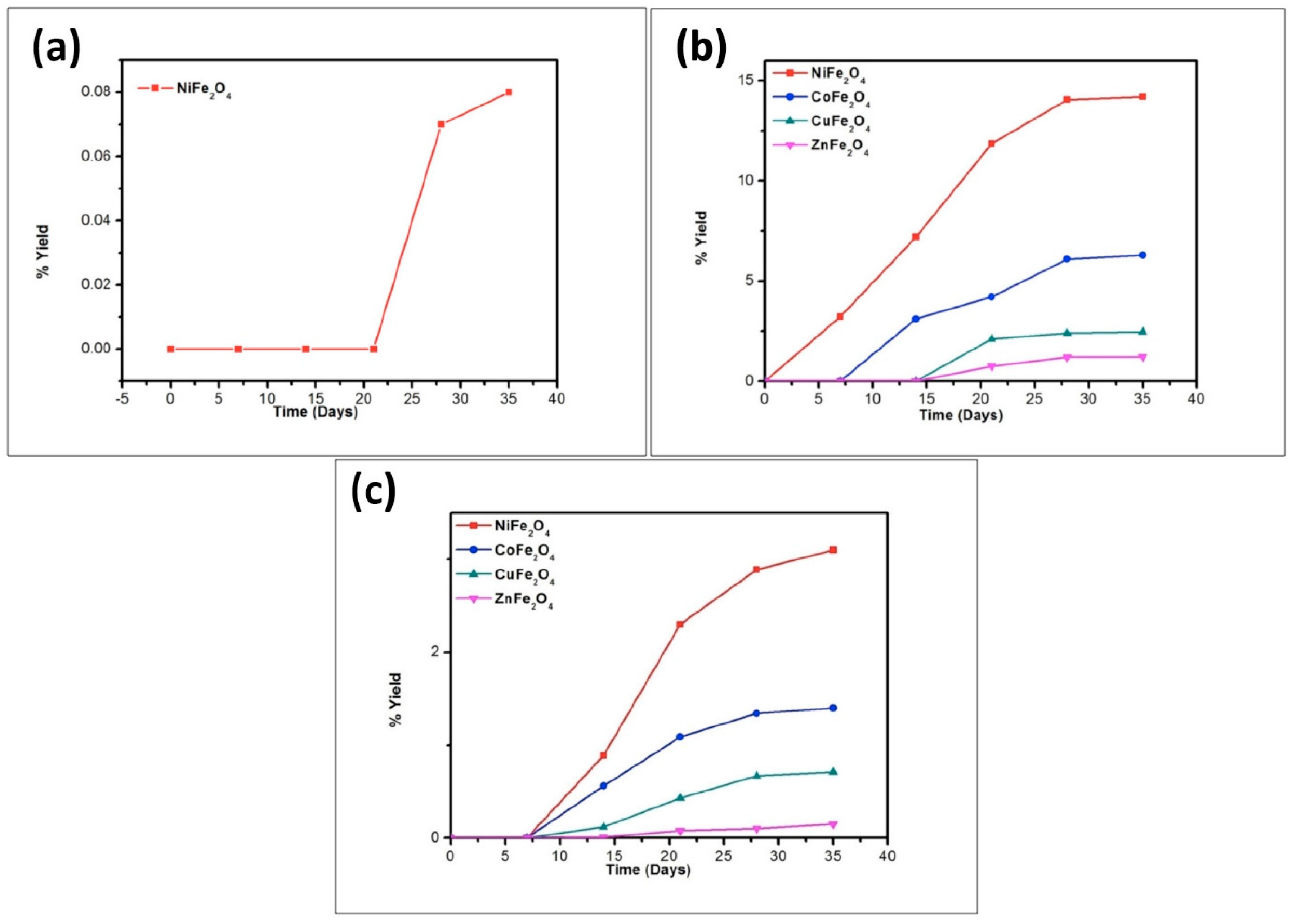
| Compound | BET (Brunauer–Emmett–Teller) Surface Area (m2/g) |
|---|---|
| NiFe2O4 | 80.64 |
| CoFe2O4 | 53.66 |
| CuFe2O4 | 34.67 |
| ZnFe2O4 | 28.54 |
| MnFe2O4 | 22.97 |
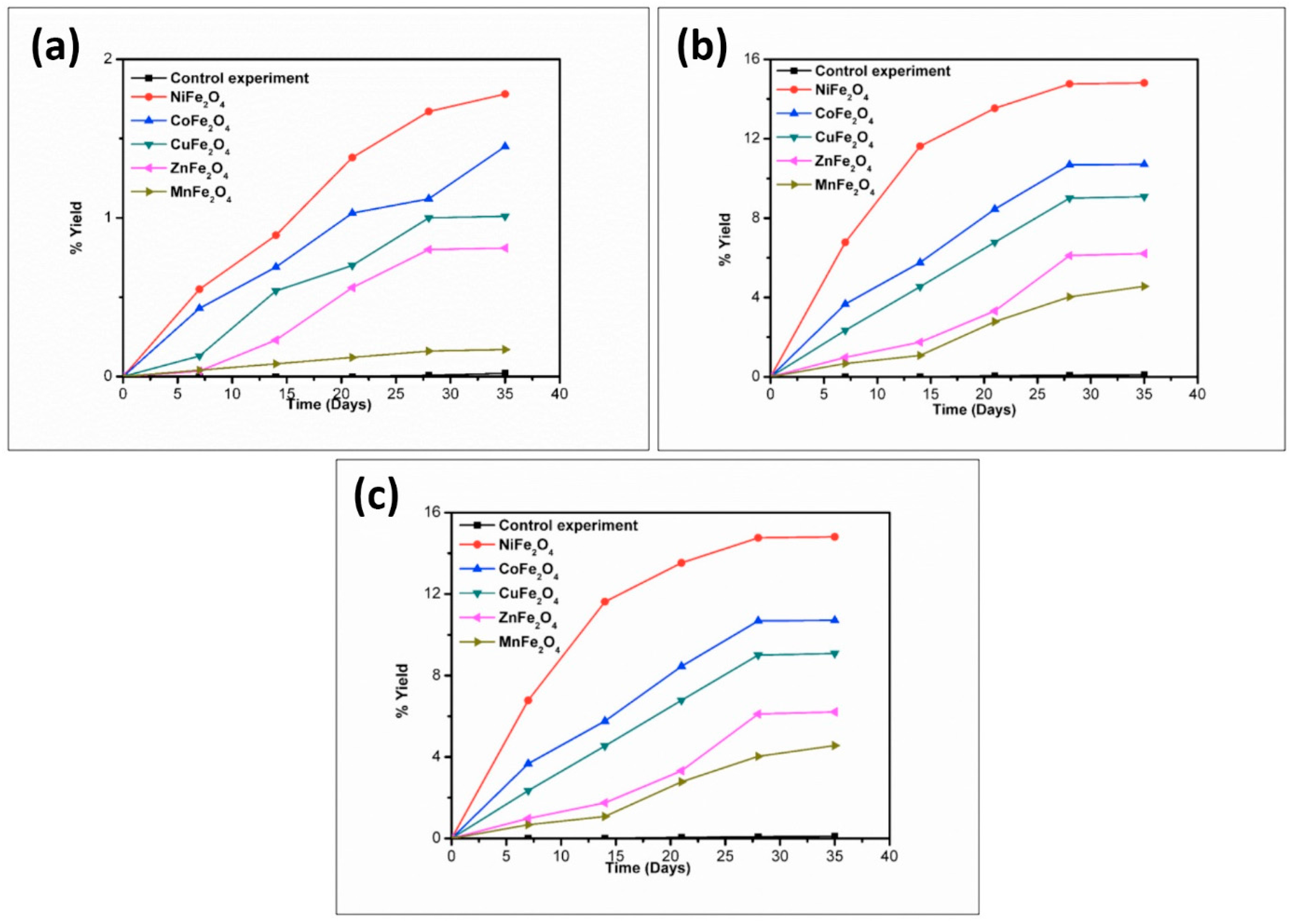
| Catalyst | % Yield of the Products Formed When Glycine Was Heated at 50, 90, and 120 °C for 35 Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DKP (Diketopiperazine) (Gly) | (Gly)2 | (Gly)3 | |||||||
| 50 °C | 90 °C | 120 °C | 50 °C | 90 °C | 120 °C | 50 °C | 90 °C | 120 °C | |
| No catalyst | 0.05 | 0.12 | 0.43 | n.d | 0.40 | 0.20 | n.d | n.d | n.d |
| NiFe2O4 | 4.50 | 17.15 | 22.01 | 14.71 | 25.70 | 20.10 | trace | 14.2 | 3.10 |
| CoFe2O4 | 3.30 | 16.13 | 20.84 | 10.91 | 22.98 | 16.01 | * n.d | 6.30 | 1.40 |
| CuFe2O4 | 1.78 | 13.89 | 17.23 | 7.11 | 19.72 | 12.71 | n.d | 2.47 | 0.71 |
| ZnFe2O4 | 1.41 | 12.01 | 15.67 | 4.07 | 18.70 | 11.11 | n.d | 1.23 | 0.15 |
| MnFe2O4 | 0.31 | 9.80 | 12.45 | 0.41 | 15.01 | 8.90 | n.d | n.d | n.d |
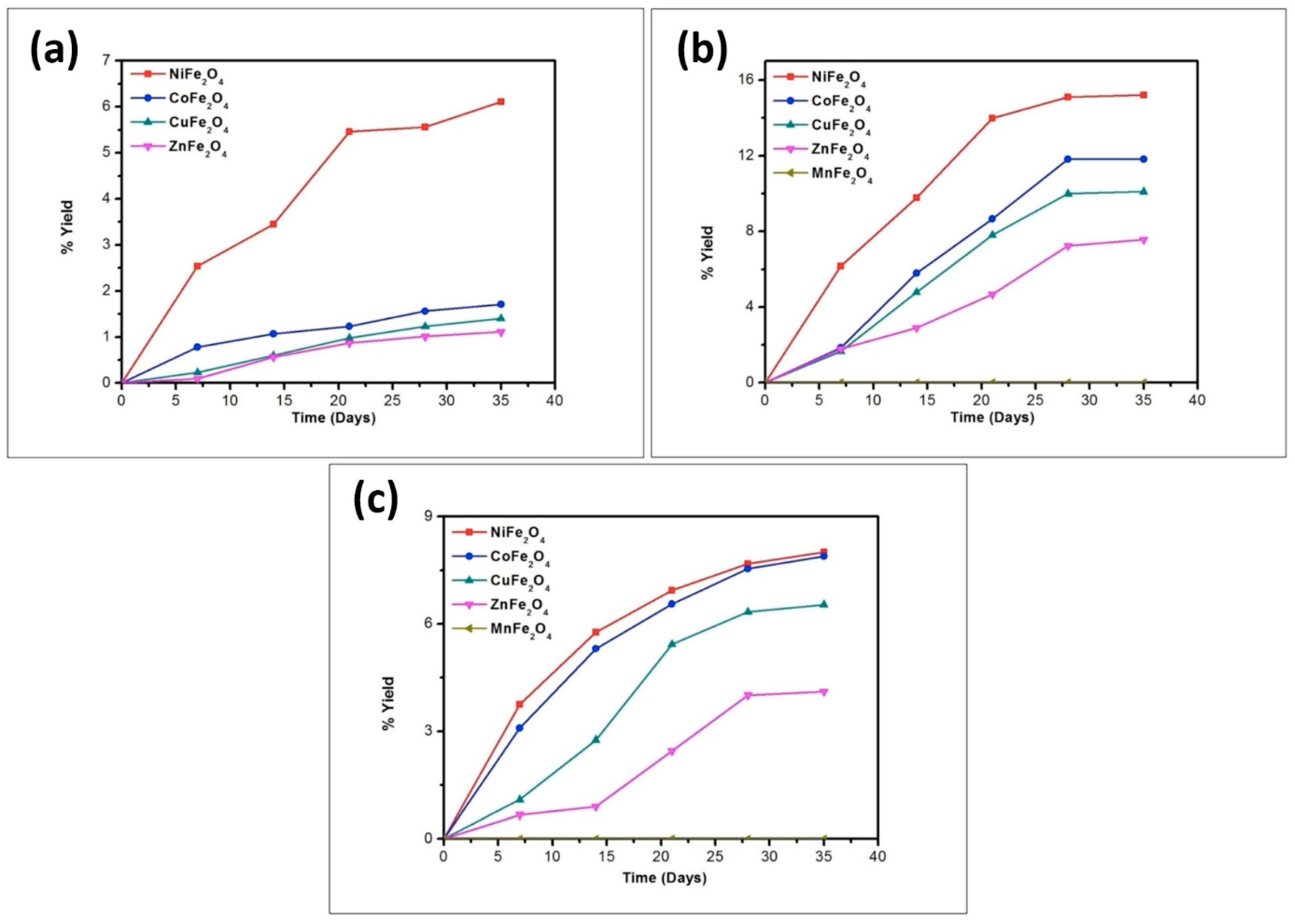
| Catalyst | % Yield of the Products Formed When Alanine Was Heated at 50, 90 and 120 °C for 35 Days | |||||
|---|---|---|---|---|---|---|
| DKP(Ala) | (Ala)2 | |||||
| 50 °C | 90 °C | 120 °C | 50 °C | 90 °C | 120 °C | |
| No catalyst | 0.02 | 0.07 | 0.11 | n.d | n.d | n.d |
| NiFe2O4 | 1.78 | 13.11 | 14.81 | 6.11 | 15.21 | 8.01 |
| CoFe2O4 | 1.45 | 9.23 | 10.71 | 1.71 | 11.81 | 7.89 |
| CuFe2O4 | 1.01 | 8.81 | 9.08 | 1.40 | 10.1 | 6.54 |
| ZnFe2O4 | 0.81 | 4.78 | 6.21 | 1.11 | 7.57 | 4.11 |
| MnFe2O4 | 0.17 | 3.89 | 4.56 | * n.d | trace | trace |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqubal, M.A.; Sharma, R.; Jheeta, S.; Kamaluddin. Thermal Condensation of Glycine and Alanine on Metal Ferrite Surface: Primitive Peptide Bond Formation Scenario. Life 2017, 7, 15. https://doi.org/10.3390/life7020015
Iqubal MA, Sharma R, Jheeta S, Kamaluddin. Thermal Condensation of Glycine and Alanine on Metal Ferrite Surface: Primitive Peptide Bond Formation Scenario. Life. 2017; 7(2):15. https://doi.org/10.3390/life7020015
Chicago/Turabian StyleIqubal, Md. Asif, Rachana Sharma, Sohan Jheeta, and Kamaluddin. 2017. "Thermal Condensation of Glycine and Alanine on Metal Ferrite Surface: Primitive Peptide Bond Formation Scenario" Life 7, no. 2: 15. https://doi.org/10.3390/life7020015