A Case Study of Landfill Leachate Using Coal Bottom Ash for the Removal of Cd2+, Zn2+ and Ni2+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Leaching Test
2.3. Batch Adsorption Experiments
3. Results and Discussion
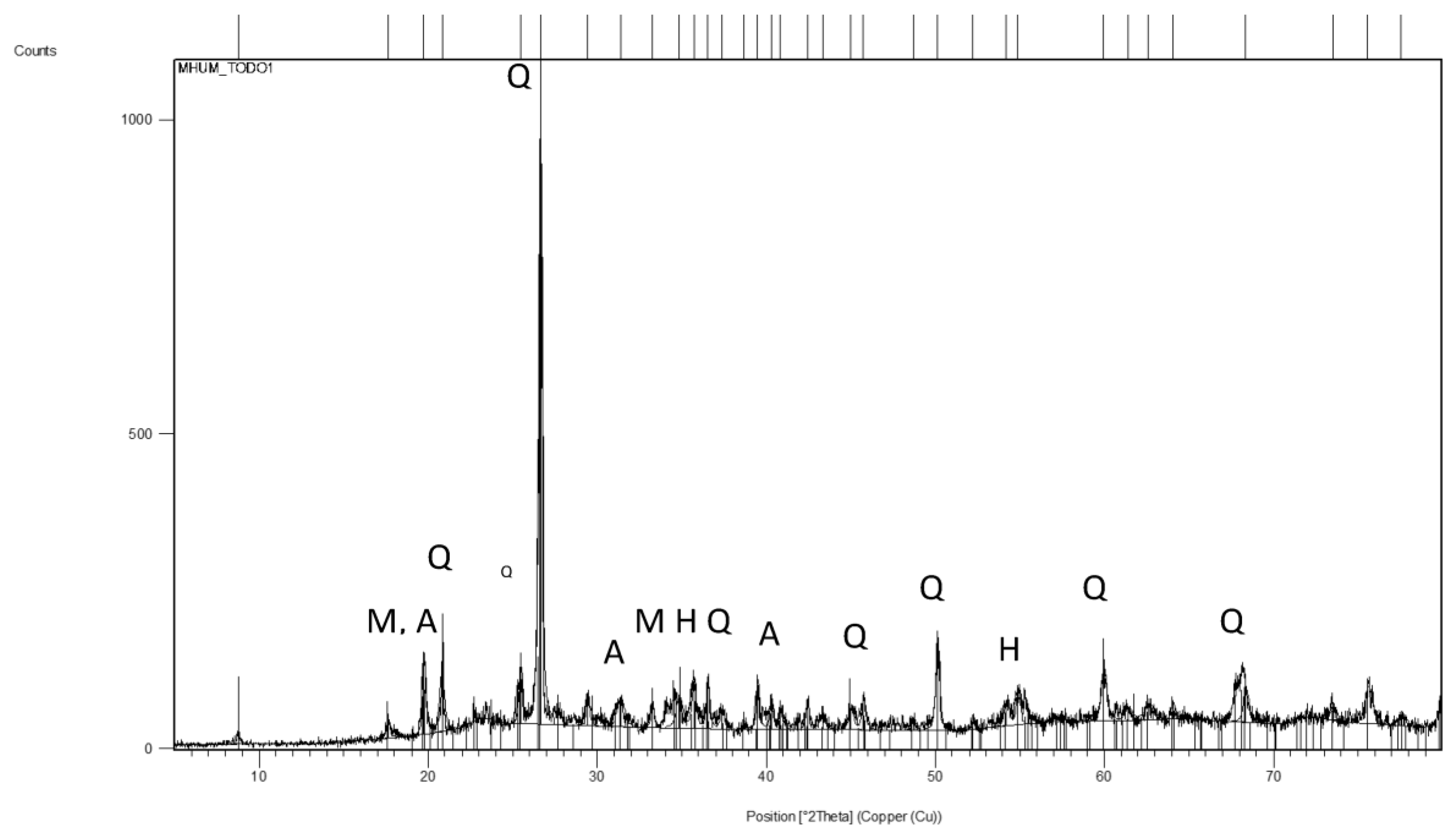
3.1. Characterization of the Bottom Ash Sample
3.2. Leaching Test
3.3. Batch Adsorption Experiments
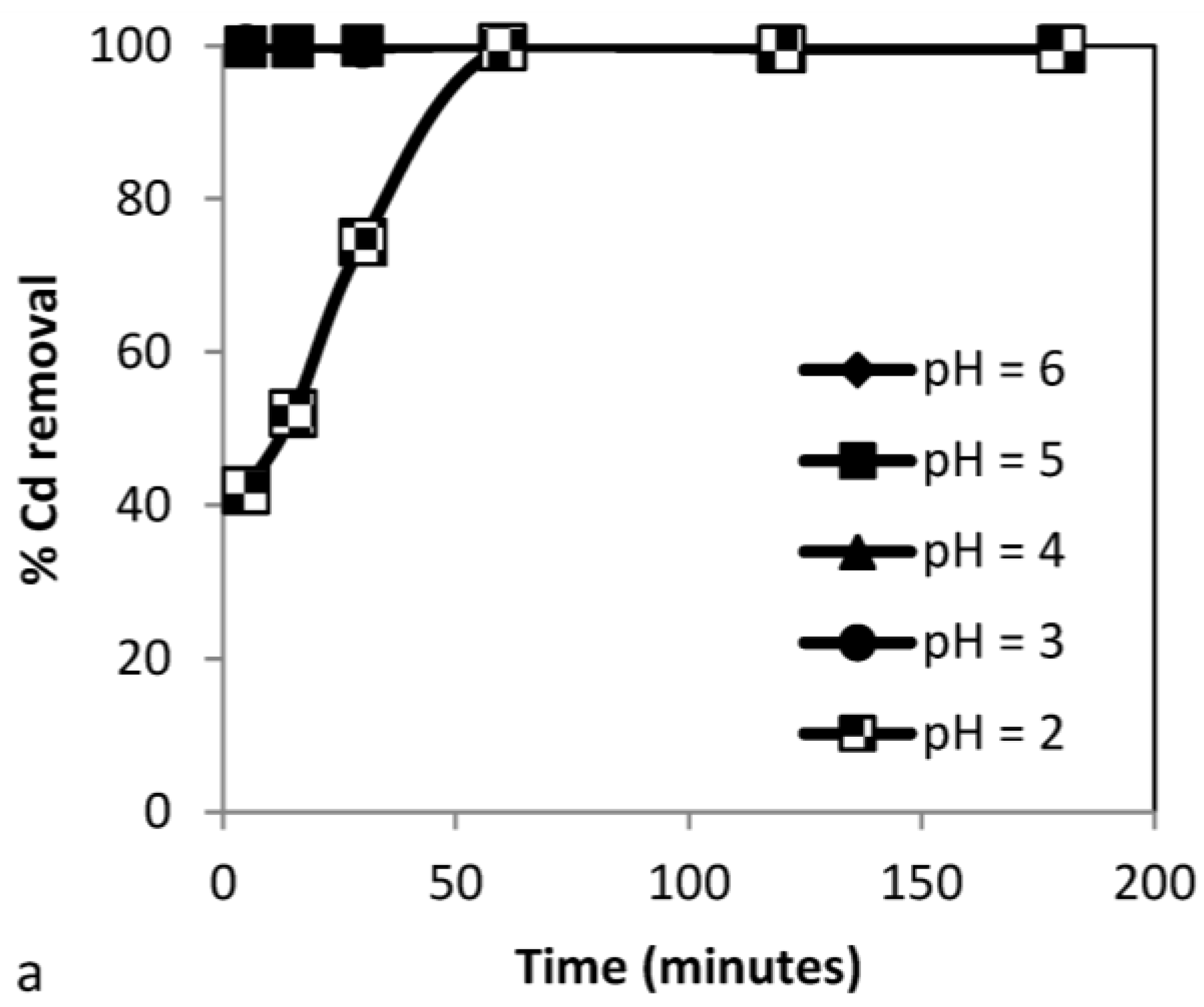
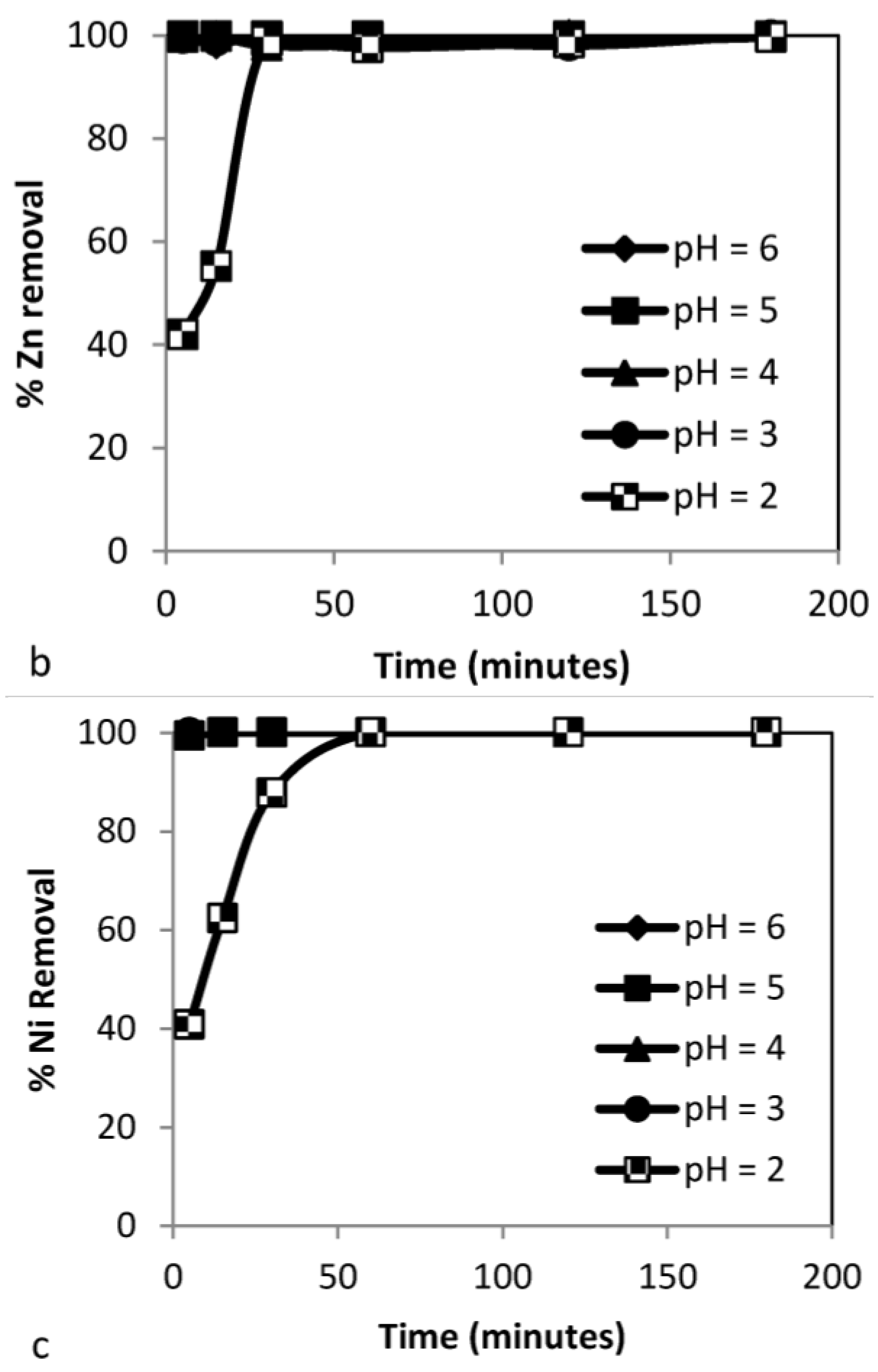
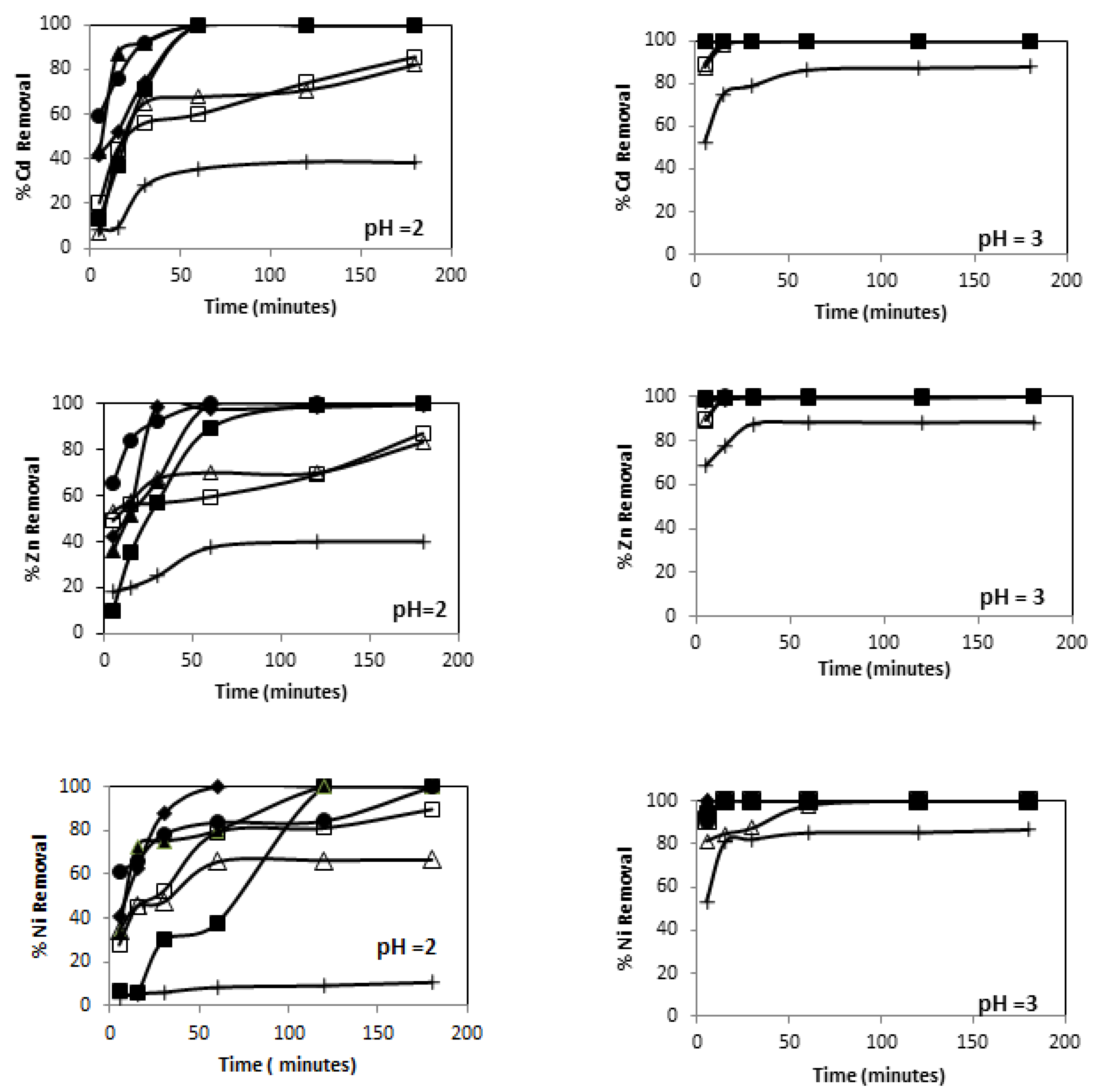
3.3.1. Effect of pH
3.3.2. Effect of the Initial Concentration
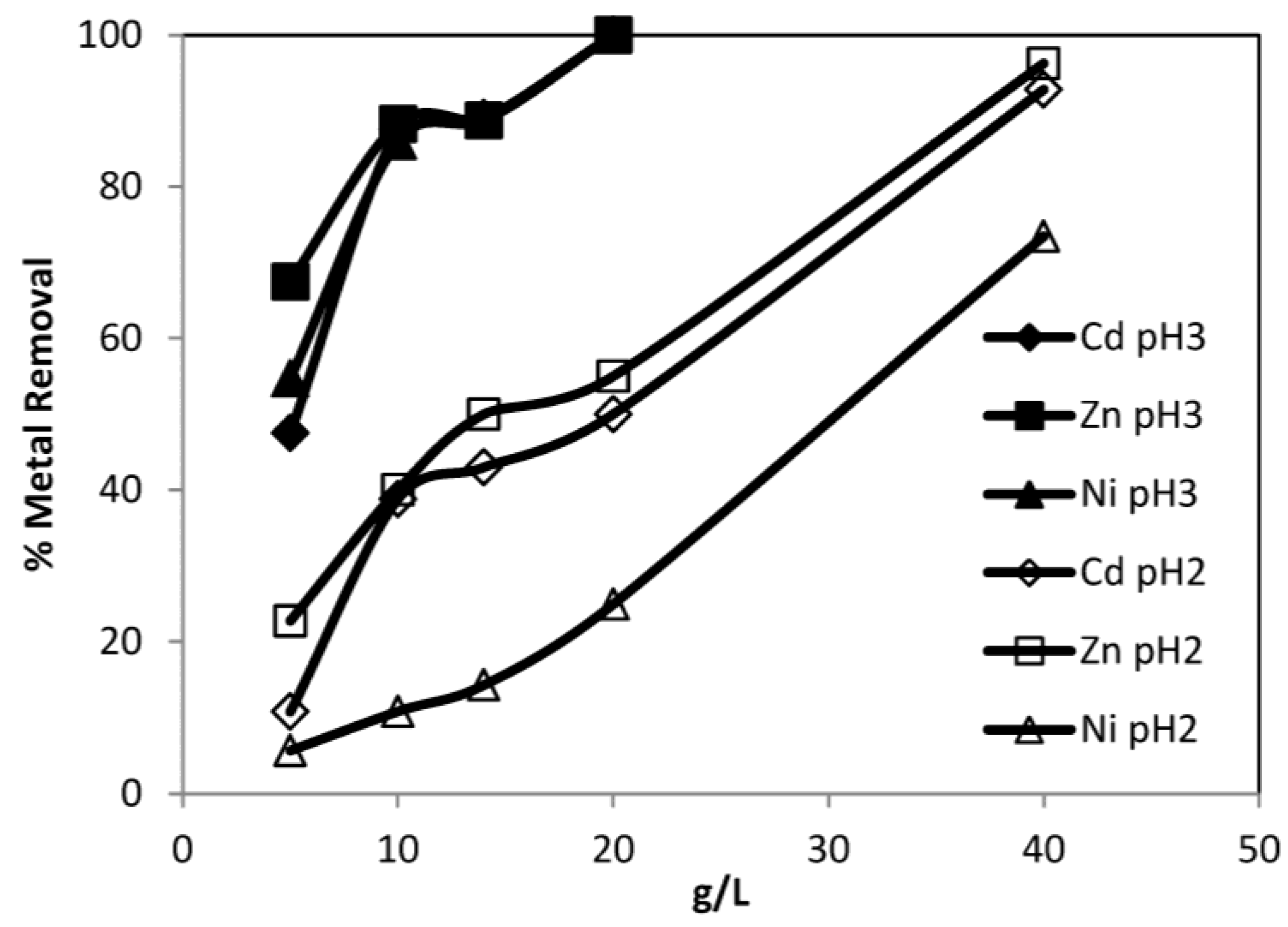
3.3.3. Effect of Dosage
3.3.4. Effect of Ionic Strength
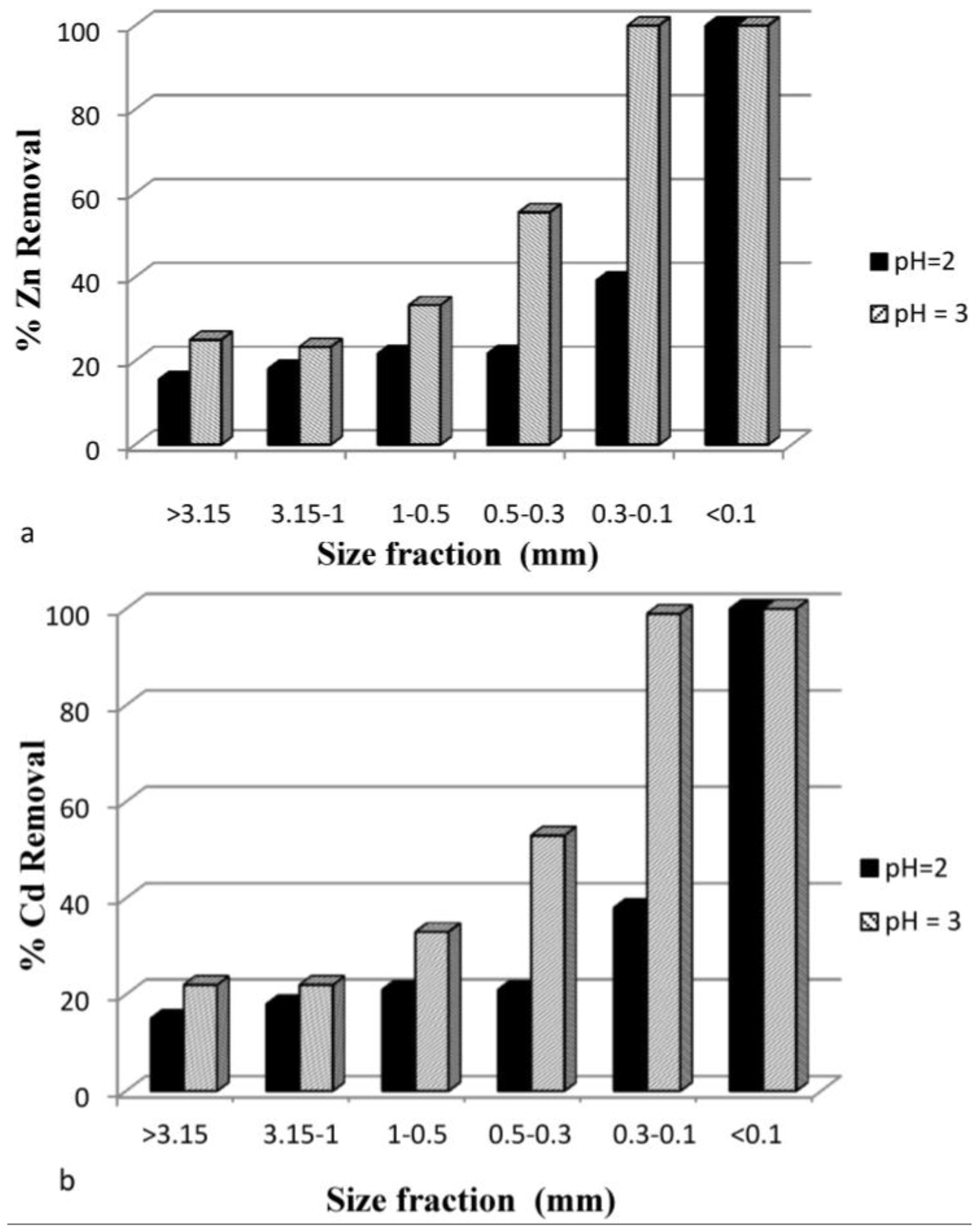
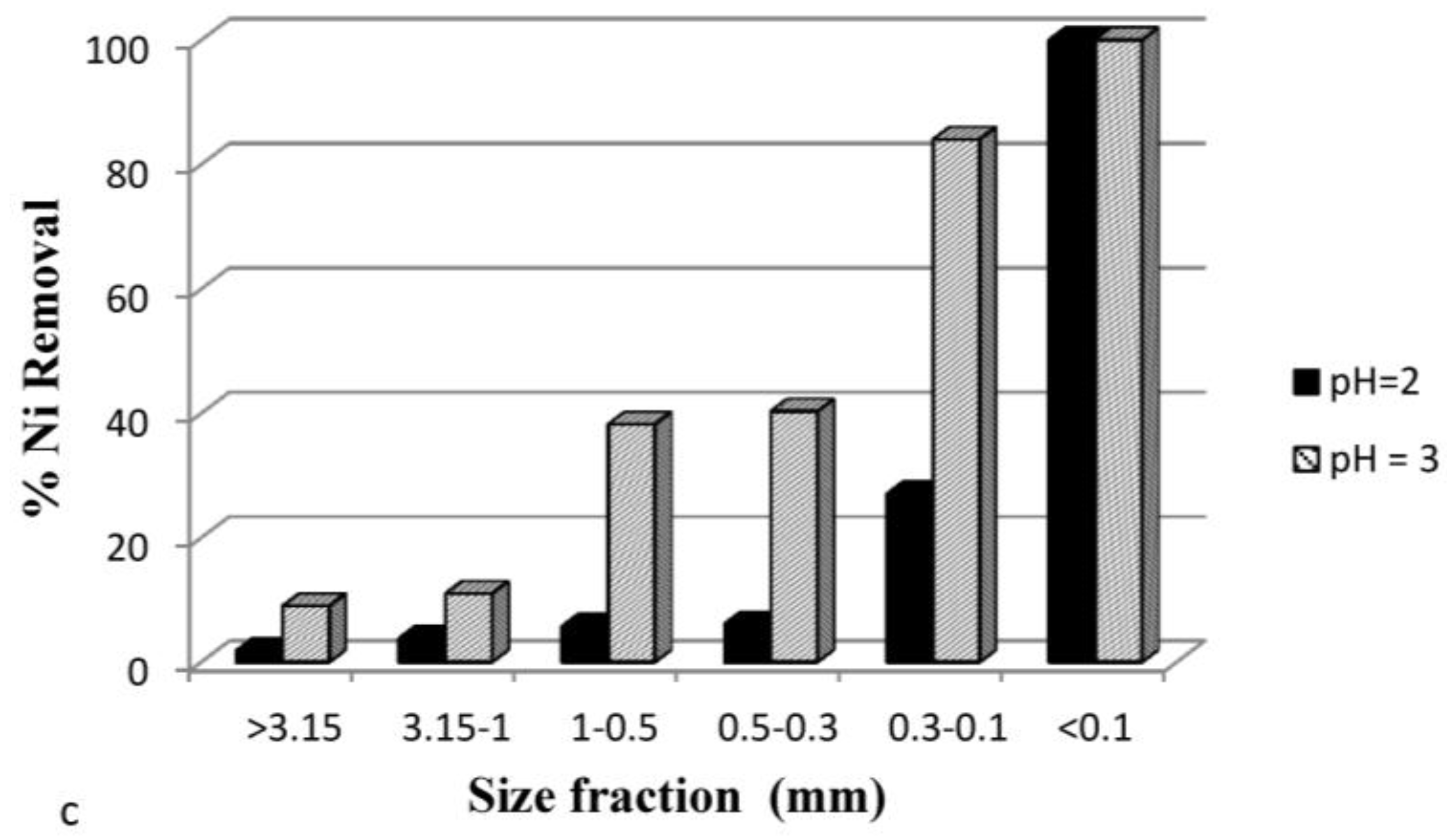
3.3.5. Effect of Particle Size
3.3.6. Multi-Component Systems
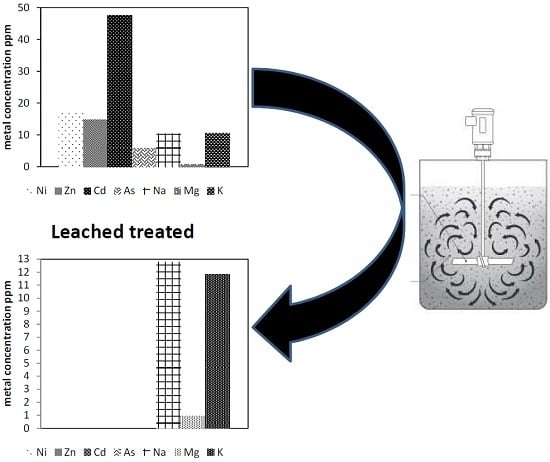
3.3.7. Treatment of Industrial Wastewater
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Owsianiak, M.; Holm, P.E.; Fantke, P.; Christiansen, K.S.; Borggaard, O.K.; Hauschild, M.Z. Assessing comparative terrestrial ecotoxicity of Cd, Co, Cu, Ni, Pb, and Zn: The influence of aging and emission source. Environ. Pollut. 2015, 206, 400–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirbas, A.; Pehlivan, E.; Gode, F.; Altun, T.; Arslan, G. Adsorption behavior of Cu(II), Zn(II), Ni(II), Pb(II), and Cd(II) from aqueous solution on Amberlite IR-120 synthetic resin. J. Colloid Interface Sci. 2005, 282, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Balomajumder, C.; Mondal, P. Application of Agro-Based Biomasses for Zinc Removal from Wastewater—A Review. Clean Soil Air Water 2011, 39, 641–652. [Google Scholar] [CrossRef]
- Choi, S.B.; Yun, Y.S. Biosorption of cadmium by various types of dried sludge: An equilibrium study and investigation of mechanisms. J. Hazard. Mater. 2006, 138, 378–383. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, E.I. Sorption of Cd(II) and Se(IV) from aqueous solution using modified rice husk. J. Hazard. Mater. 2007, 147, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Fuerhacker, M.; Haile, T.M.; Kogelnig, D. Application of ionic liquids for the removal of heavy metals from wastewater and activated sludge. Water Sci. Technol. 2012, 65, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Coman, V.; Robotin, B.; Ilea, P. Nickel recovery/removal from industrial wastes: A review. Resour. Conserv. Recycl. 2013, 73, 229–238. [Google Scholar] [CrossRef]
- Li, L.; Takahashi, N.; Kaneko, K. A novel method for nickel recovery and phosphorus removal from spent electroless nickel-plating solution. Sep. Purif. Technol. 2015, 147, 237–244. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Kusumastuti, A.; Derek, C.J.C. Emulsion liquid membranes for cadmium removal: Studies of extraction efficiency. Membr. Water Treat. 2013, 4, 11–25. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Bhushan, B.; Agarwal, S. A critical analysis on the efficiency of activated carbons from low-cost precursors for heavy metals remediation. Crit. Rev. Environ. Sci. Technol. 2015, 45, 613–668. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Yuan, D.; Yan, J. Removal of nickel from aqueous solution using cathodic deposition of nickel hydroxide at a modified electrode. J. Chem. Technol. Biotechnol. 2013, 88, 2193–2200. [Google Scholar] [CrossRef]
- Purkayastha, D.; Mishra, U.; Biswas, S. A comprehensive review on Cd(II) removal from aqueous solution. J. Water Process Eng. 2014, 2, 105–128. [Google Scholar] [CrossRef]
- Saravanan, M.; Chiya, A.B.; Velan, M. Removal of copper, nickel, and zinc ions from electroplating rinse water. Clean Soil Air Water 2012, 40, 66–79. [Google Scholar]
- Ayala, J.; Blanco, F.; García, P.; Rodríguez, P.; Sancho, J. Asturian (Spanish) fly ash as heavy metals removal materials. Fuel 1998, 77, 1147–1154. [Google Scholar] [CrossRef]
- Adamczuk, A.; Kołodyńska, D. Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chem. Eng. J. 2015, 274, 200–212. [Google Scholar] [CrossRef]
- Cho, H.; Oh, D.; Kim, K. A study on removal characteristics of heavy metals from aqueous solution by fly ash. J. Hazard. Mater. 2005, 127, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Montagnaro, F.; Santoro, L. Reuse of coal combustion ashes as dyes and heavy metal adsorbents: Effect of sieving and demineralization on waste properties and adsorption capacity. Chem. Eng. J. 2009, 150, 174–180. [Google Scholar] [CrossRef]
- Cetin, S.; Pehlivan, E. The use of fly ash as a low cost, environmentally friendly alternative to activated carbon for the removal of heavy metals from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2007, 298, 83–87. [Google Scholar] [CrossRef]
- Mohan, S.; Gandhimathi, R. Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. J. Hazard. Mater. 2009, 169, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Alinnor, I.J. Adsorption of heavy metal ions from aqueous solution by fly ash. Fuel 2007, 86, 853–857. [Google Scholar] [CrossRef]
- Bayat, B. Combined removal of zinc(II) and cadmium(II) from aqueous solutions by adsorption onto high-calcium Turkish fly ash. Water Air Soil Pollut. 2002, 136, 69–92. [Google Scholar] [CrossRef]
- Nascimento, M.; Soares, P.S.M.; de Souza, V.P. Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method. Fuel 2009, 88, 1714–1719. [Google Scholar] [CrossRef]
- Bayat, B. Comparative study of adsorption properties of Turkish fly ashes: I. The case of nickel(II), copper(II) and zinc(II). J. Hazard. Mater. 2002, 95, 251–273. [Google Scholar] [CrossRef]
- Tofan, L.; Paduraru, C.; Bilba, D.; Rotariu, M. Thermal power plants ash as sorbent for the removal of Cu(II) and Zn(II) ions from wastewaters. J. Hazard. Mater. 2008, 156, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sočo, E.; Kalembkiewicz, J. Adsorption of nickel(II) and copper(II) ions from aqueous solution by coal fly ash. J. Environ. Chem. Eng. 2013, 1, 581–588. [Google Scholar] [CrossRef]
- Visa, M.; Isac, L.; Duta, A. Fly ash adsorbents for multi-cation wastewater treatment. Appl. Surf. Sci. 2012, 258, 6345–6352. [Google Scholar] [CrossRef]
- Gitari, W.M.; Petrik, L.F.; Etchebers, O.; Key, D.I.; Okujeni, C. Utilization of fly ash for treatment of coal mines wastewater: Solubility controls on major inorganic contaminants. Fuel 2008, 87, 2450–2462. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Tripathy, S.; Panigrahi, M.K.; Equeenuddin, S.M. Evaluation of the use of an alkali modified fly ash as a potential adsorbent for the removal of metals from acid mine drainage. Appl. Water Sci. 2013, 3, 567–576. [Google Scholar] [CrossRef]
- American Coal Ash Association (ACAA). 2013 Coal Combustion Product (CCP) Production and Use; American Coal Ash Association: Aurora, CO, USA, 2013. [Google Scholar]
- Singh, M.; Siddique, R. Properties of concrete containing high volumes of coal bottom ash as fine aggregate. J. Clean Prod. 2015, 91, 269–278. [Google Scholar] [CrossRef]
- Kim, K.K.; Lee, H.K. Use of power plant bottom ash as fine and coarse aggregates in high-strength concrete. Constr. Build. Mater. 2011, 25, 1115–1122. [Google Scholar] [CrossRef]
- Kou, S.-C.; Poo, C.-S. Properties of concrete prepared with crushed fine stone, furnace bottom ash and fine recycled aggregate as fine aggregates. Constr. Build. Mater. 2009, 23, 2877–2886. [Google Scholar] [CrossRef]
- Bilir, T. Effects of non-ground slag and bottom ash as fine aggregate on concrete permeability properties. Constr. Build. Mater. 2012, 26, 730–734. [Google Scholar] [CrossRef]
- Andrade, L.B.; Rocha, J.C.; Cheriaf, M. Influence of coal bottom ash as fine aggregate on fresh properties of concrete. Constr. Build. Mater. 2009, 23, 609–614. [Google Scholar] [CrossRef]
- Sun, W.-L.; Qu, Y.-Z.; Yu, Q.; Ni, J.-R. Adsorption of organic pollutants from coking and papermaking wastewaters by bottom ash. J. Hazard. Mater. 2008, 154, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Grupta, V.K.; Mittal, A.; Krishnan, L.; Gajbe, V. Adsorption kinetics and column operations for the removal and recovery of malachite green from wastewater using bottom ash. Sep. Purif. Technol. 2004, 40, 87–96. [Google Scholar] [CrossRef]
- Mittal, A.; Gupta, V.K.; Malviya, A.; Mittal, J. Process development for the batch and bulk removal and recovery of a hazardous, water-soluble azo dye (Metanil Yellow) by adsorption over waste materials (Bottom Ash and De-Oiled Soya). J. Hazard. Mater. 2008, 151, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Kaur, D.; Mittal, J. Applicability of waste materials—Bottom ash and de-oiled soya—As adsorbents for the removal and recovery of a hazardous dye, brilliant green. J. Colloid Interface Sci. 2008, 326, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Mittal, A.; Jhare, D.; Mittal, J. Batch and bulk removal of hazardous colouring agent Rose Bengal by adsorption techniques using bottom ash as adsorbent. RSC Adv. 2012, 22, 8381–8389. [Google Scholar] [CrossRef]
- Gorme, J.B.; Maniquiz, M.C.; Kim, S.S.; Son, Y.G.; Kim, Y.-T.; Kim, L. Characterization of bottom ash as an adsorbent of lead from aqueous solutions. Environ. Eng. Res. 2010, 15, 207–213. [Google Scholar] [CrossRef]
- Sukpreabprom, H.; Arquero, O.-A.; Naksata, W.; Sooksamiti, P.; Janhom, S. Isotherm, Kinetic and Thermodynamic Studies on the Adsorption of Cd(II) and Zn(II) ions from Aqueous Solutions onto Bottom Ash. Int. J. Environ. Sci. Dev. 2014, 5, 165–170. [Google Scholar] [CrossRef]
- Asokbunyarat, V.; van Hullebusch, E.D.; Lens, P.N.L.; Annachhatre, A.P. Coal Bottom Ash as Sorbing Material for Fe(II), Cu(II), Mn(II), and Zn(II) Removal from Aqueous Solutions. Water Air Soil Pollut. 2015, 226, 1–17. [Google Scholar] [CrossRef]
- Joint EURELECTRIC/ECOBA Briefing: The Classification of Coal Combustion Products under the Revised Waste Framework Directive (2008/98/EC). Available online: www.ecoba.org (accessed on 7 October 2015).
- Tiwari, M.K.; Bajpai, S.; Dewangan, U.K.; Tamraka, R.K. Suitability of leaching test methods for fly ash and slag: A review. J. Radiat. Res. Appl. Sci. 2015, 8, 523–537. [Google Scholar] [CrossRef]
- Erol, M.; Küçükbayrak, S.; Meriçcoboyu, A.E.; Ulubaş, T. Removal of Cu2+ and Pb2+ in aqueous solutions by fly ash. Energy Convers. Manag. 2005, 46, 1319–1331. [Google Scholar] [CrossRef]
- Hoeffer, P.R.; Lecuyer, I.; Cloirec, P.L. Experimental design methodology applied to adsorption of metallic ions onto fly ash. Water Res. 2001, 35, 965–976. [Google Scholar] [CrossRef]
- Bartoňová, L.; Klika, Z. Effect of CaO on retention of S, Cl, Br, As, Mn, V, Cr, Ni, Cu, Zn, W and Pb in bottom ashes from fluidized-bed coal combustion power station. J. Environ. Sci. 2014, 26, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.-S.; Kim, Y.-K.; Kong, S.-H.; Rhee, S.-W.; Lee, W.-K. The adsorption characteristics of heavy metals by various particle sizes of MSWI bottom ash. Waste Manag. 2003, 23, 851–857. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse Fan agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Antagonistic Competitive Equilibrium Modeling for the Adsorption of Ternary Metal Ion Mixtures from Aqueous Solution onto Bagasse Fly Ash. Ind. Eng. Chem. Res. 2008, 47, 3129–3137. [Google Scholar] [CrossRef]
- Imessaoudene, D.; Hanini, S.; Bouzidi, A. Biosorption of strontium from aqueous solutions onto spent coffee grounds. J. Radioanal. Nucl. Chem. 2013, 298, 893–902. [Google Scholar] [CrossRef]
- Wu, C.-H.; Kuo, C.-Y.; Guan, S.-S. Adsorption Kinetics of Lead and Zinc Ions by Coffee Residues. Pol. J. Environ. Stud. 2015, 24, 761–767. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, J.; Zhao, X.; Li, F.; Jiang, F.; Zhang, M.; Cheng, X. Competitive adsorption of strontium and cobalt onto tin antimonite. Chem. Eng. J. 2016, 285, 679–689. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Esposito, A.; Toro, L.; Vegliò, F. Metal speciation and pH effect on Pb, Cu, Zn and Cd biosorption onto Sphaerotilus natans: Langmuir-type empirical. Water Res. 2003, 37, 627–633. [Google Scholar] [CrossRef]

| Sample | SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | MnO (%) | MgO (%) | CaO (%) | Na2O (%) | K2O (%) | TiO2 (%) | P2O5 (%) | L.O.I. (%) | SO3 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. ash <100 µm | 54.1 | 24.1 | 6.0 | 0.06 | 1.56 | 4.11 | 0.58 | 4.05 | 0.92 | 0.13 | 2.22 | 2.4 |
| 20.8 | 10.6 | 3.4 | 0.05 | 0.86 | 36.9 | 0.31 | 1.58 | 0.40 | 0.06 | 9.84 | 15.2 |
| Size (mm) | wt. % | LOI % |
|---|---|---|
| >3.15 | 14.01 | 1.3 |
| 3.15–1 | 42.20 | 1.15 |
| 1–0.5 | 13.23 | 1.16 |
| 0.5–0.3 | 8.90 | 1.12 |
| 0.3–0.1 | 17.50 | 0.92 |
| <0.1 | 4.16 | 9.84 |
| Water Solution | Na | K | Ca | Mg | Ni | Cd | Zn | Cu | Mn | Fe | pHfinal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | ||
| Deionized | 5.2 | 5.8 | 416 | 0.1 | 0.1 | 0 | 0–0.1 | 0.1 | 0.2 | 0 | 12.71 |
| Deionized-pH-2 | 5.8 | 6.6 | 506 | 0.3 | 0.2 | 0 | 0.1 | 0.1 | 0.3 | 0.1 | 11.57 |
| Size (mm) | Na | K | Ca | Mg | Ni | Cd | Zn | Cu | Mn | Fe | pHfinal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | ||
| >3.15 | 12.5 | 20.1 | 132 | 28.5 | 0.1 | 0–0.1 | 0.4 | 0.1 | 1.3 | 70 | 4.05 |
| 3.15–1 | 11.6 | 19 | 228 | 24 | 0.2 | 0 | 0.5 | 0.2 | 0.6 | 2.1 | 4.51 |
| 1–0.5 | 6.9 | 12.2 | 360 | 0.1 | 0–0.1 | 0 | 0 | 0–0.1 | 0 | 0 | 11.89 |
| 0.5–0.3 | 5.3 | 8.4 | 490 | 0.2 | 0.1 | 0 | 0 | 0 | 0 | 0 | 11.69 |
| 0.3–0.1 | 5.0 | 7.0 | 1160 | 0.1 | 0.1 | 0 | 0.1 | 0 | 0 | 0 | 12.52 |
| <0.1 | 4.6 | 5.9 | 1500 | 0.1 | 0.1 | 0 | 0.1 | 0.1 | 0 | 0 | 12.98 |
| Time (min) | pHi | pHf | % RCd | % RCd | % RCd | % RNi | % RNi | % RNi | % RZn | % RZn | % RZn | % R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 mg/L | 300 mg/L | Multi | 100 mg/L | 300 mg/L | Multi | 100 mg/L | 300 mg/L | Multi | Total | |||
| 5 | 3 | 6.67 | 99.8 | 87.7 | 45.6 | 94.3 | 81.3 | 89.1 | 99.5 | 89.3 | 100 | 78.23 |
| 15 | 3 | 6.93 | 100 | 97.7 | 60.6 | 100 | 84.7 | 95.9 | 99.7 | 99.3 | 100 | 85.5 |
| 30 | 3 | 7.98 | 100 | 99.8 | 73.2 | 100 | 87.3 | 98.9 | 99.7 | 100 | 100 | 90.7 |
| 60 | 3 | 9.62 | 100 | 100 | 88.7 | 100 | 97.7 | 99.7 | 99.8 | 100 | 100 | 96.13 |
| 120 | 3 | 10.71 | 100 | 100 | 99.9 | 100 | 100 | 99.8 | 99.8 | 100 | 100 | 99.9 |
| 180 | 3 | 11.32 | 100 | 100 | 100 | 100 | 100 | 99.9 | 100 | 100 | 100 | 99.97 |
| 5 | 2 | 6.83 | 11 | 6.7 | 11.7 | 6.8 | 34.7 | 17.9 | 30 | 53.3 | 35 | 21.53 |
| 15 | 2 | 6.88 | 34.7 | 42 | 11.2 | 6 | 45.5 | 20.4 | 40 | 58 | 38 | 23.2 |
| 30 | 2 | 7.04 | 60.8 | 65 | 14.5 | 30.2 | 47.3 | 25.5 | 60 | 67.5 | 48 | 29.33 |
| 60 | 2 | 7.04 | 90 | 67.6 | 16.6 | 37.9 | 65.7 | 34.9 | 89.6 | 70.1 | 53 | 34.83 |
| 120 | 2 | 7.07 | 100 | 70 | 21.1 | 100 | 66.3 | 35.9 | 99.8 | 70 | 68.6 | 41.87 |
| 180 | 2 | 7.27 | 100 | 82 | 27.6 | 100 | 66.7 | 56.9 | 100 | 83.3 | 92 | 58.83 |
| Element | Major Component (ppm) | Element | Minor Component (ppb) | ||
|---|---|---|---|---|---|
| Untreated | Treated | Untreated | Treated | ||
| Ni | 17.4 | 0.003 | Mn | 9.23 | 0.3 |
| Zn | 14.8 | 0.003 | Cu | 41.5 | 5.9 |
| Cd | 47.5 | 0.006 | Co | 0.45 | 0.07 |
| As | 5.9 | 0.002 | Se | 5.5 | 1.2 |
| Na | 10.37 | 12.79 | Hg | 4 | 0.34 |
| Mg | 0.9 | 0.95 | Ag | 17.2 | 0.2 |
| K | 10.7 | 11.84 | Pb | 1.9 | 1.47 |
| Ca | 51.04 | 207.5 | U | 0.04 | 0 |
| Tl | 0.04 | 0.02 | |||
| Fe | 0.67 | 1.14 | |||
| Sb | 28 | 9 | |||
| B | 20 | 25 | |||
| Al | 460 | 9 | |||
| Ti | 0.06 | 0.33 | |||
| V | 1 | 1 | |||
| Cr | 11 | 8 | |||
| Mo | 3.4 | 4.7 | |||
| Sr | 171 | 629 | |||
| Sn | 0.06 | 0.17 | |||
| Ba | 8 | 38 | |||
| Time (min) | % Cd Removal | % Ni Removal | % Zn Removal |
|---|---|---|---|
| 5 | 61.54 | 54.4 | 91.45 |
| 15 | 99.99 | 99.98 | 99.98 |
| 30 | 100 | 100 | 100 |
| 60 | 100 | 100 | 100 |
| 120 | 100 | 100 | 100 |
| 180 | 100 | 100 | 100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala, J.; Fernández, B. A Case Study of Landfill Leachate Using Coal Bottom Ash for the Removal of Cd2+, Zn2+ and Ni2+. Metals 2016, 6, 300. https://doi.org/10.3390/met6120300
Ayala J, Fernández B. A Case Study of Landfill Leachate Using Coal Bottom Ash for the Removal of Cd2+, Zn2+ and Ni2+. Metals. 2016; 6(12):300. https://doi.org/10.3390/met6120300
Chicago/Turabian StyleAyala, Julia, and Begoña Fernández. 2016. "A Case Study of Landfill Leachate Using Coal Bottom Ash for the Removal of Cd2+, Zn2+ and Ni2+" Metals 6, no. 12: 300. https://doi.org/10.3390/met6120300