Characterization of the Microstructure, Mechanical Properties, and Corrosion Resistance of a Friction-Stir-Welded Joint of Hyper Duplex Stainless Steel
Abstract
:1. Introduction
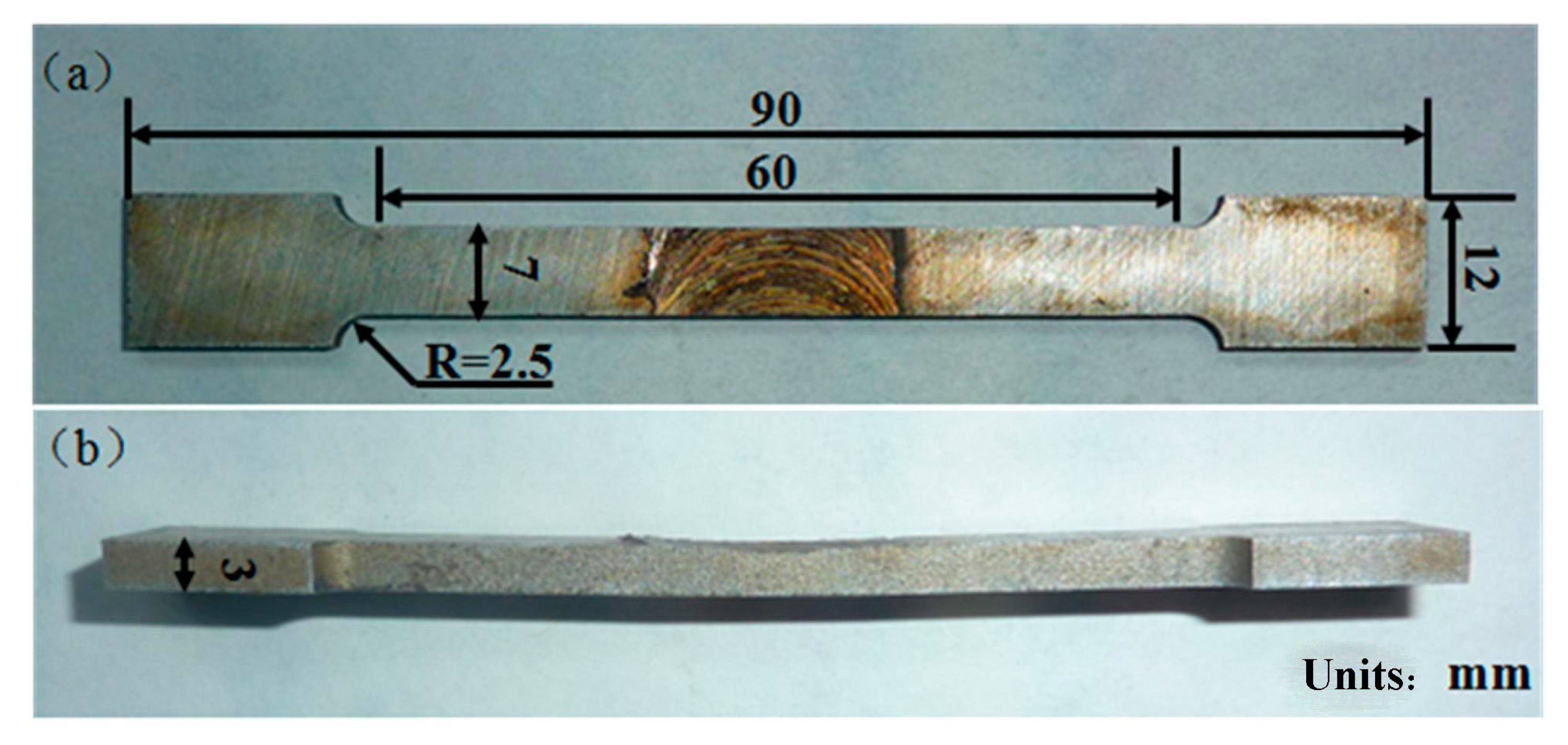
2. Materials & Experiment
3. Results and Discussion
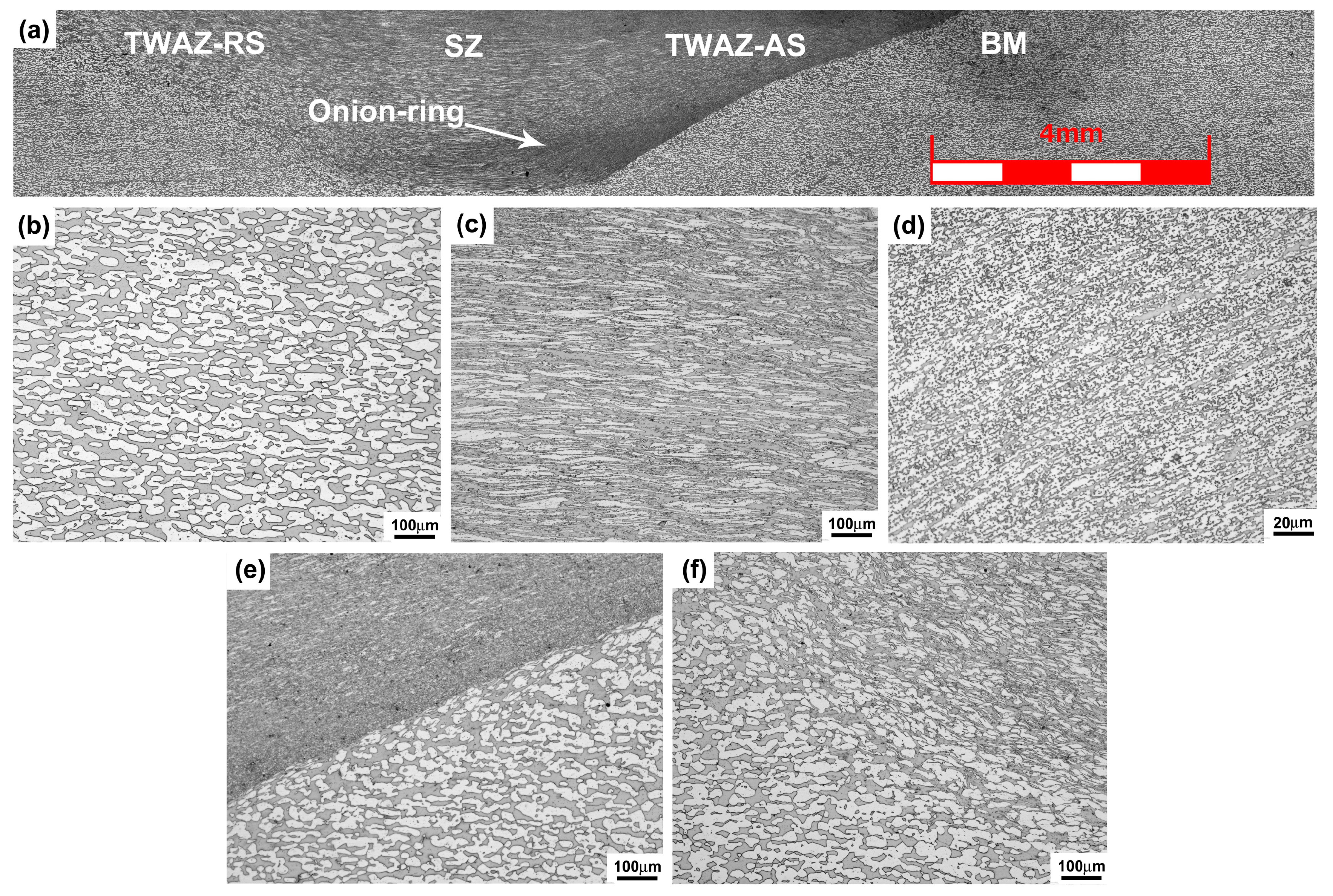
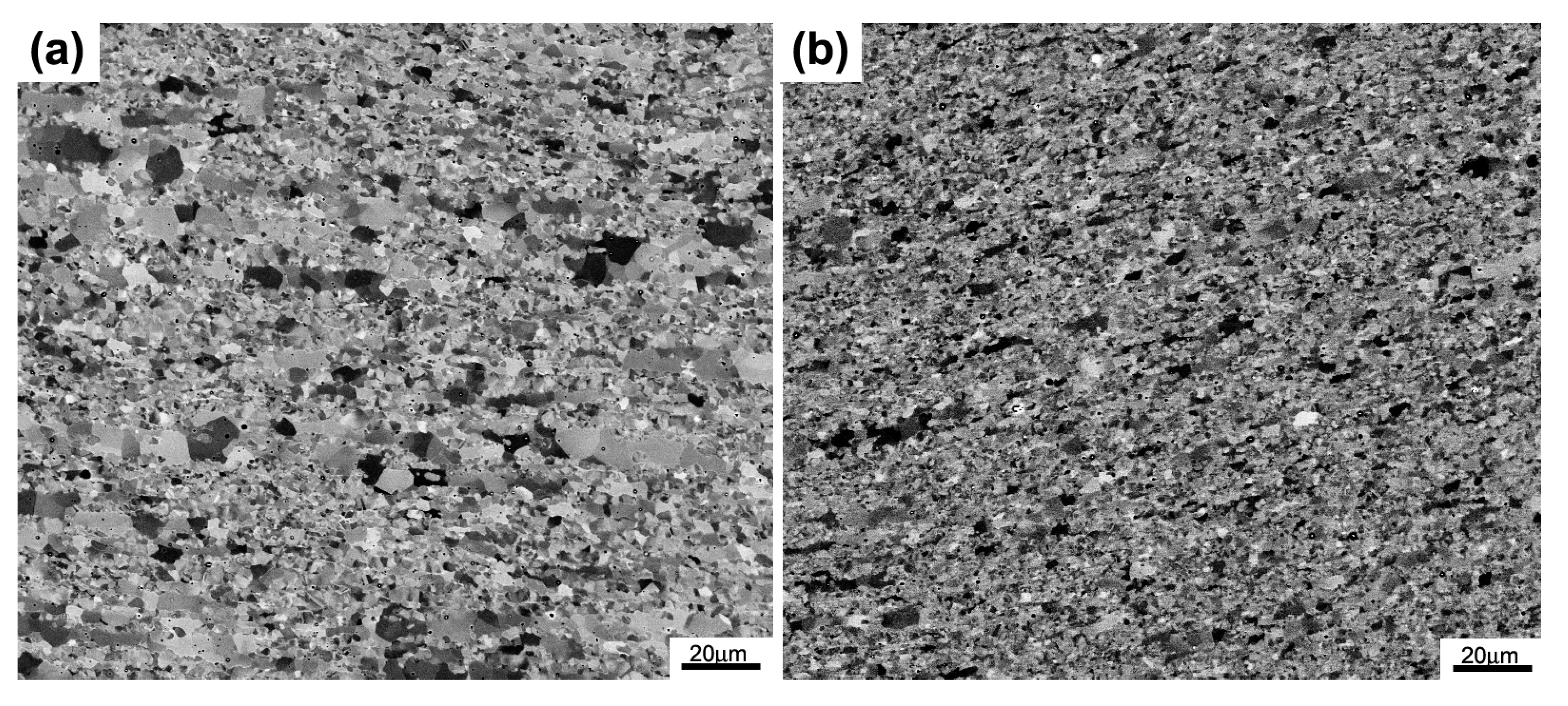
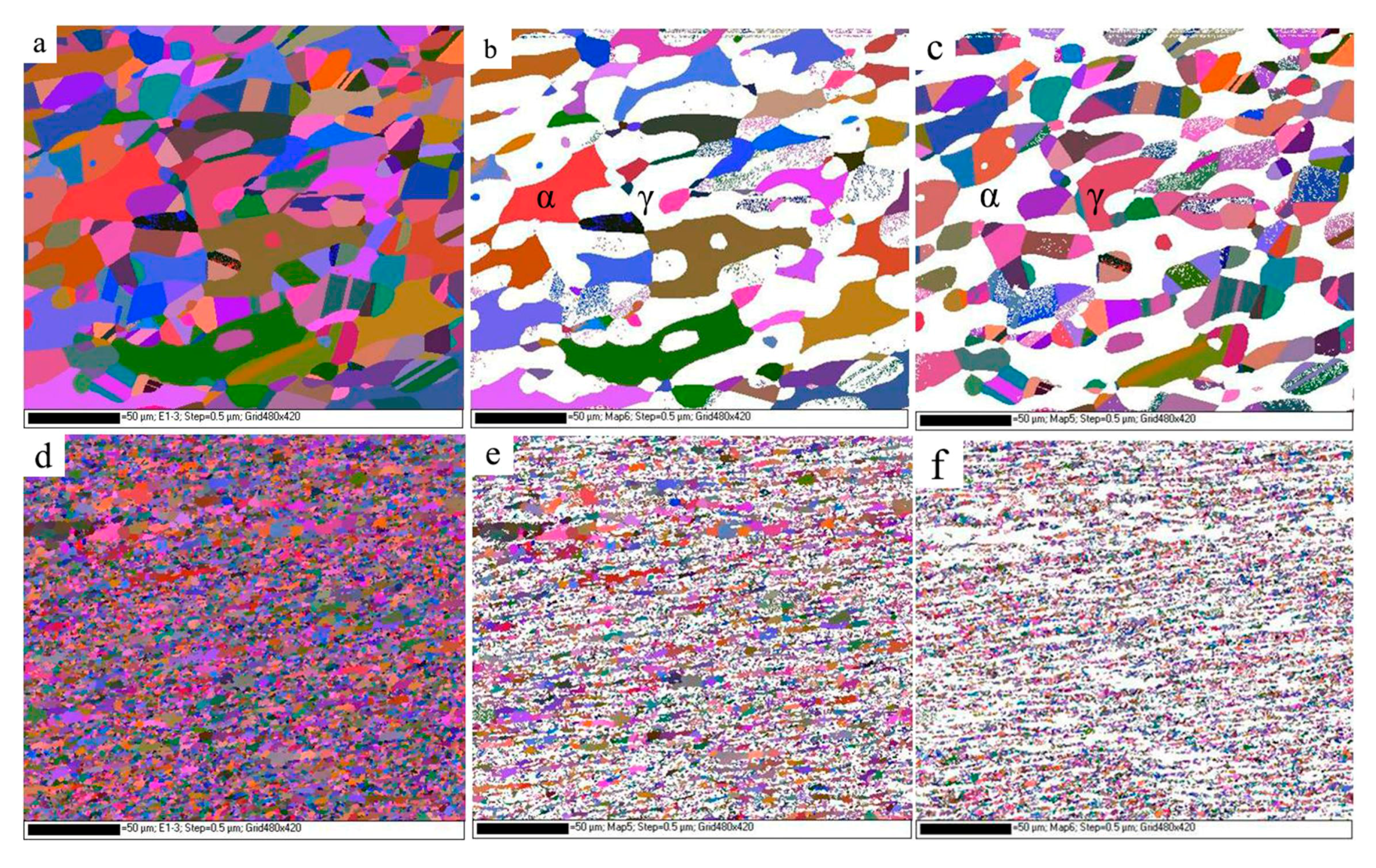
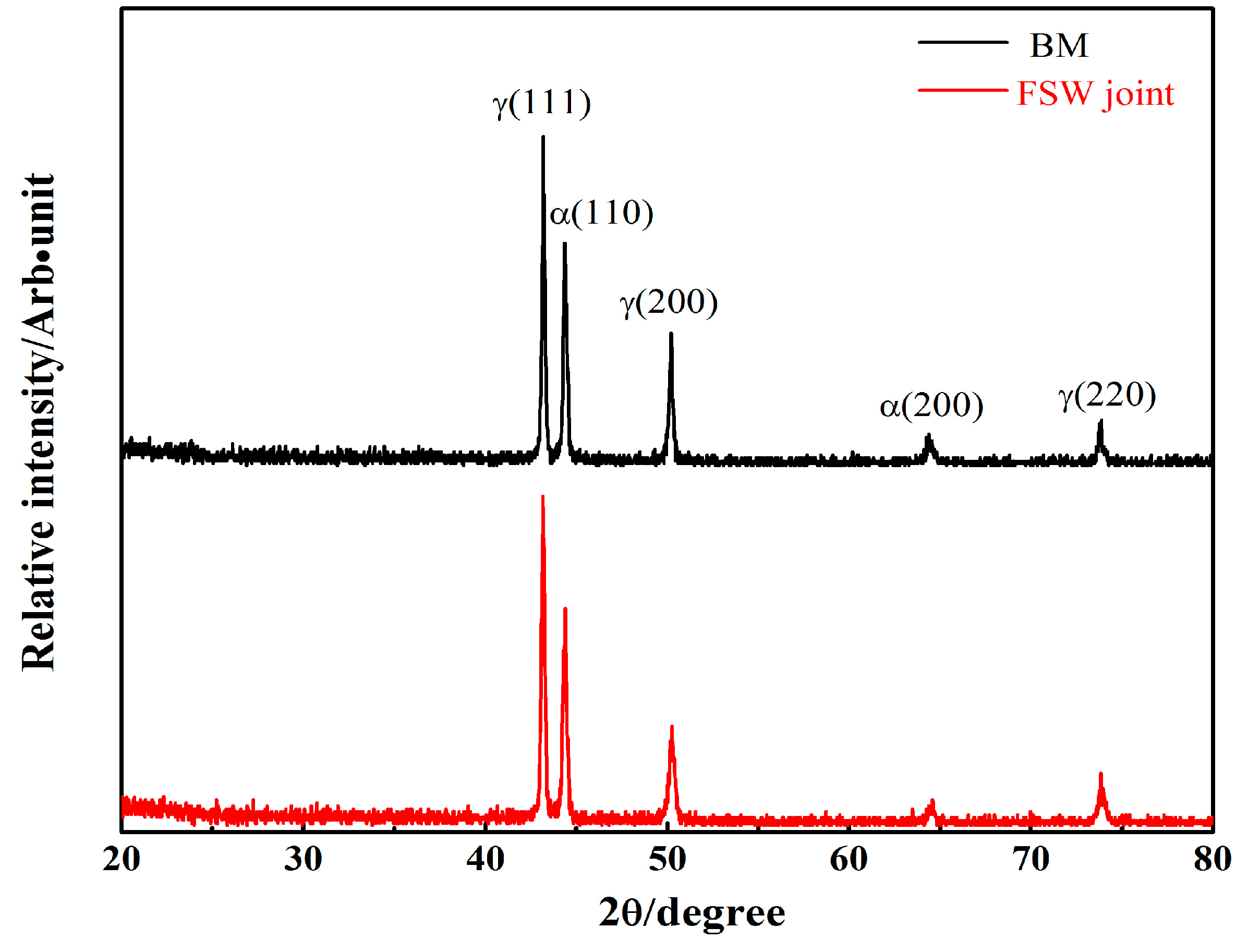
3.1. Microstructural Evolution
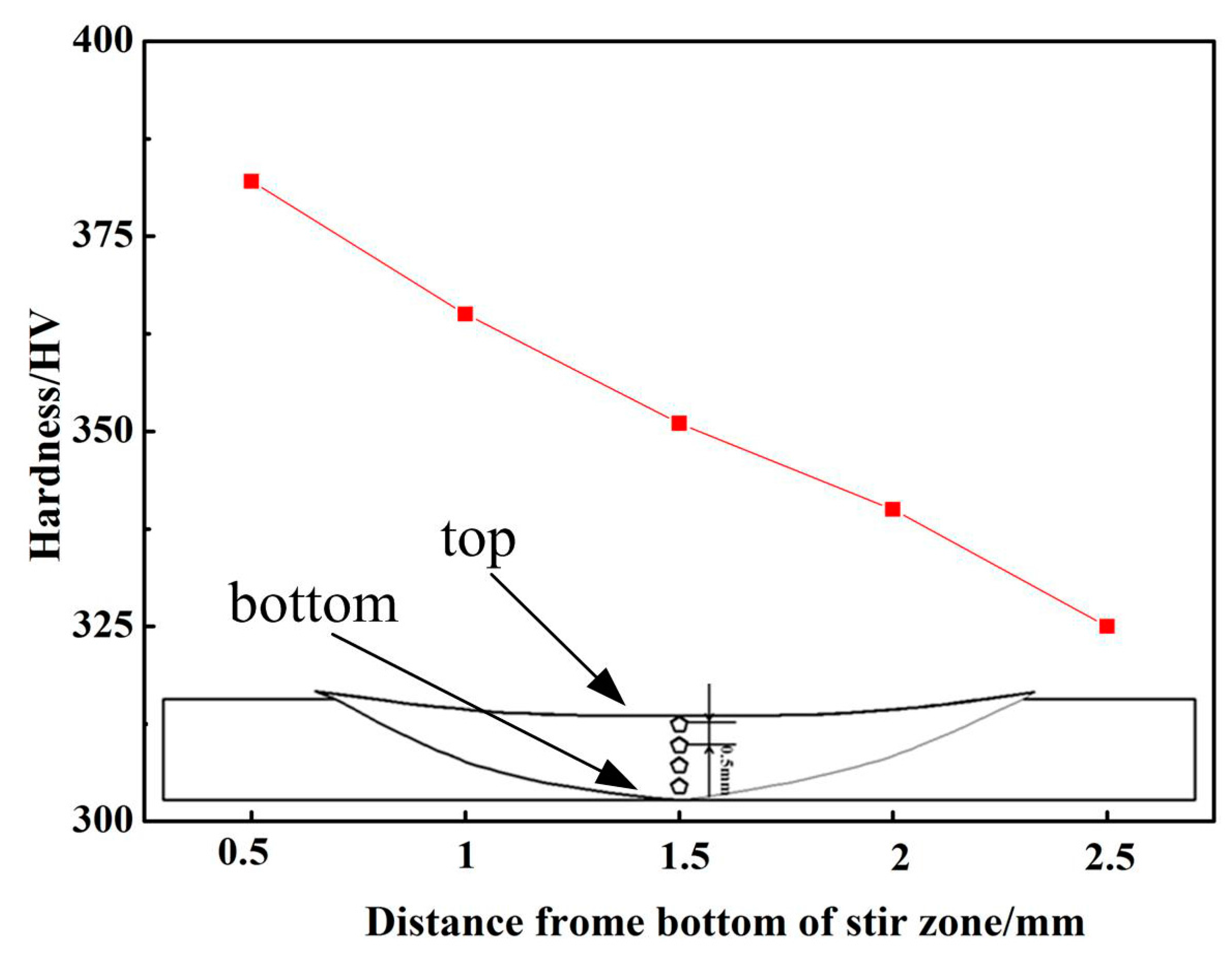
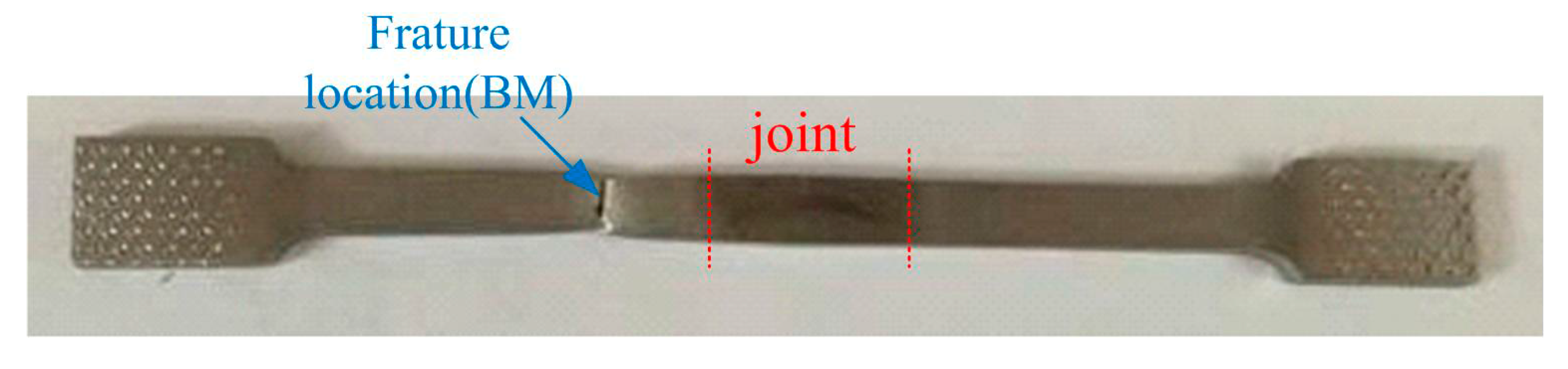
3.2. Mechanical Characterization
3.3. Corrosion Resistance
4. Conclusions
- (1)
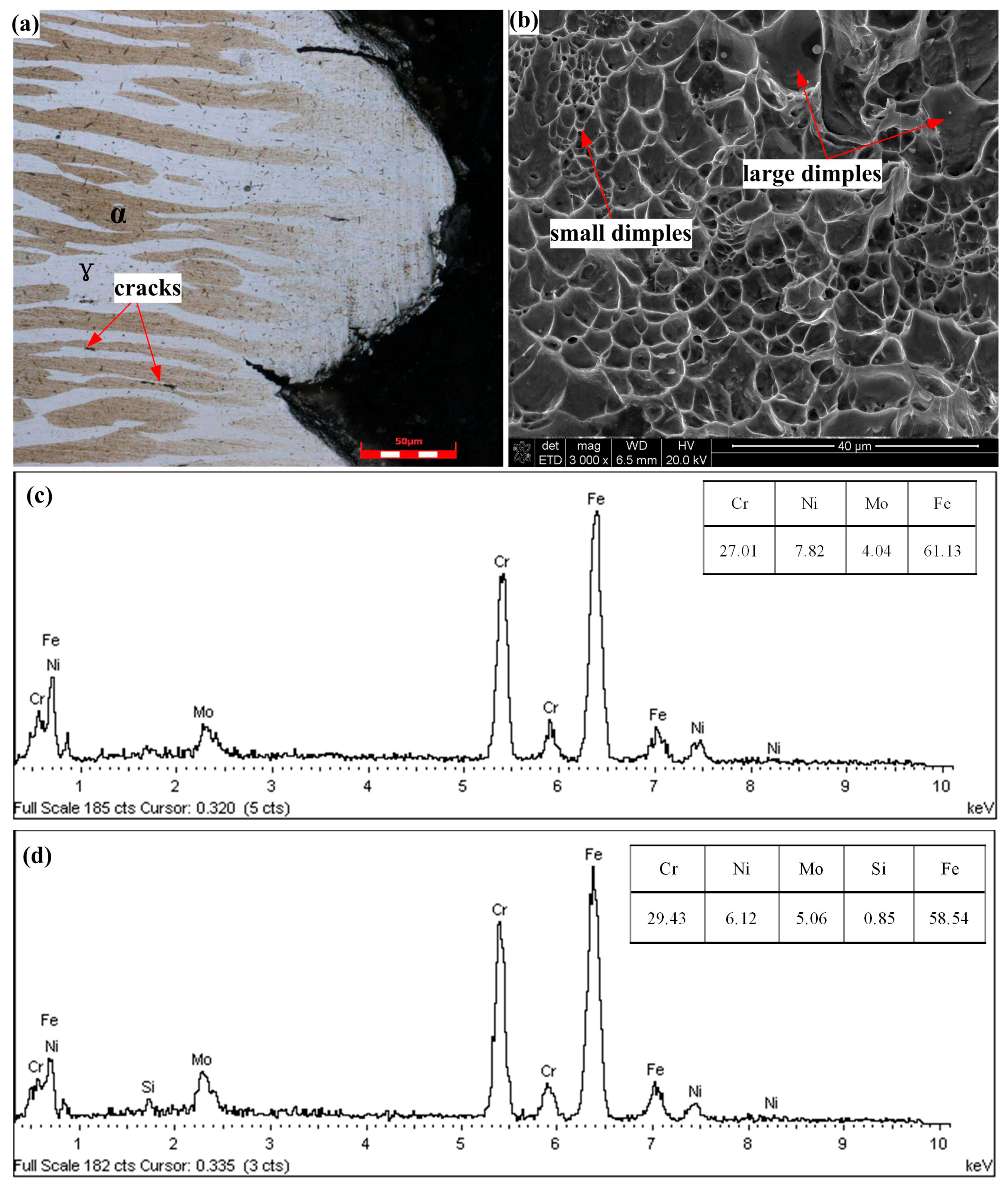
- A high-quality FSW joint without deleterious phases is obtained by rapidly cooling the samples. The welded joint microstructure contains fine equiaxed grains in the SZ. The grains are exceptionally pronounced in the austenite phase.
- (2)
- Failure of the tensile specimens is consistently located in the BM zone, suggesting that the welded joint overly matches the BM. Owing to their fine grains, the welded joints display a higher tensile strength than their BMs.
- (3)
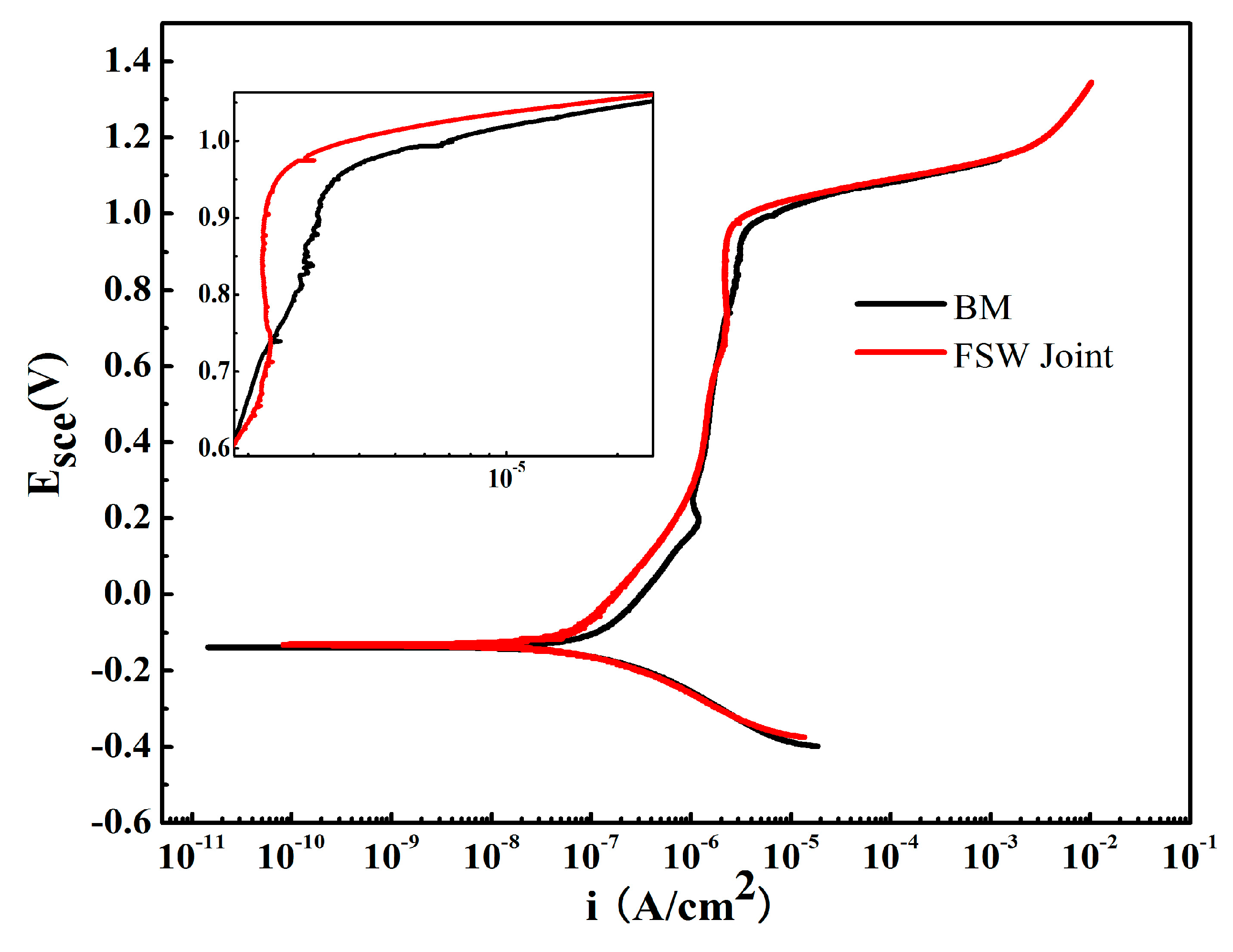
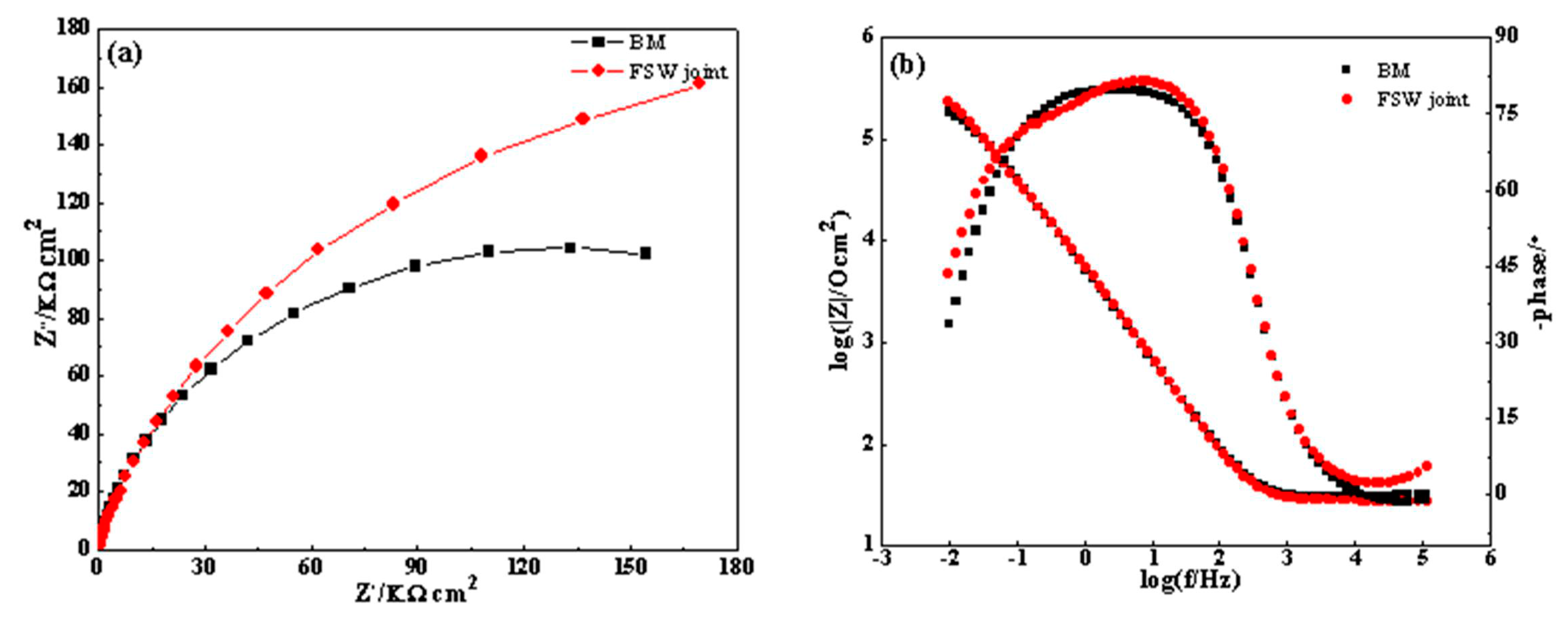
- In the potentiodynamic polarization and impedance spectroscopy tests in NaCl solution, all SZs exhibit corrosion resistance superior to that of the BM.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hayes, F.H. Phase equilibria in duplex stainless steel. J. Less Common Met. 1985, 114, 89–96. [Google Scholar] [CrossRef]
- Weibull, I. Duplex stainless steels and their application, particularly in centrifugal separators: Part B Corrosion resistance. Mater. Des. 1987, 8, 82–88. [Google Scholar] [CrossRef]
- Kenneth, G.; Marie-Louise, N.; Martin, H.; Eduardo, G. Sandvik SAF 2707HD (UNS S32707)—A Hyper-Duplex Stainless Steel for Severe Chloride Containing Environments; Internal Lecture No. S-51-63; Sandvik Materials Technology: Sandviken, Sweden, 2006. [Google Scholar]
- Chail, G.; Kangas, P. Super and hyper duplex stainless steels: Structure, properties and application. Procedia Struct. Integr. 2016, 2, 1755–1762. [Google Scholar] [CrossRef]
- Ha, H.; Jang, M.; Lee, T.; Moon, J. Understanding the relation between phase fraction and pitting corrosion resistance of UNS S32759 stainless steel. Mater. Charact. 2015, 106, 338–345. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Mondal, S. High temperature ageing behaviour of a duplex stainless steel. Mater. Charact. 2008, 59, 1776–1783. [Google Scholar] [CrossRef]
- Sathirachinda, N.; Pettersson, R.; Pan, J. Depletion effects at phase boundaries in 2205 duplex stainless steel characterized with SKPFM and TEM/EDS. Corros. Sci. 2009, 51, 1850–1860. [Google Scholar] [CrossRef]
- Deng, B.; Wang, Z.; Jiang, Y.; Sun, T.; Xu, J.; Li, J. Effect of cycles on the corrosion on the corrosion and mechanical properties of UNS S31803 duplex stainless steel. Corros. Sci. 2009, 51, 2969–2975. [Google Scholar] [CrossRef]
- Badji, R.; Bouabdallah, M.; Bacroix, B. Phase transformation and mechanical behavior in annealed 2205 duplex stainless seel welds. Mater. Charact. 2008, 59, 447–453. [Google Scholar] [CrossRef]
- Stenvall, P.; Holmquist, M. Weld Properties of Sandvik SAF 2707 HD; Sandvik materials technology: Sandviken, Sweden, 2008; Volume 11. [Google Scholar]
- Pramanik, A.; Littlefair, G.; Basak, A.K. Weldability of duplex stainless steel. Mater. Manuf. Process 2015, 30, 1053–1068. [Google Scholar] [CrossRef]
- Hsieh, R.I.; Liou, H.Y.; Pan, Y.T. Effects of cooling time and alloying elements on the microstructure of the Gleeble-simulated heat affected zone of 22% Cr duplex stainless steels. J. Mater. Sci. Perform. 2001, 10, 526–536. [Google Scholar] [CrossRef]
- Pardal, J.M.; Tavares, S.S.M.; Cindra Fonseca, M.; de Souza, J.A.; Côrte, R.R.A.; de Abreu, H.F.G. Influence of the grain size on deleterious phase precipitation in superduplex stainless steel UNS S32750. Mater. Charact. 2009, 60, 165–172. [Google Scholar] [CrossRef]
- Sieurin, H.; Sandstrom, R. Sigma phase precipitation in duplex stainless steel 2205. Mater. Sci. Eng. A 2007, 444, 271–276. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, J.; Tae, K.; Park, K.; Lee, C.S. Effect of Ce addition on secondary phase transformation and mechanical properties of 27Cr-7Ni hyper duplex stainless steels. Mater. Sci. Eng. A 2013, 573, 27–36. [Google Scholar] [CrossRef]
- Chen, Z.W.; Cui, S. On the forming mechanism of banded structures in aluminium alloy friction stir welds. Scr. Mater. 2008, 58, 417–420. [Google Scholar] [CrossRef]
- Hirata, T.; Oguri, T.; Hagino, H.; Tanaka, T. Influence of friction stir welding parameters on grain size and formablity in 5083 aluminum alloy. Mater. Sci. Eng. A 2007, 456, 344–349. [Google Scholar] [CrossRef]
- Sato, Y.S.; Nelson, T.W.; Sterling, C.J.; Steel, R.J.; Pettersson, C.O. Microstructure and mechanical properties of friction Stir Welded SAF 2507 super duplex stainless steel. Mater. Sci. Eng. A 2005, 397, 376–384. [Google Scholar] [CrossRef]
- Saeid, T.; Abdollah-Zadeh, A.; Shibayanagi, T.; Ikeuchi, K.; Assadi, H. On the formation of grain structure during friction stir welding of duplex stainless steel. Mater. Sci. Eng. A 2010, 527, 6484–6488. [Google Scholar] [CrossRef]
- Sarlak, H.; Atapour, M.; Esmailzadeh, M. Corrosion behavior of friction stir welded lean duplex stainless steel. Mater. Des. 2015, 66, 209–216. [Google Scholar] [CrossRef]
- Kim, S.; Jang, S.; Lee, I.; Park, Y. Effects of solution heat-treatment and nitrogen in shielding gas on the resistance to pitting corrosion of hyper hyper duplex stainless steel welds. Corros. Sci. 2011, 53, 1939–1947. [Google Scholar] [CrossRef]
- Jang, S.; Kim, S.; Lee, I.; Park, Y. Effect of shielding gas composition on phase transformation and mechanism of pitting corrosion of hyper duplex stainless steel welds. Mater. Trans. 2011, 52, 1228–1236. [Google Scholar] [CrossRef]
- Thomas, W.M.; Johnson, K.I.; Wiesner, C.S. Friction stir welding–recent developments in tool and process technologies. Adv. Eng. Mater. 2003, 5, 485–490. [Google Scholar] [CrossRef]
- Saeid, T.; Abdollah-Zadeh, A.; Assadi, H.; MalekGhaini, F. Effect of friction stir welding speed on the microstructure and mechanical properties of a duplex stainless steel. Mater. Sci. Eng. A 2008, 496, 262–268. [Google Scholar] [CrossRef]
- Wang, D.; Ni, D.R.; Xiao, B.L.; Ma, Z.Y.; Wang, W.; Yang, K. Microstructure evolution and mechanical properties of friction stir joint of Fe-Cr-Mn-Mo-N austenite stainless steel. Mater. Des. 2014, 64, 355–359. [Google Scholar] [CrossRef]
- Liu, F.C.; Nelson, T.W. In-situ material flow pattern around probe during friction stir welding of austenite stainless steel. Mater. Des. 2016, 110, 354–364. [Google Scholar] [CrossRef]
- Guerra, M.; Schmidt, C.; McClure, J.C.; Murr, L.E.; Nunes, A.C. Flow patterns during friction welding. Mater. Charact. 2003, 49, 95–101. [Google Scholar] [CrossRef]
- Fang, Y.L.; Liu, Z.Y.; Song, H.M.; Jiang, L.Z. Hot deformation behavior of a new austenite-ferrite duplex stainless containing high content of nitrogen. Mater. Sci. Eng. A 2009, 526, 128–133. [Google Scholar] [CrossRef]
- Magnani, M.; Terada, M.; Lino, A.O.; Tallo, V.P.; Fonseca, E.B.; Santos, T.F.; Ramirez, A.J. Microstructural and electrochemical characterization of friction stir welded duplex stainless steels. Int. J. Electrochem. Sci. 2014, 9, 2966–2977. [Google Scholar]
- Santos, T.F.; López, E.A.; Fonseca, E.B.; Ramirez, A.J. Friction stir welding of duplex and superduplex stainless steel and some aspects of microstructural characterization and mechanical performance. Mater. Res. 2016, 19, 117–131. [Google Scholar] [CrossRef]
- Esmailzadeh, M.; Shamanian, M.; Kermanpur, A.; Saeid, T. Microstructure and mechanical properties of friction Stir Welded lean duplex stainless steel. Mater. Sci. Eng. A 2013, 561, 486–491. [Google Scholar] [CrossRef]
- Sato, Y.S.; Harayama, N.; Kokawa, H.; Inoue, H.; Tadokoro, Y.; Tsuge, S. Evaluation of microstructure and properties in friction stir welded superaustenitic stainless steel. Sci. Technol. Weld. Join. 2009, 14, 202–209. [Google Scholar] [CrossRef]
- Ramkumar, K.D.; Thiruvengatam, G.; Sudharsan, S.P.; Mishra, D.; Arivazhagan, N.; Sridhar, R. Characterization of weld strength and impact toughness in the multi-pass welding of super-duplex stainless steel UNS2750. Mater. Des. 2014, 60, 125–135. [Google Scholar] [CrossRef]
- Hajian, M.; Abdollah, A.; Rezaei-Nejad, S.S.; Assaddi, H.; Hadavi, S.M.M.; Chung, K.; Shokouhimehr, M. Microstructure and mechanical properties of friction stir processed AISI 316L stainless steel. Mater. Des. 2015, 67, 82–94. [Google Scholar] [CrossRef]
- Li, H.B.; Jiang, Z.H.; Feng, H.; Zhang, S.C.; Li, L.; Han, P.D. Microstructure, mechanical, and corrosion properties of friction Stir Welded high nitrogen nickel-free austenitic stainless steel. Mater. Des. 2015, 84, 291–299. [Google Scholar] [CrossRef]
- Rlston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Jinlong, L.; Tongxiang, L.; Chen, W.; Limin, D. Comparision of corrosion properties of passive films formed on coarse grained and ultrafined grained AISI 2205 duplex stainless steels. J. Electroanal. 2015, 757, 263–269. [Google Scholar] [CrossRef]
- Luo, H.; Dong, C.F.; Xiao, K.; Li, X.G. Characterization of passive film on 2205 duplex stainless steel in sodium thiosulphate solution. Appl. Surf. Sci. 2011, 258, 631–639. [Google Scholar] [CrossRef]
- Eghlimi, A.; Shamanian, M.; Raeissi, K. Effect of current type on microstructure and corrosion resistance of super duplex stainless steel claddings produced by the tungsten arc welding process. Surf. Coat. Technol. 2014, 244, 45–51. [Google Scholar] [CrossRef]
- Jinlong, L.; Tongxiang, L.; Limin, D.; Chen, W. Influence of sensitization on microstructure and passive property of AISI 2205duplex stainless steel. Corros. Sci. 2016, 104, 144–151. [Google Scholar] [CrossRef]
- Donik, Č.; Kocijan, A.; Grant, J.T.; Jenko, M.; Drenik, A.; Pihlar, B. XPS study of duplex stainless steel oxidized by oxygen atoms. Corros. Sci. 2009, 51, 827–832. [Google Scholar] [CrossRef]



| Elements | C | N | Si | Mn | Cr | Ni | Co | Mo | Cu | Ce | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt. %) | 0.02 | 0.47 | 0.42 | 1.00 | 27.07 | 6.71 | 0.99 | 4.66 | 0.85 | 0.03 | Bal. |
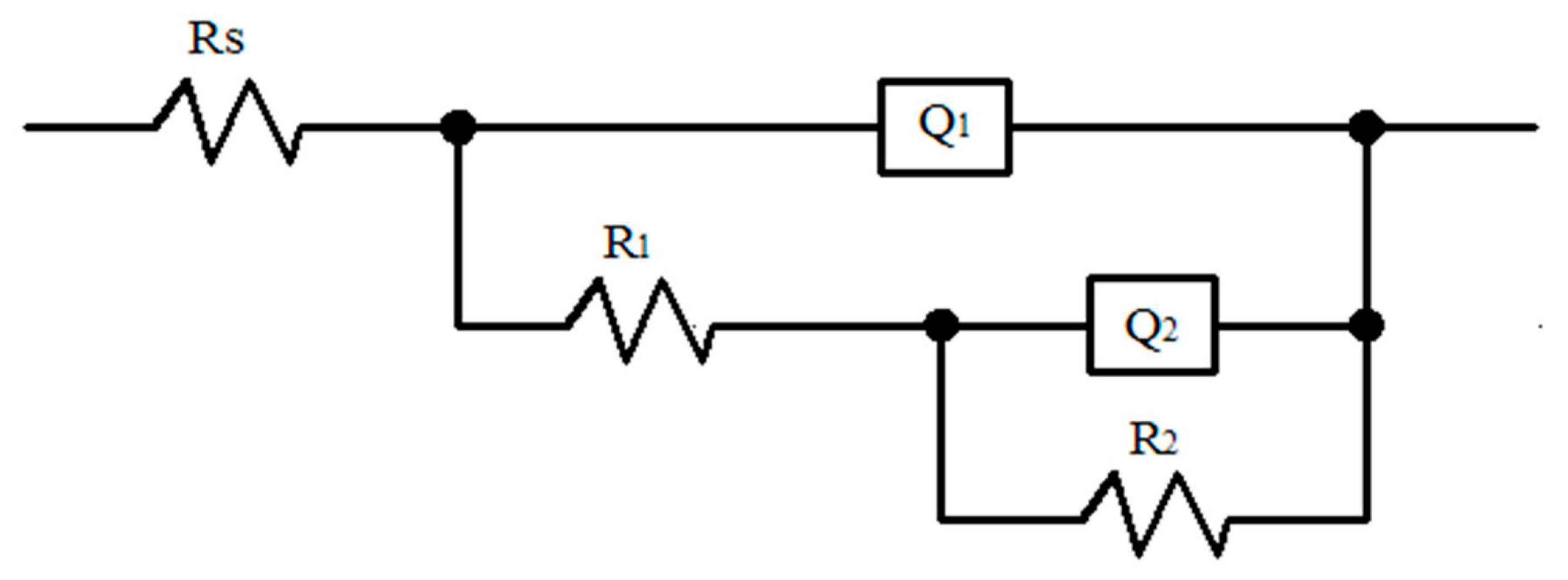
| Area | Rs (Ω∙cm2) | Q1 (uF∙cm−2) | R1 (kΩ∙cm2) | n1 | R2 (kΩ∙cm2) | Q2 (uF∙cm−2) | n2 |
|---|---|---|---|---|---|---|---|
| BM | 30.53 | 19.10 | 0.51 | 0.98 | 262.50 | 16.50 | 0.77 |
| SZ | 27.61 | 29.25 | 47.35 | 0.94 | 304.50 | 12.94 | 0.84 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, X.; Li, G.; Han, P.; Liang, W. Characterization of the Microstructure, Mechanical Properties, and Corrosion Resistance of a Friction-Stir-Welded Joint of Hyper Duplex Stainless Steel. Metals 2017, 7, 138. https://doi.org/10.3390/met7040138
Li J, Liu X, Li G, Han P, Liang W. Characterization of the Microstructure, Mechanical Properties, and Corrosion Resistance of a Friction-Stir-Welded Joint of Hyper Duplex Stainless Steel. Metals. 2017; 7(4):138. https://doi.org/10.3390/met7040138
Chicago/Turabian StyleLi, Jianchun, Xinglong Liu, Guoping Li, Peide Han, and Wei Liang. 2017. "Characterization of the Microstructure, Mechanical Properties, and Corrosion Resistance of a Friction-Stir-Welded Joint of Hyper Duplex Stainless Steel" Metals 7, no. 4: 138. https://doi.org/10.3390/met7040138





