Study of the Precipitation Hardening Behaviour and Intergranular Corrosion of Al-Mg-Si Alloys with Differing Si Contents
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials and Procedures
2.2. Corrosion Tests
2.3. Characterization
3. Results
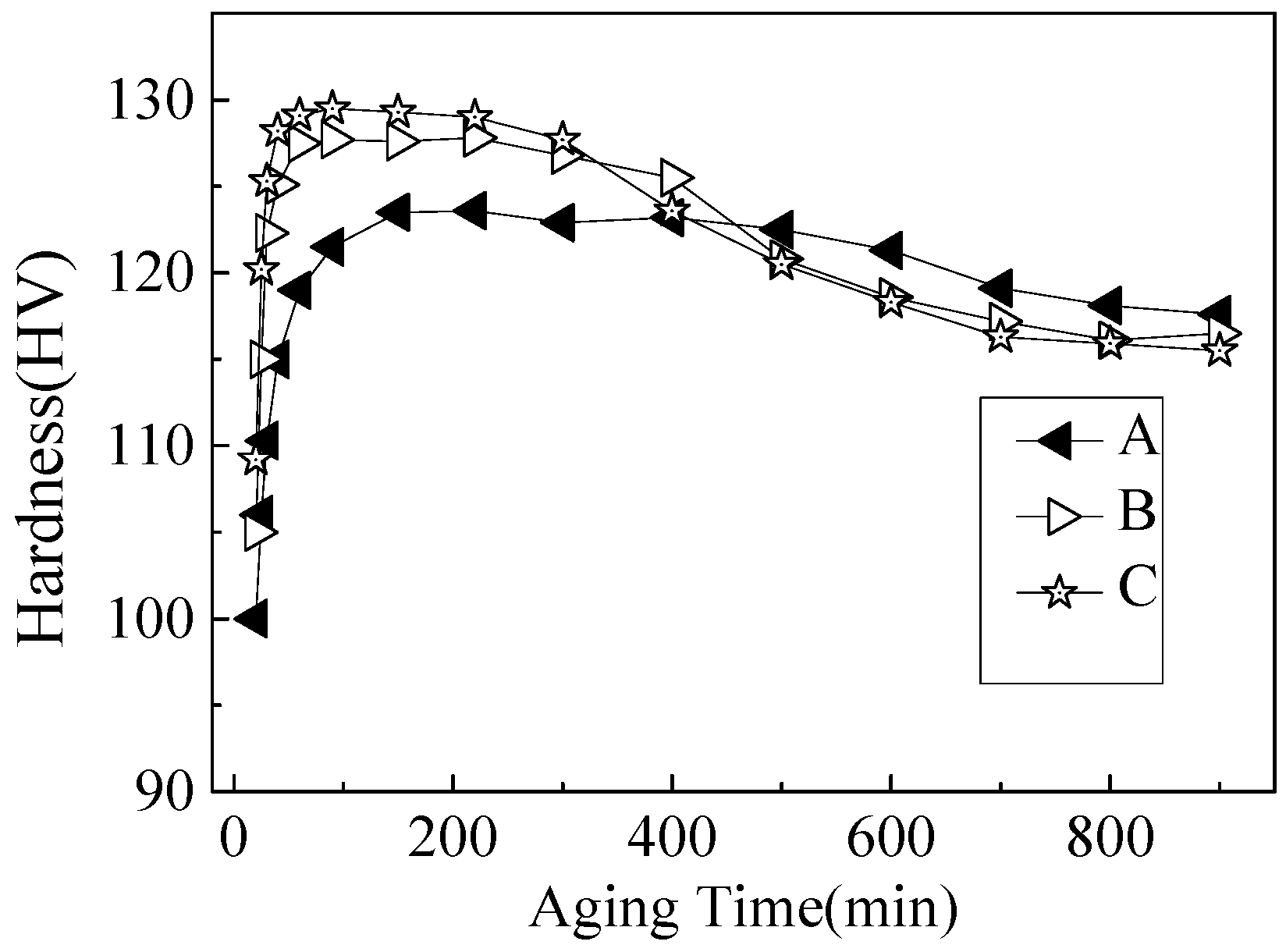
3.1. Hardness Evolution During Ageing at 170 °C
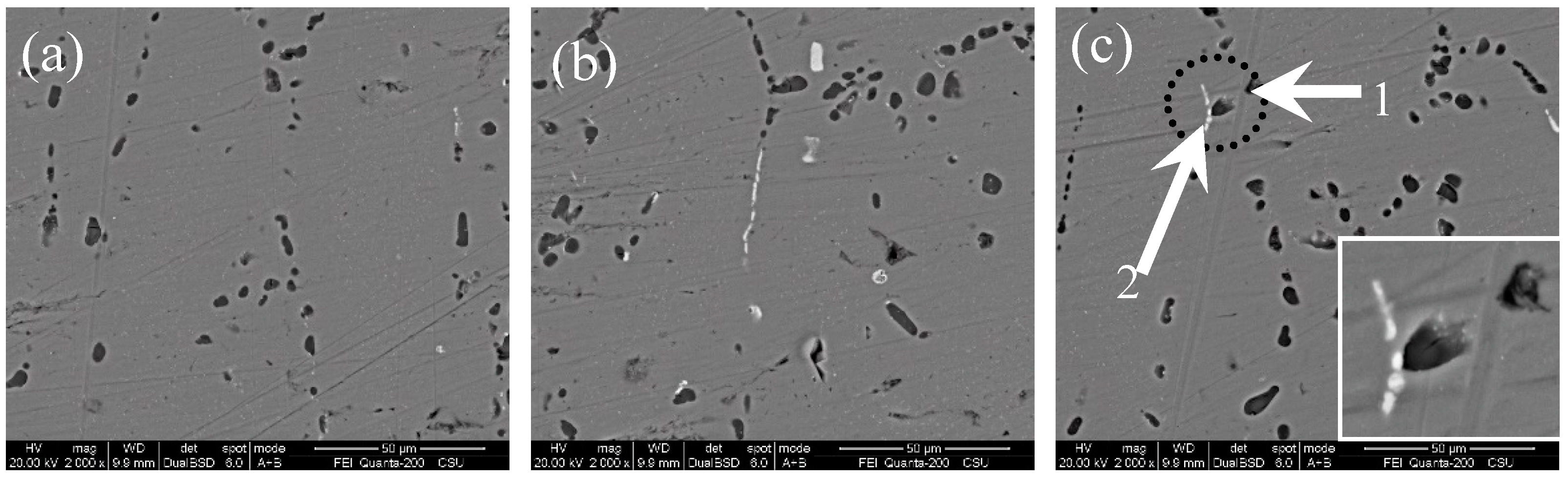
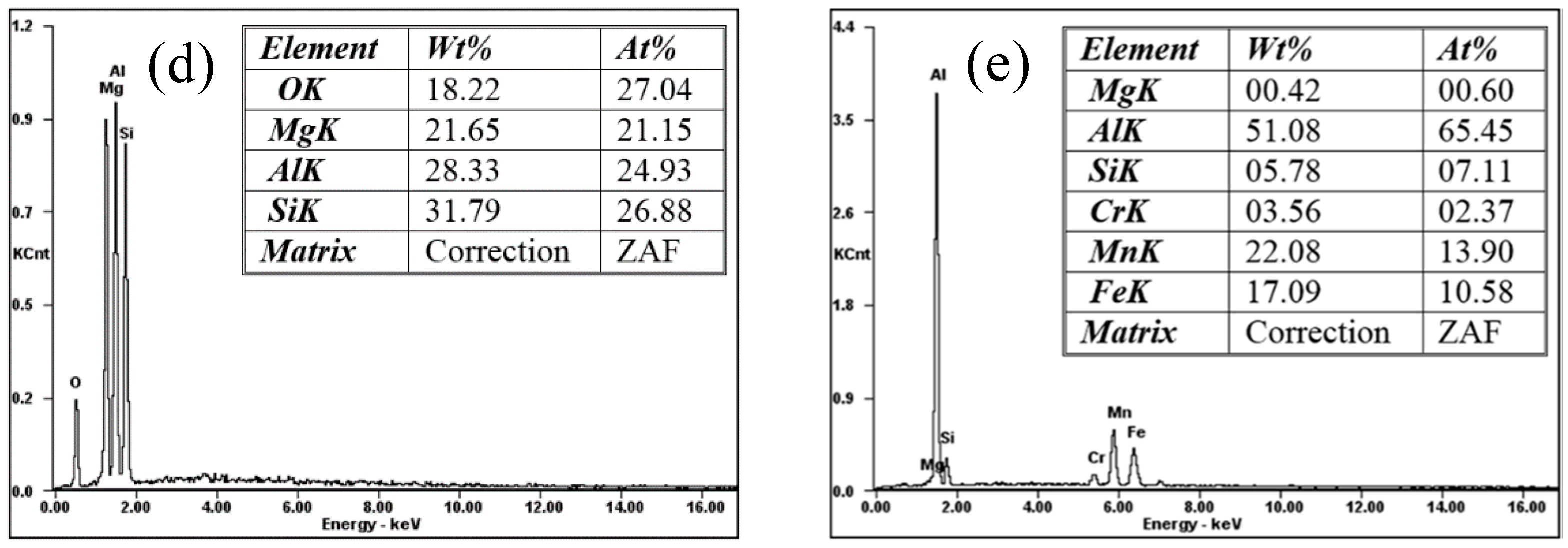
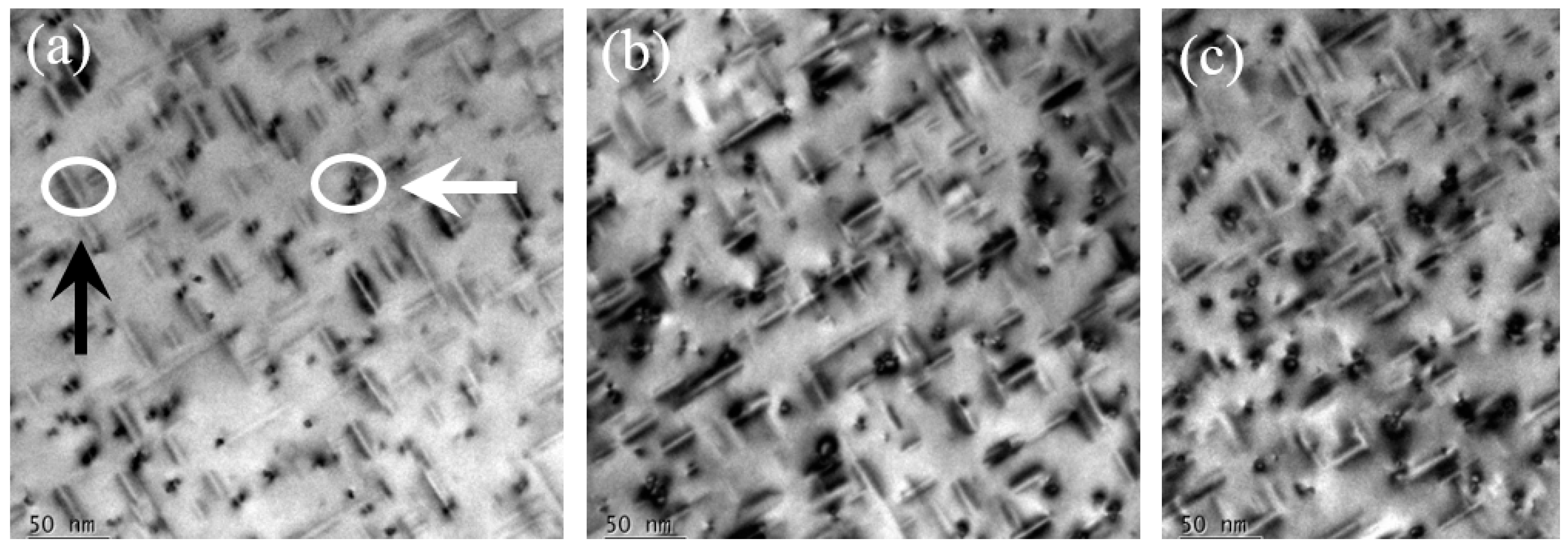
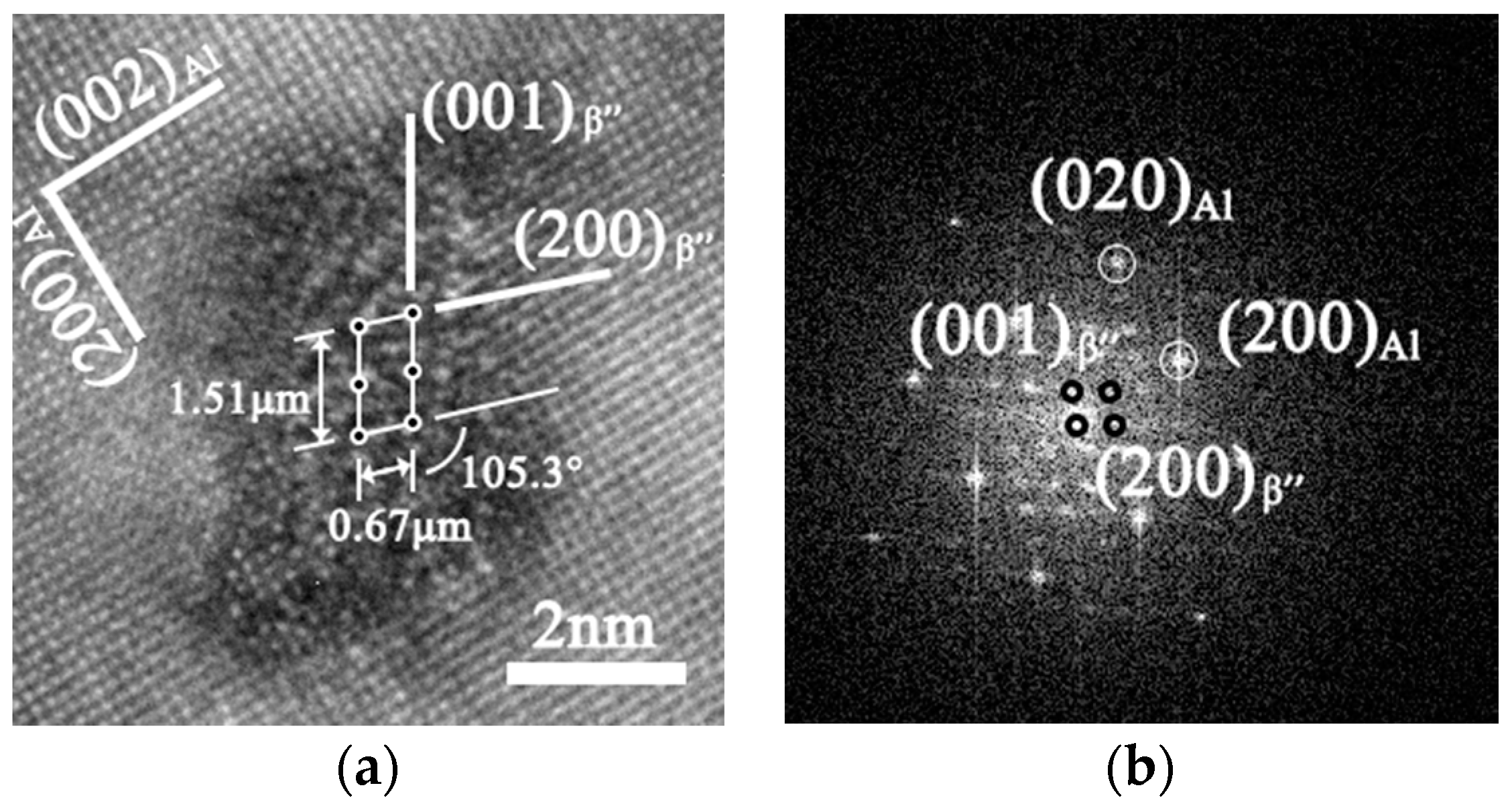
3.2. Microstructure
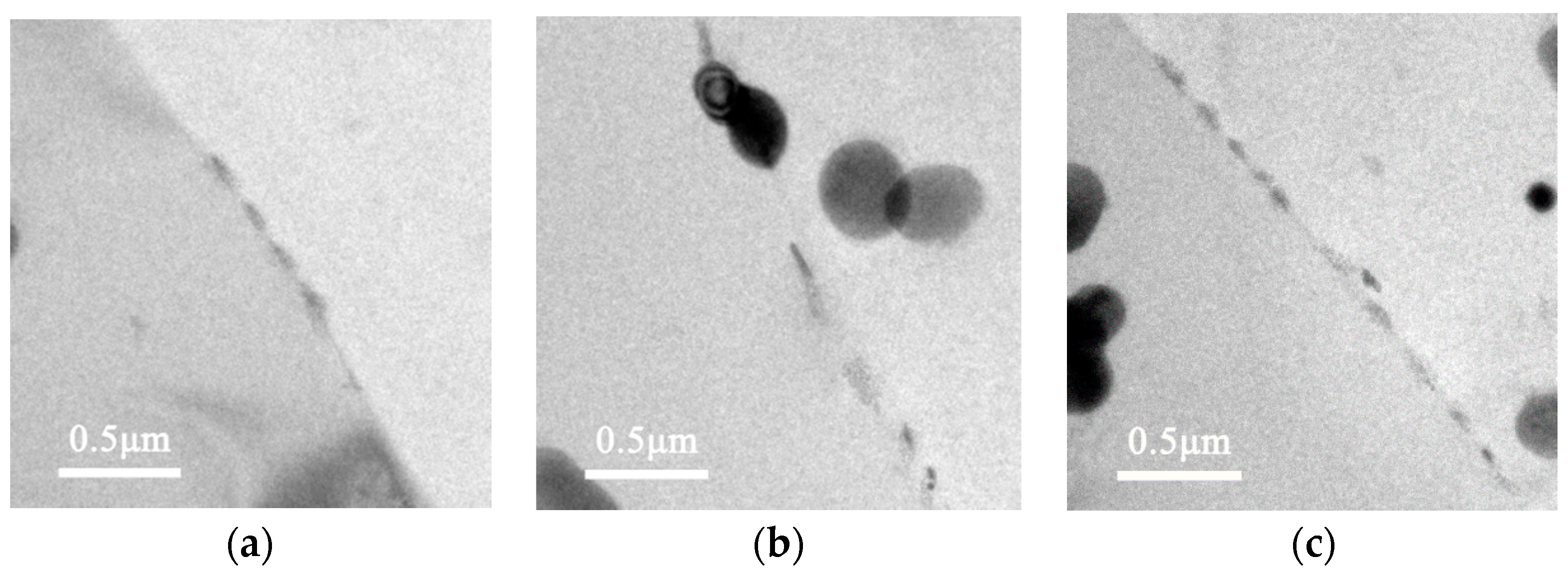
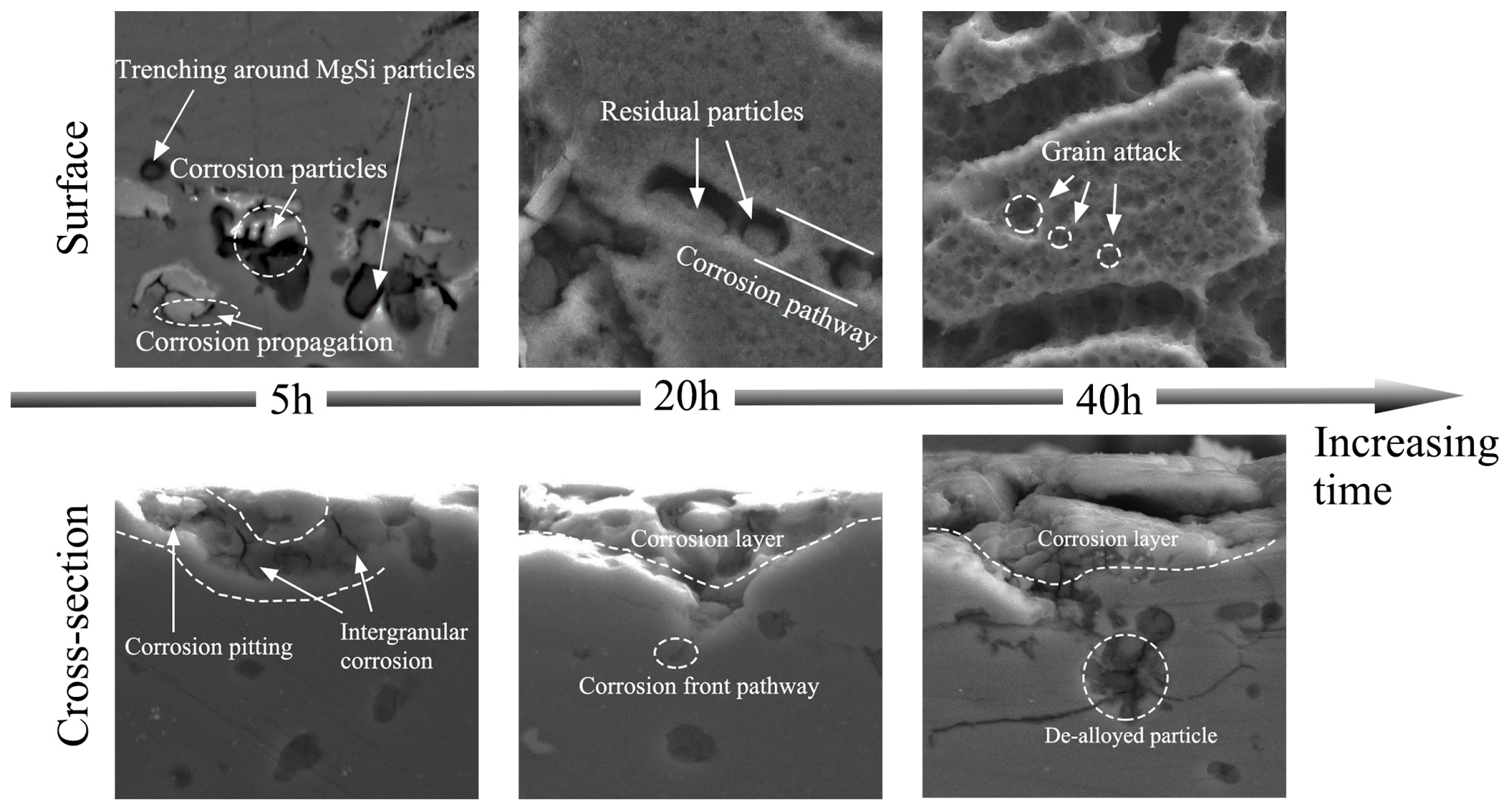
3.3. Corrosion Behaviour
3.4. Potentiodynamic Polarization Tests
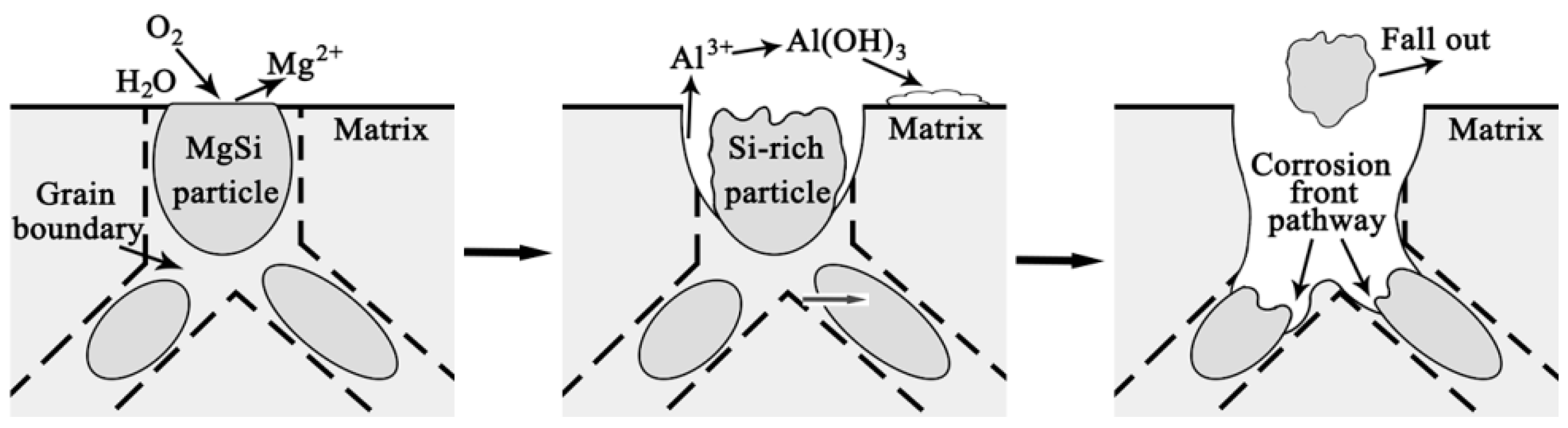
3.5. Corrosion Process
4. Discussion
5. Conclusions
- The hardness of the Al-Mg-Si alloys with peak-ageing treatments mainly originated from contribution from the β′′ phase. With an increased Si content, the age-hardening response improved, and the hardness value also increased by enhancing the quantity and density of the β′′ strengthening phase.
- The microstructures affecting the IGC performances the Al-Mg-Si alloys consisted of MgSi particles, Al-Fe-Mn-Si intermetallics, and the PFZ. The IGC susceptibility of the Al-Mg-Si alloys was mainly attributed to the high electrochemical potential difference between the MgSi particles and solute-depleted zones.
- Corrosion priority initiated from the grain boundary PFZ adjacent to the Al-Fe-Mn-Si intermetallics or from the MgSi precipitate peripheries forming a trench around the particles. Meanwhile, some intermetallics and precipitates displayed self-corrosion until dislodging after forming continuous corrosion channels. With an extended corrosion time, IGC constantly proceeded along the corrosion front pathway.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hua, D.R.; Yang, W.S.; Yu, Z.H.; Wu, P.; Hussain, M.; Jiang, L.T.; Wu, G.H. Aging behavior of 6061Al matrix composite reinforced with high content SiC nanowires. J. Alloys Compd. 2015, 649, 1037–1042. [Google Scholar]
- Lin, C.W.; Hung, F.Y.; Lui, T.S.; Chen, L.H. Microstructure Evolution and High-Temperature Compressibility of Modified Two-Step Strain-Induced Melt Activation-Processed Al-Mg-Si Aluminum Alloy. Metals 2016, 6, 113. [Google Scholar] [CrossRef]
- Kaseem, M.; Choi, K.; Ko, Y.G. A highly compact coating responsible for enhancing corrosion properties of Al-Mg-Si alloy. Mater. Lett. 2017, 196, 316–319. [Google Scholar] [CrossRef]
- Li, J.R.; Kai, W.; Jiang, Q.T.; Sun, H.Y.; Li, Y.T.; Hou, B.R.; Li, W.Z.; Liu, M. Corrosion and Discharge Behaviors of Mg-Al-Zn and Mg-Al-Zn-In Alloys as Anode Materials. Metals 2016, 6, 65. [Google Scholar] [CrossRef]
- Yin, D.; Xiao, Q.; Chen, Y.Q.; Liu, H.Q.; Yi, D.Q.; Wang, B.; Pan, S.P. Effect of natural ageing and pre-straining on the hardening behavior and microstructural response during artificial ageing of an Al-Mg-Si-Cu alloy. Mater. Des. 2016, 95, 329–339. [Google Scholar] [CrossRef]
- Farlkoosh, A.R.; Pekguleryuz, M. Enhanced mechanical properties of an Al-Si-Cu-Mg alloy at 300 °C: Effects of Mg and the Q-precipitate phase. Mater. Sci. Eng. A 2015, 621, 277–286. [Google Scholar] [CrossRef]
- Yin, M.J.; Chen, J.H.; Wang, S.B.; Liu, Z.R.; Cha, L.M.; Duan, S.Y.; Wu, C.L. Anisotropic and temperature-dependent growth mechanism of S-phase precipitates in Al-Cu-Mg alloy in relation with GPB zones. Trans. Nonferrous Met. Soc. China 2016, 26, 1–11. [Google Scholar] [CrossRef]
- Xu, X.X.; Yang, Z.; Ye, Y.L.; Wang, G.X.; He, X.L. Effects of various Mg/Si ratios on microstructure and performance property of Al-Mg-Si alloy cables. Mater. Charact. 2016, 119, 114–119. [Google Scholar] [CrossRef]
- EcKermannae, F.; Sutera, T.; Uggowitzerv, P.J.; Afseth, A.; Schmutz, P. The influence of MgSi particle reactivity and dissolution processes on corrosion in Al-Mg-Si alloys. Electrochim. Acta 2008, 54, 844–855. [Google Scholar] [CrossRef]
- Chrominski, W.; Lewandowska, M. Precipitation phenomena in ultrafine grained Al-Mg-Si alloy with heterogeneous microstructure. Acta Mater. 2016, 703, 547–557. [Google Scholar] [CrossRef]
- Nassef, A.; El-Garaihy, W.H.; El-Hadek, M. Mechanical and Corrosion Behavior of Al-Zn-Cr Family Alloys. Metals 2017, 7, 171. [Google Scholar] [CrossRef]
- Hai, L.; Mao, Q.Z.; Wang, Z.X.; Miao, F.F.; Fang, B.J.; Song, R.G.; Zheng, Z.Q. Enhancing mechanical properties of Al-Mg-Si-Cu sheets by solution treatment substituting for recrystallization annealing before the final cold-rolling. Mater. Sci. Eng. A 2014, 620, 204–212. [Google Scholar]
- Ding, L.P.; Jia, Z.H.; Zhang, Z.Q.; Sanders, R.E.; Liu, Q.; Yang, G. The natural aging and precipitation hardening behavior of Al-Mg-Si-Cu alloys with different Mg/Si ratios and Cu additions. Mater. Sci. Eng. A 2015, 627, 119–126. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, D.; Wang, X.; Ma, Q.B.; Zhuang, L.Z.; Zhang, J.S. Effects of Cu addition on the precipitation hardening response and intergranular corrosion of Al-5.2Mg-2.0Zn (wt %) alloy. Mater. Charact. 2016, 122, 177–182. [Google Scholar] [CrossRef]
- Jiang, J.T.; Xiao, W.Q.; Yang, L.; Shao, W.Z.; Yuan, S.J.; Zhen, L. Ageing behavior and stress corrosion cracking resistance of a non-isothermally aged Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2014, 605, 167–175. [Google Scholar] [CrossRef]
- Song, F.X.; Zhang, X.M.; Liu, S.D.; Tan, Q.; Li, D.F. The effect of quench rate and overaging temper on the corrosion behavior of AA7050. Corros. Sci. 2014, 78, 276–286. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhou, X.R.; Hashimoto, T.; Liu, B. Localized corrosion in AA2024-T351 aluminum alloy: Transition from intergranular corrosion to crystallographic pitting. Mater. Charact. 2017, 130, 230–236. [Google Scholar] [CrossRef]
- Bonfils-Lahovary, M.L.; Laffont, L.; Blanc, C. Characterization of intergranular corrosion defects in a 2024 T351 aluminum alloy. Corros. Sci. 2017, 119, 60–67. [Google Scholar] [CrossRef]
- Bonzom, R.; Oltra, R. Droplet cell investigation of intergranular corrosion on AA2024. Electrochem. Commun. 2017, 81, 84–87. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, L.J.; Du, Y. Diffusivities in liquid and fcc Al-Mg-Si alloys and their application to the simulation of solidification and dissolution processes. Calphad 2015, 49, 58–66. [Google Scholar] [CrossRef]
- Ferguson, J.B.; Lopez, H.E.; Cho, K.; Kim, C.S.; Farlkoosh, A.R.; Pekguleryuz, M. Temperature Effects on the Tensile Properties of Precipitation-Hardened AI-Mg-Cu-Si Alloys. Metals 2016, 6, 43. [Google Scholar] [CrossRef]
- Kaseem, M.; Min, J.H.; Ko, Y.G. Corrosion behavior of Al-1 wt % Mg-0.85 wt % Si alloy coated by micro-arc-oxidation using TiO2 and Na2MoO4 additives: Role of current density. J. Alloys Compd. 2017, 723, 448–455. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Nordlien, J.H. Nisancioglu, K. Effect of high temperature heat treatment on intergranular corrosion of AlMgSi(Cu) model alloy. Corros. Sci. 2006, 48, 258–272. [Google Scholar] [CrossRef]
- Mol, J.M.C.; Langkruis, J.; Wit, J.H.W.; Zwaag, S. An integrated study on the effect of pre-and post-extrusion heat treatments and surface treatment on the filiform corrosion properties of an aluminum extrusion alloy. Corros. Sci. 2005, 47, 2711–2730. [Google Scholar] [CrossRef]
- Li, J.F.; Maier, B.; Frankel, G.S. Corrosion of an Al-Mg-Si alloy under MgCl2 solution droplets. Corros. Sci. 2011, 53, 2142–2151. [Google Scholar] [CrossRef]
- Mizuno, K.; Nylund, A.; Olefjord, I. Surface reactions during pickling of an aluminum-magnesium-silicon alloy in phosphoric acid. Corros. Sci. 2001, 43, 381–396. [Google Scholar] [CrossRef]
- Li, C.; Sun, J.Y.; Li, Z.D.; Gao, Z.M.; Liu, Y.C.; Yu, L.M.; Li, H.J. Microstructure and corrosion behavior of Al-10%Mg2Si cast alloy after heat treatment. Mater. Charact. 2016, 122, 142–147. [Google Scholar] [CrossRef]
- Zeng, L.F.; Wei, Z.L.; Li, J.F.; Li, C.X.; Xing, T.; Zhao, Z.; Zheng, Z.Q. Corrosion mechanism associated with Mg2Si and Si particles in Al-Mg-Si alloys. Trans. Nonferrous Met. Soc. China 2011, 21, 2559–2567. [Google Scholar] [CrossRef]
- Li, C.X.; Li, J.F.; Birbilis, N.; Jia, Z.Q.; Zheng, Z.Q. Synergetic effect of Mg2Si and Si particles on intergranular corrosion of Al-Mg-Si alloys through multi-electrode coupling system. J. Chin. Soc. Corros. Prot. 2010, 30, 107–113. [Google Scholar]
- Blanc, C.; Roques, Y.; Mankowski, G. Application of phase shifting interferometric microscopy to studies of the behavior of coarse intermetallic particles in 6056 aluminum alloys. Corros. Sci. 1998, 40, 1019–1035. [Google Scholar] [CrossRef]
- Ahlatci, H. Production and corrosion behaviors of the Al-12Si-XMg alloys containing in situ Mg2Si particles. J. Alloys Compd. 2010, 503, 122–126. [Google Scholar] [CrossRef]
- Bhattamishra, A.K.; Lal, K. Microstructural studies on the effect of Si and Cr on the intergranular corrosion in Al-Mg-Si alloys. Mater. Des. 1997, 27, 25–28. [Google Scholar] [CrossRef]
- Liang, W.J.; Rometsch, P.A.; Cao, L.F.; Birbilis, N. General aspects related to the corrosion of 6xxx series aluminum alloys: Exploring the influence of Mg/Si ratio and Cu. Corros. Sci. 2013, 76, 119–128. [Google Scholar] [CrossRef]
- Wang, Z.X.; Li, H.; Miao, F.F.; Sun, W.Z.; Fang, B.F.; Song, R.G.; Zheng, Z.Q. Improving the intergranular corrosion resistance of Al-Mg-Si-Cu alloys without strength loss by a two-step aging treatment. Mater. Sci. Eng. A 2014, 590, 267–273. [Google Scholar] [CrossRef]
- Masuda, T.; Takaki, Y.; Sakurai, T.; Hirosawa, S. Combined effect of pre-straining and pre-aging on bake-hardening behavior of an A1-0.6 massy Mg-1.0 massy Si alloy. Mater. Trans. 2010, 51, 325–332. [Google Scholar] [CrossRef]
- Yassar, R.S.; Cai, M.; Field, D.P.; Chen, X.; Asay, J. The effect of shock-loading aging behavior of an Al-Mg-Si alloy. J. Mater. Sci. 2006, 41, 1711–1720. [Google Scholar] [CrossRef]
- Buchanan, K.; Colas, K.; Ribis, J.; Lopez, A.; Gamier, J. Analysis of the metastable precipitates in peak-hardness aged Al-Mg-Si(-Cu) alloys with differing Si contents. Acta Mater. 2017, 132, 209–221. [Google Scholar] [CrossRef]
- Ding, X.P.; Cui, H.; Zhang, J.X.; Li, H.X.; Guo, M.X.; Lin, Z.; Zhuang, L.Z.; Zhang, J.S. The effect of Zn on the age hardening response in an Al-Mg-Si alloy. Mater. Charact. 2015, 65, 1229–1235. [Google Scholar] [CrossRef]
- Chen, J.H.; Costan, E.; Van-huis, M.A.; Xu, Q.; Zandbergen, H.W. Atomic Pillar-Based Nanoprecipitates Strengthen AlMgSi Alloys. Science 2006, 312, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Qing, L.; Jia, Z.H.; Xing, Y.; Ding, L.P.; Wang, X.L. The intergranular corrosion behavior of 6000-series alloys with different Mg/Si and Cu content. Appl. Surf. Sci. 2017, 405, 489–496. [Google Scholar] [CrossRef]


| Alloys (Composition in wt %) | Heat Treatment Conditions | Corrosion Form | Corrosion Mechanism |
|---|---|---|---|
| Al-6.34Mg-3.66Si | 520 °C/6 h (ST) + aged at 200 °C/6 h | IGC | Propagation of corrosion pits in the corrosive media occurs along the interface between the MgSi particles and α-Al [27]. |
| Al-0.63Mg-0.28Si and Al-0.63Mg-0.88Si | ST + aged at 175 °C/1 h | The ratio of Mg to Si less than 1.73 resulted in IGC | Corrosion initiates on the MgSi surface and PFZ. Corrosion develops along the grain boundary PFZ at the adjacent MgSi precipitates [28]. |
| Al-1.31Si-0.4Mg and Al-0.6Si-0.52Mg-0.18Cu | 540 °C/30 min (ST) + aged at 185 °C | Aged sample is susceptible to IGC | IGC caused by micro-galvanic coupling between the cathodic Cu-containing precipitates and solute-depleted active zone [29]. |
| Al-0.86Mg-0.92Si | 550 °C/ST + aged at 175 °C/8 h | - | Very strong dissolution of the Al-Mg- and Si-containing particles along the grain boundaries [30]. |
| Al-12Si-0Mg and Al-12Si-5Mg and Al-12Si-10Mg and Al-12Si-20Mg | Casting | - | Corrosion primarily initiates from the Al matrix adjacent to the primary Mg2Si particles [31]. |
| Al-0.4Mg-1.0Si | 550 °C/1 h + aged at 175 °C/16 h | IGC | Precipitation of MgSi at the grain boundary favours intergranular corrosion attack [32]. |
| Al-0.6Mg-0.5Si | T6 | - | MgSi particles were anodically precipitated compared to the Al matrix in acidic and neutral pH [33]. |
| Sample | Chemical Compositions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mg | Si | Fe | Cr | Mn | Zr | Ti | Ag | Al | |
| A | 1.91 | 1.21 | ≤0.1 | 0.13 | 0.29 | 0.098 | 0.1 | 0.1 | Bal. |
| B | 1.91 | 1.73 | ≤0.1 | 0.12 | 0.29 | 0.11 | 0.1 | 0.1 | Bal. |
| C | 1.90 | 2.52 | ≤0.1 | 0.14 | 0.28 | 0.10 | 0.1 | 0.1 | Bal. |
| Alloy | Maximum Corrosion Depth (μm) | IGC Level |
|---|---|---|
| A | 65 | 3 |
| B | 89 | 3 |
| C | 96 | 4 |
| Alloy | Corrosion Potential, Ecorr (mV) | Corrosion Current Density, Icorr (μA/cm2) | Pitting Potential, Epit (V) |
|---|---|---|---|
| A | −532 ± 6 | 0.47 ± 0.02 | –0.408 |
| B | −612 ± 4 | 0.55 ± 0.06 | –0.440 |
| C | −606 ± 6 | 0.57 ± 0.04 | –0.453 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Luo, B.; Bai, Z.; Wang, J.; Yin, Y. Study of the Precipitation Hardening Behaviour and Intergranular Corrosion of Al-Mg-Si Alloys with Differing Si Contents. Metals 2017, 7, 387. https://doi.org/10.3390/met7100387
Zheng Y, Luo B, Bai Z, Wang J, Yin Y. Study of the Precipitation Hardening Behaviour and Intergranular Corrosion of Al-Mg-Si Alloys with Differing Si Contents. Metals. 2017; 7(10):387. https://doi.org/10.3390/met7100387
Chicago/Turabian StyleZheng, Yaya, Binghui Luo, Zhenhai Bai, Juan Wang, and Yuan Yin. 2017. "Study of the Precipitation Hardening Behaviour and Intergranular Corrosion of Al-Mg-Si Alloys with Differing Si Contents" Metals 7, no. 10: 387. https://doi.org/10.3390/met7100387




