Formation and Corrosion Behavior of Mechanically-Alloyed Cu–Zr–Ti Bulk Metallic Glasses
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
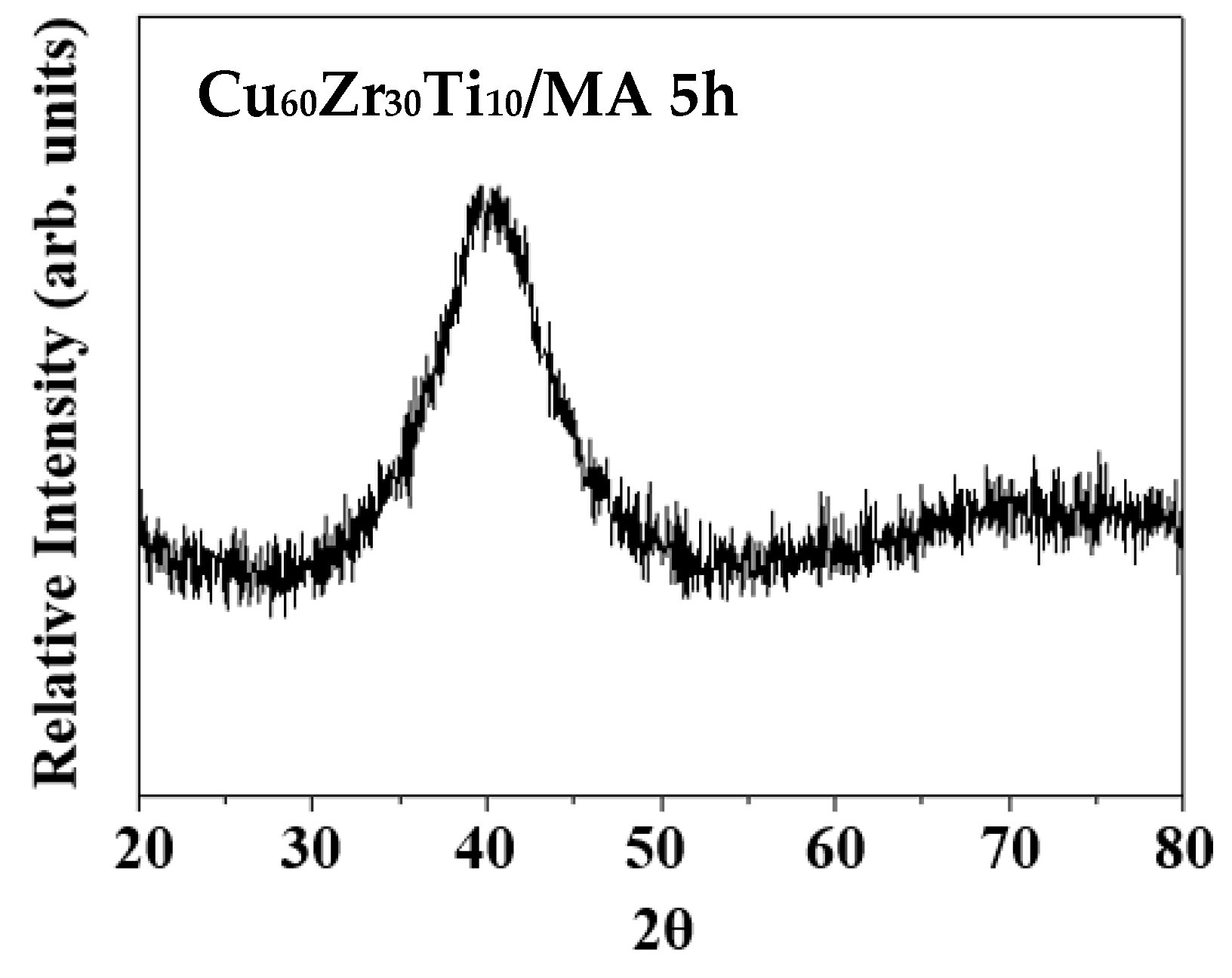
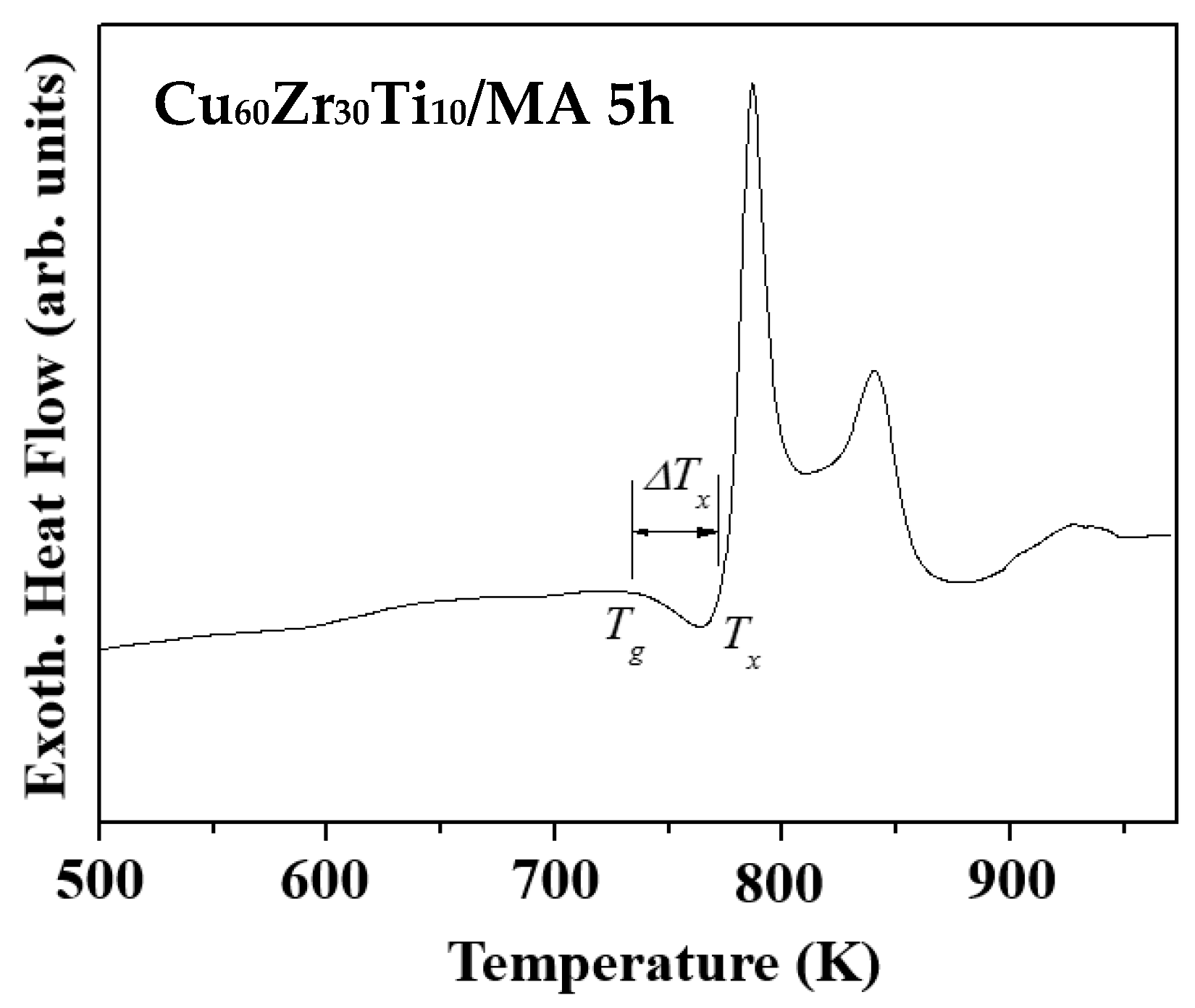
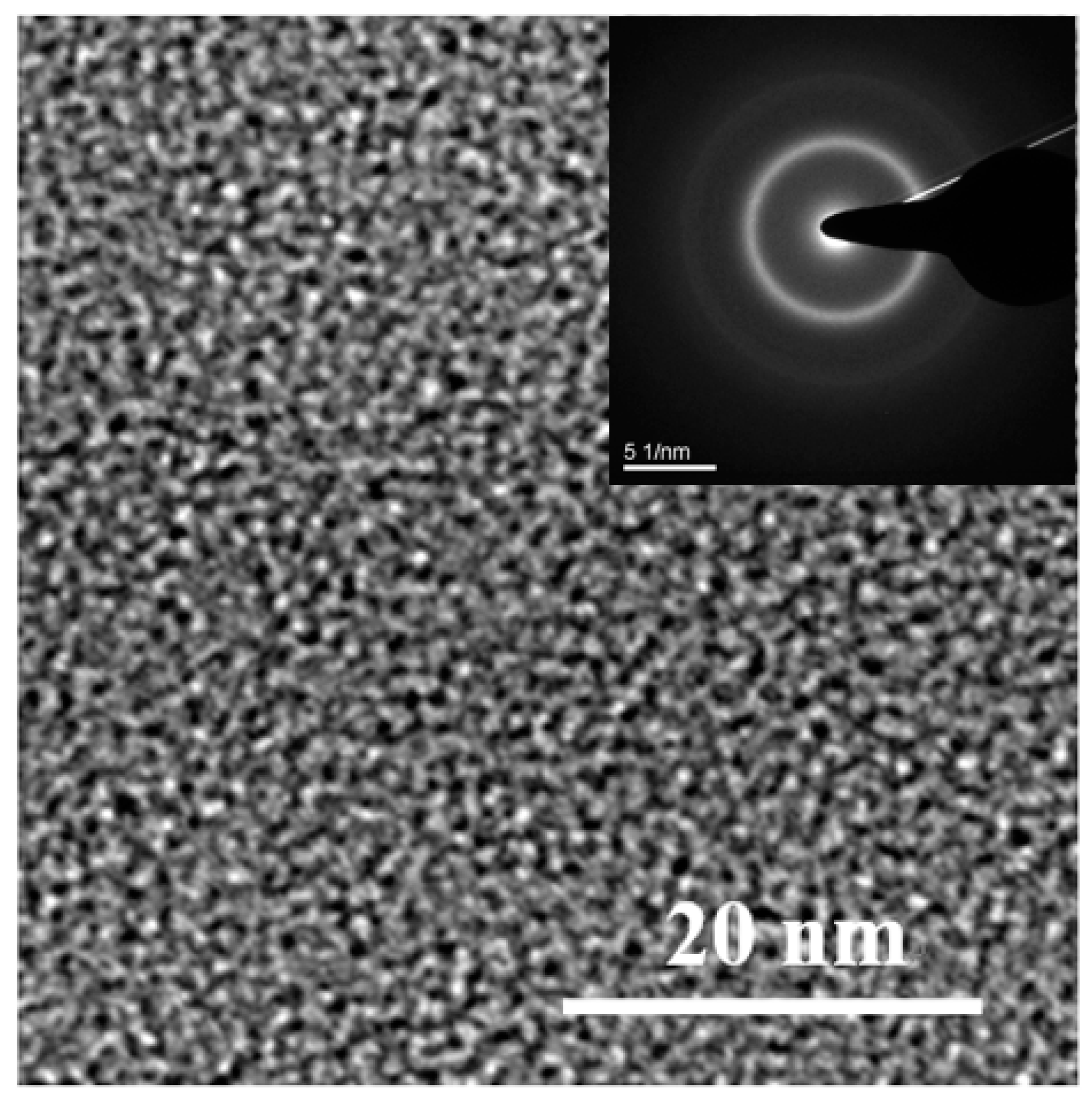
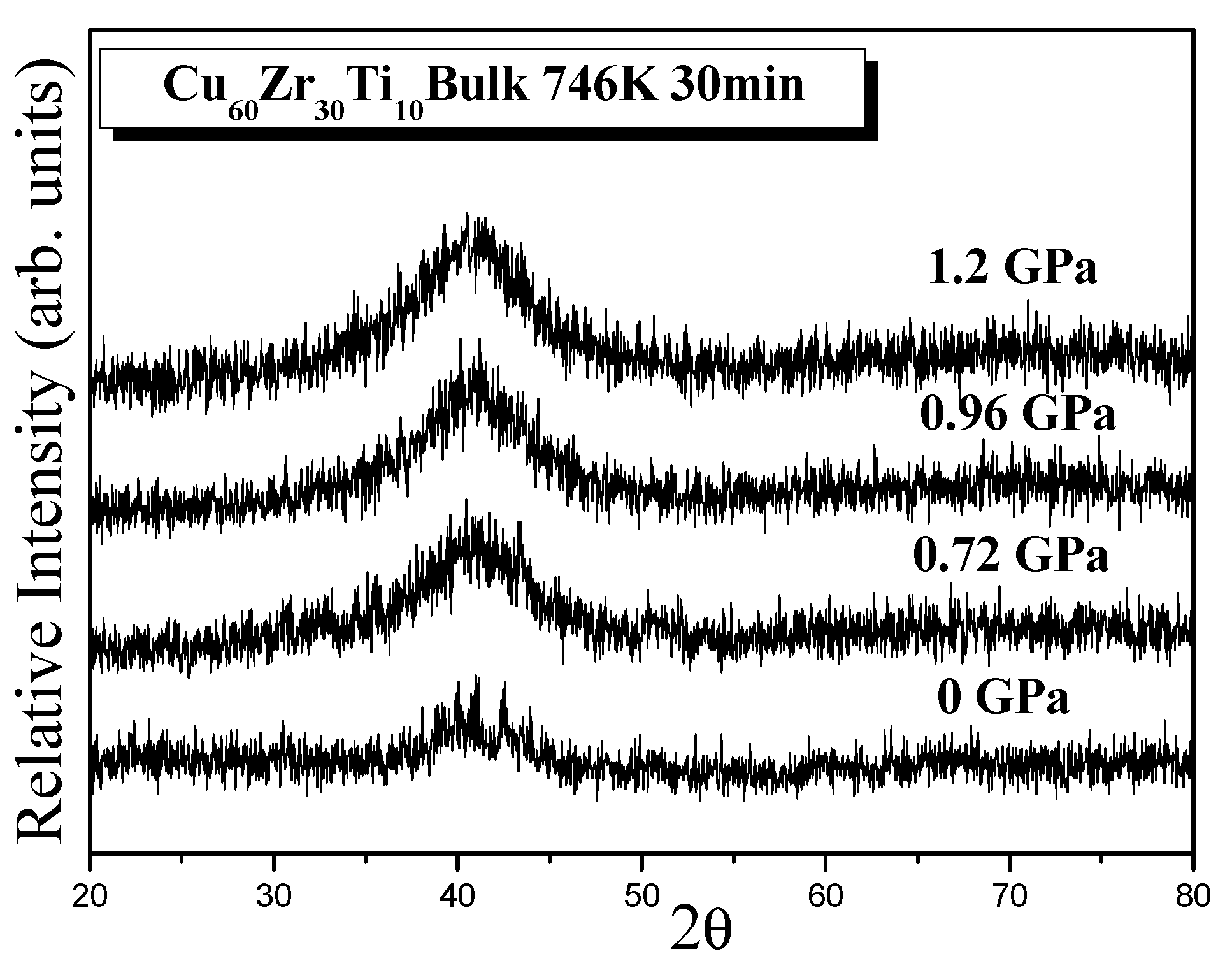
3.1. Ball-Milled Powder and Consolidated BMG
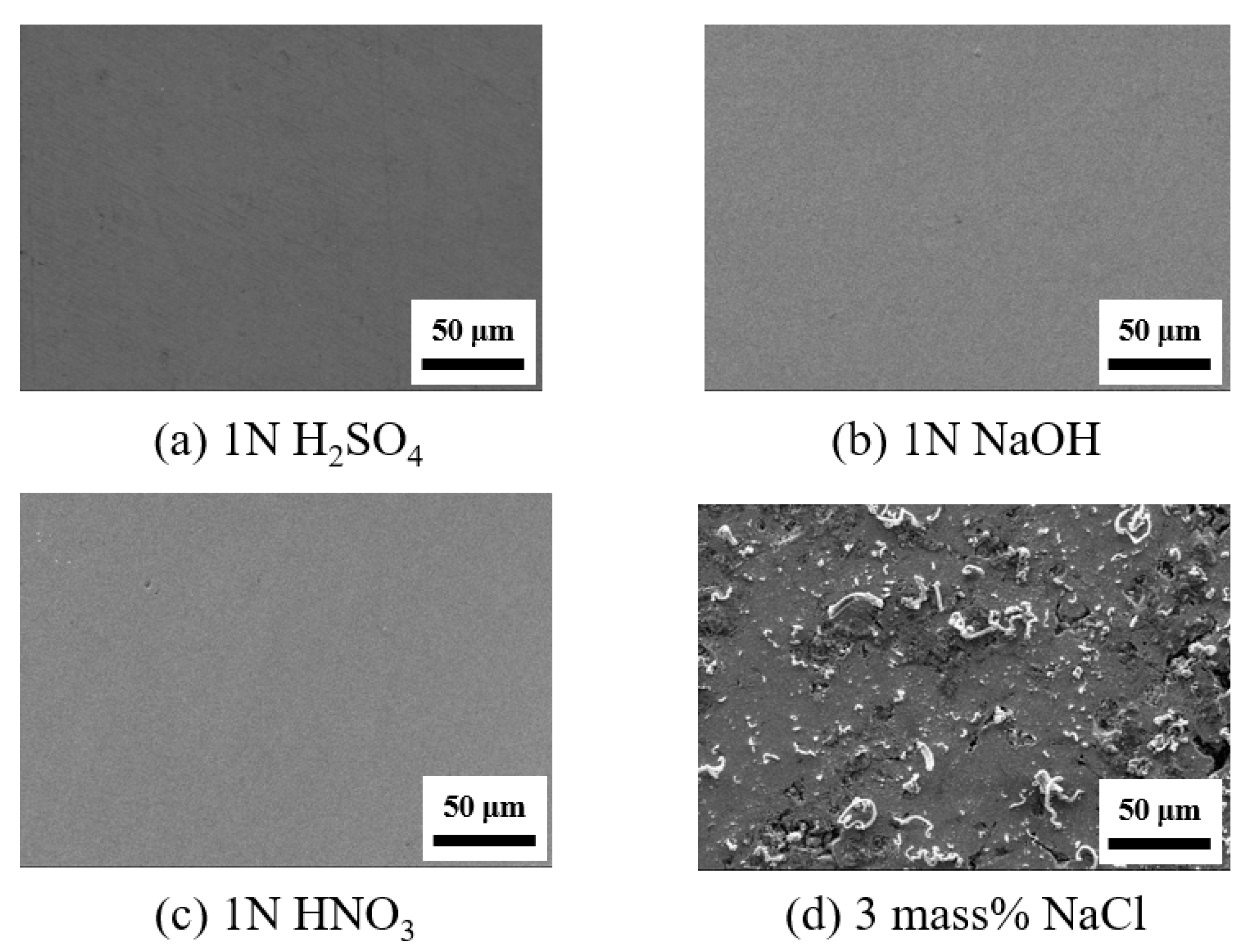
3.2. Corrosion Behavior of Cu–Zr–Ti BMG Alloys
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, X.H.; Johnson, W.L. Formation of Ti–Zr–Cu–Ni bulk metallic glasses. J. Appl. Phys. 1995, 78, 5614–5619. [Google Scholar] [CrossRef]
- Li, C.; Saida, J.; Kiminami, M.; Inoue, A. Dynamic crystallization process in a supercooled liquid region of Cu40Ti30Ni15Zr10Sn5 amorphous alloy. J. Non-Cryst. Solids 2000, 261, 108–114. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, W.; Zhang, T.; Kurosaka, K. Cu-based bulk glassy alloys with high tensile strength of over 2000 MPa. J. Non-Cryst. Solids 2002, 304, 200–2009. [Google Scholar] [CrossRef]
- Ashby, M.F.; Greer, A.L. Metallic glasses as structural materials. Scr. Mater. 2006, 54, 321–326. [Google Scholar] [CrossRef]
- Lindsay Greer, A. Metallic glasses on the threshold. Mater. Today 2009, 12, 14–22. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, C.L.; Chen, Q.; Zhang, S.M. Corrosion behavior of Zr-based bulk metallic glasses in different artificial body fluids. J. Alloys Compd. 2006, 425, 268–273. [Google Scholar] [CrossRef]
- Gebert, A.; Haehnel, V.; Park, E.S.; Kim, D.H.; Schultz, L. Corrosion behavior of Mg65Cu7.5Ni7.5Ag5Zn5Gd5Y5 bulk metallic glass in aqueous environments. Electrochim. Acta 2008, 53, 3403–3411. [Google Scholar] [CrossRef]
- Ghidelli, M.; Gravier, S.; Blandin, J.-J.; Djemia, P.; Mompiou, F.; Abadias, G.; Raskin, J.-P.; Pardoen, T. Extrinsic mechanical size effects in thin ZrNi metallic glass films. Acta Mater. 2015, 90, 232–241. [Google Scholar] [CrossRef]
- Ghidelli, M.; Volland, A.; Blandin, J.-J.; Pardoen, T.; Raskin, J.-P.; Mompiou, F.; Djemia, P.; Gravier, S. Exploring the mechanical size effects in Zr65Ni35 thin film metallic glasses. J. Alloys Compd. 2014, 615 (Suppl. S1), 90–92. [Google Scholar] [CrossRef]
- Chu, J.P.; Jang, J.S.C.; Huang, J.C.; Chou, H.S.; Yang, Y.; Ye, J.C.; Wang, Y.C.; Lee, J.W.; Liu, F.X.; Liaw, P.K.; et al. Thin film metallic glasses: Unique properties and potential applications. Thin Solid Films 2012, 520, 5097–5122. [Google Scholar] [CrossRef]
- Song, S.X.; Bei, H.; Wadsworth, J.; Nieh, T.G. Flow serration in a Zr-based bulk metallic glass in compression at low strain rates. Intermetallics 2008, 16, 813–818. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ma, E. Atomic-level structure and structure-property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Hofmann, D.C.; Suh, J.-Y.; Wiest, A.; Duan, G.; Lind, M.-L.; Demetriou, M.D.; Johnson, W.L. Designing metallic glass matrix composites with high toughness and tensile ductility. Nature 2008, 451, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Pauly, S.; Gorantla, S.; Wang, G.; Kühn, U.; Eckert, J. Transformation-mediated ductility in CuZr-based bulk metallic glasses. Nat. Mater. 2010, 9, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chan, K.C.; Yu, P.; Wang, G. Encapsulated Zr-based bulk metallic glass with large plasticity. Mater. Sci. Eng. A 2011, 528, 2988–2994. [Google Scholar] [CrossRef]
- Johnson, W.L. Thermodynamic and kinetic aspects of the crystal to glass transformation in metallic materials. Prog. Mater. Sci. 1986, 30, 81–134. [Google Scholar] [CrossRef]
- Lin, C.K.; Liu, S.W.; Lee, P.Y. Preparation and thermal stability of Zr–Ti–Al–Ni–Cu amorphous powders by mechanical alloying. Metall. Mater. Trans. A 2001, 32, 1777–1786. [Google Scholar] [CrossRef]
- Jeng, I.K.; Lee, P.Y.; Chen, J.S.; Jeng, R.R.; Yeh, C.H.; Lin, C.K. Mechanical alloyed Ti–Cu–Ni–Si–B amorphous alloys with significant supercooled liquid region. Intermetallics 2002, 10, 1271–1276. [Google Scholar] [CrossRef]
- Yi, S.; Lee, J.K.; Kim, W.T.; Kim, D.H. Ni-based bulk amorphous alloys in the Ni–Ti–Zr–Si system. J. Non-Cryst. Solids 2001, 291, 132–136. [Google Scholar] [CrossRef]
- Lee, P.Y.; Hung, S.S.; Hsieh, J.T.; Lin, Y.L.; Lin, C.K. Consolidation of amorphous Ni–Zr–Ti–Si powders by vacuum hot-Pressing method. Intermetallics 2002, 10, 1277–1282. [Google Scholar] [CrossRef]
- Hu, C.J.; Lee, P.Y. Formation of Cu–Zr–Ni amorphous powders with significant supercooled liquid region by mechanical alloying technique. Mater. Chem. Phys. 2002, 74, 13–18. [Google Scholar] [CrossRef]
- Qin, C.; Katsuhiko, A.; Zhang, T.; Zhang, W.; Inoue, A. Corrosion Behavior of Cu–Zr–Ti–Nb Bulk Glassy Alloys. Mater. Trans. 2003, 44, 749–753. [Google Scholar] [CrossRef]
- Asami, K.; Qin, C.-L.; Zhang, T.; Inoue, A. Effect of additional elements on the corrosion behavior of a CuTiZr bulk glass. Mater. Sci. Eng. A 2004, 375–377, 235–239. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C.; Zou, L.M.; Qu, S.G.; Li, X.Q.; Li, Y.Y. Ti-based bulk metallic glass matrix composites with in situ precipitated β-Ti phase fabricated by spark plasma sintering. J. Non-Cryst. Solids 2013, 359, 15–20. [Google Scholar] [CrossRef]
- Wang, W.H.; He, D.W.; Zhao, D.Q.; Yao, Y.S.; He, M. Nanocrystallization of ZrTiCuNiBeC bulk metallic glass under high pressure. Appl. Phys. Lett. 1999, 75, 2770–2772. [Google Scholar] [CrossRef]
- Wang, W.H.; Pan, M.X.; Zhao, D.Q.; Wen, P.; Zhang, Y.; Zhuang, Y.X. Crystallization of ZrTiCuNiBe bulk metallic glasses. J. Metastab. Nano-Cryst. Mater. 2003, 15–16, 73–85. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Olsen, J.S.; Gerward, L.; Abdali, S.; Eckert, J.; Schlorke-de Boer, N.; Schultz, L.; Truckenbrodt, J.; Shi, P.X. Pressure effect on crystallization of metallic glass Fe72P11C6Al5B4Ga2 alloy with wide supercooled liquid region. J. Appl. Phys. 2000, 87, 2664–2671. [Google Scholar] [CrossRef]
- Gu, X.J.; Jin, H.J.; Zhang, H.W.; Wang, J.Q.; Lu, K. Pressure-enhanced thermal stability against eutectic crystallization in Al-based metallic glasses. Scr. Mater. 2001, 45, 1091–1097. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, K.Q.; Wang, A.M.; Zhang, H.F.; Quan, M.X.; Hu, Z.Q. Pressure-induced nanocrystallization of Zr55Al10Ni5Cu30 bulk metallic glass. J. Mater. Res. 2002, 17, 2935–2939. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, W.; Dong, C.; Qiang, J.B.; Fukuhara, M.; Makino, A.; Inoue, A. Effects of Ni addition on the glass-forming ability, mechanical properties and corrosion resistance of Zr–Cu–Al bulk metallic glasses. Mater. Sci. Eng. A 2011, 528, 8551–8556. [Google Scholar] [CrossRef]
- Qin, C.L.; Oak, J.J.; Ohtsu, N.; Asami, K.; Inoue, A. XPS study on the surface films of a newly designed Ni-free Ti-based bulk metallic glass. Acta Mater. 2007, 55, 2057–2063. [Google Scholar] [CrossRef]
- Pang, S.; Zhang, T.; Asami, K.; Inoue, A. Bulk glassy Ni(Co–)Nb–Ti–Zr alloys with high corrosion resistance and high strength. Mater. Sci. Eng. 2004, 375–377, 368–371. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.Q.; Chen, L.X.; Yuan, J.; Zhang, Z.H.; Wang, Q.D. The effect of partial substitution of Zr for Ti on the electrochemical properties and surface passivation film of Mg35Ti10−xZrxNi55 (x = 1, 3, 5, 7, 9) electrode alloys. J. Alloys Compd. 2002, 337, 296–302. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L. The effect of microalloying on thermal stability and corrosion resistance of Cu-based bulk metallic glasses. Mater. Sci. Eng. 2006, 415, 286–290. [Google Scholar] [CrossRef]
- Zander, D.; Heisterkamp, B.; Gallino, I. Corrosion resistance of Cu–Zr–Al–Y and Zr–Cu–Ni–Al–Nb bulk metallic glasses. J. Alloys Compd. 2007, 434–435, 234–236. [Google Scholar] [CrossRef]
- Mondal, K.; Murty, B.S.; Chatterjee, U.K. Electrochemical behavior of multicomponent amorphous and nanocrystalline Zr-based alloys in different environments. Corros. Sci. 2006, 48, 2212–2225. [Google Scholar] [CrossRef]
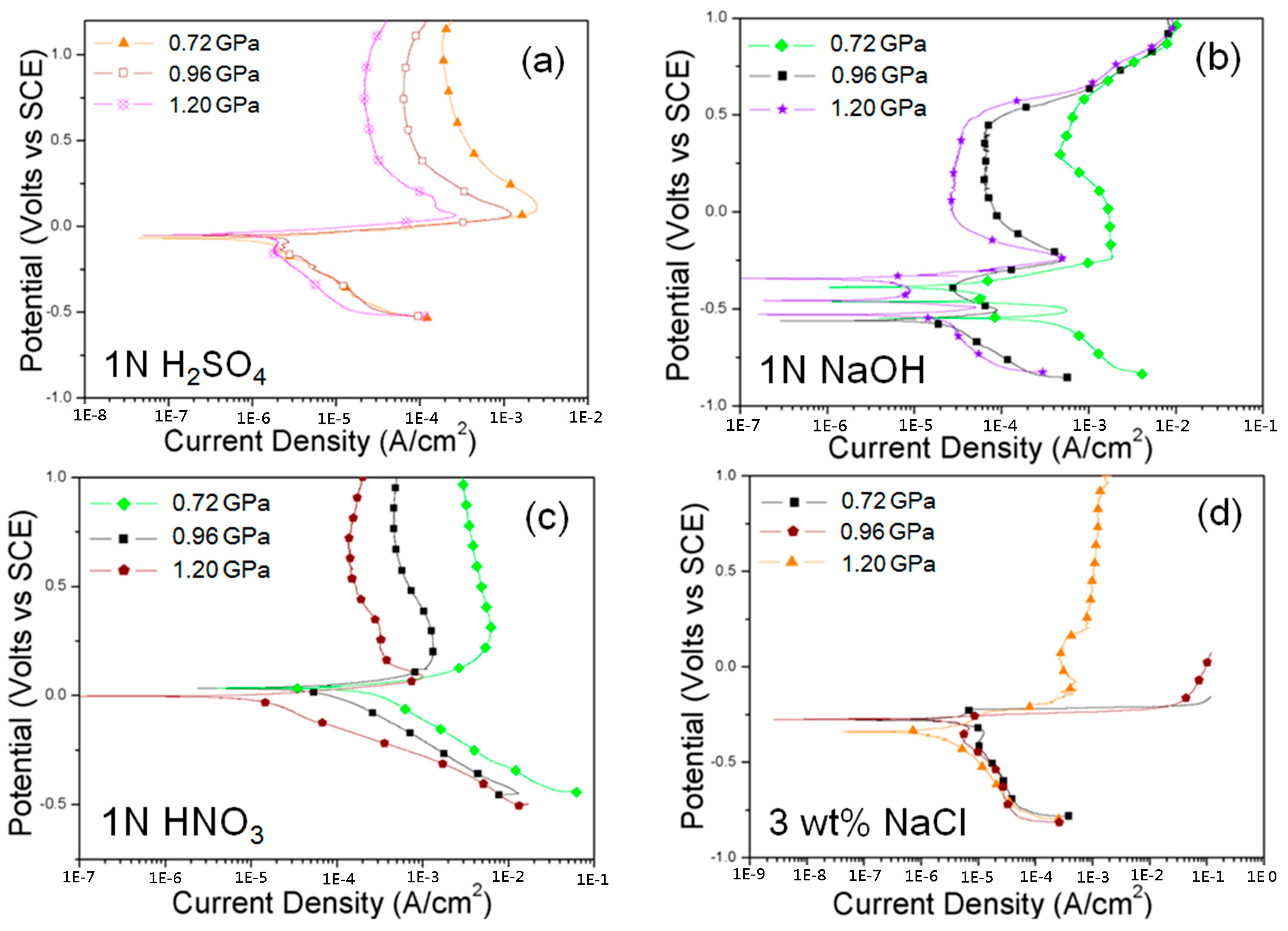
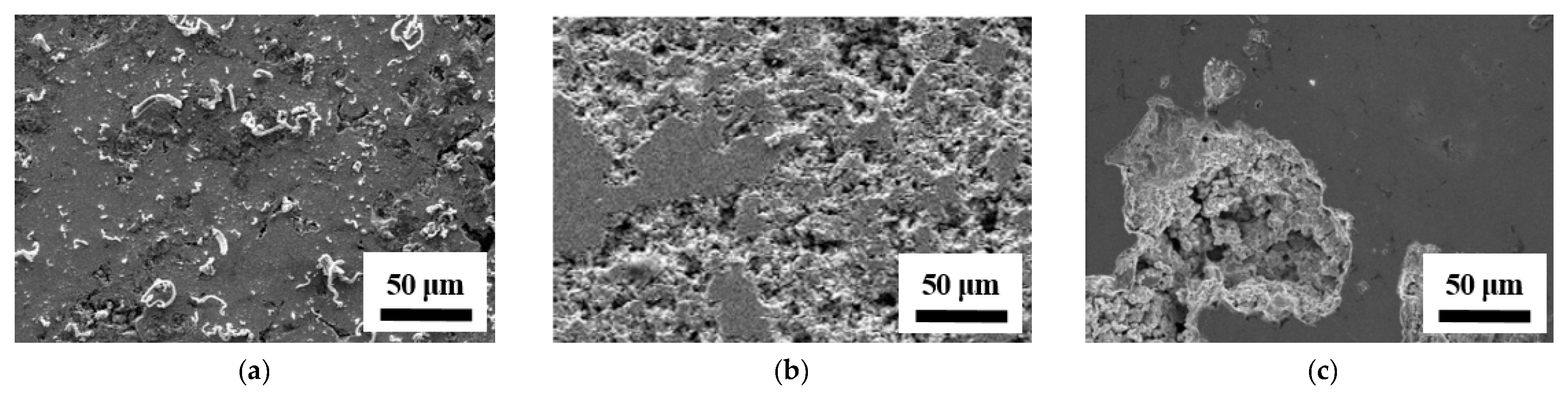
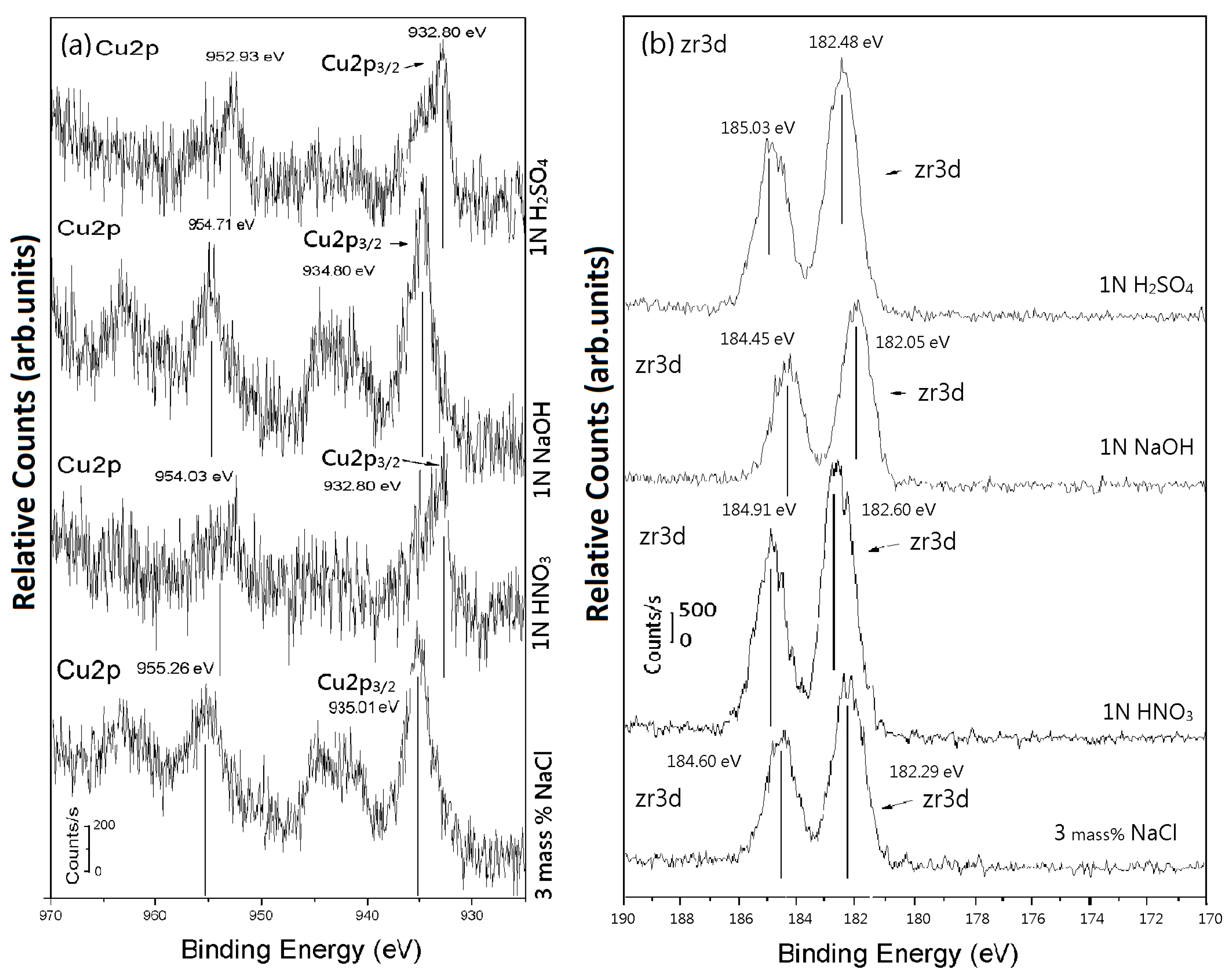
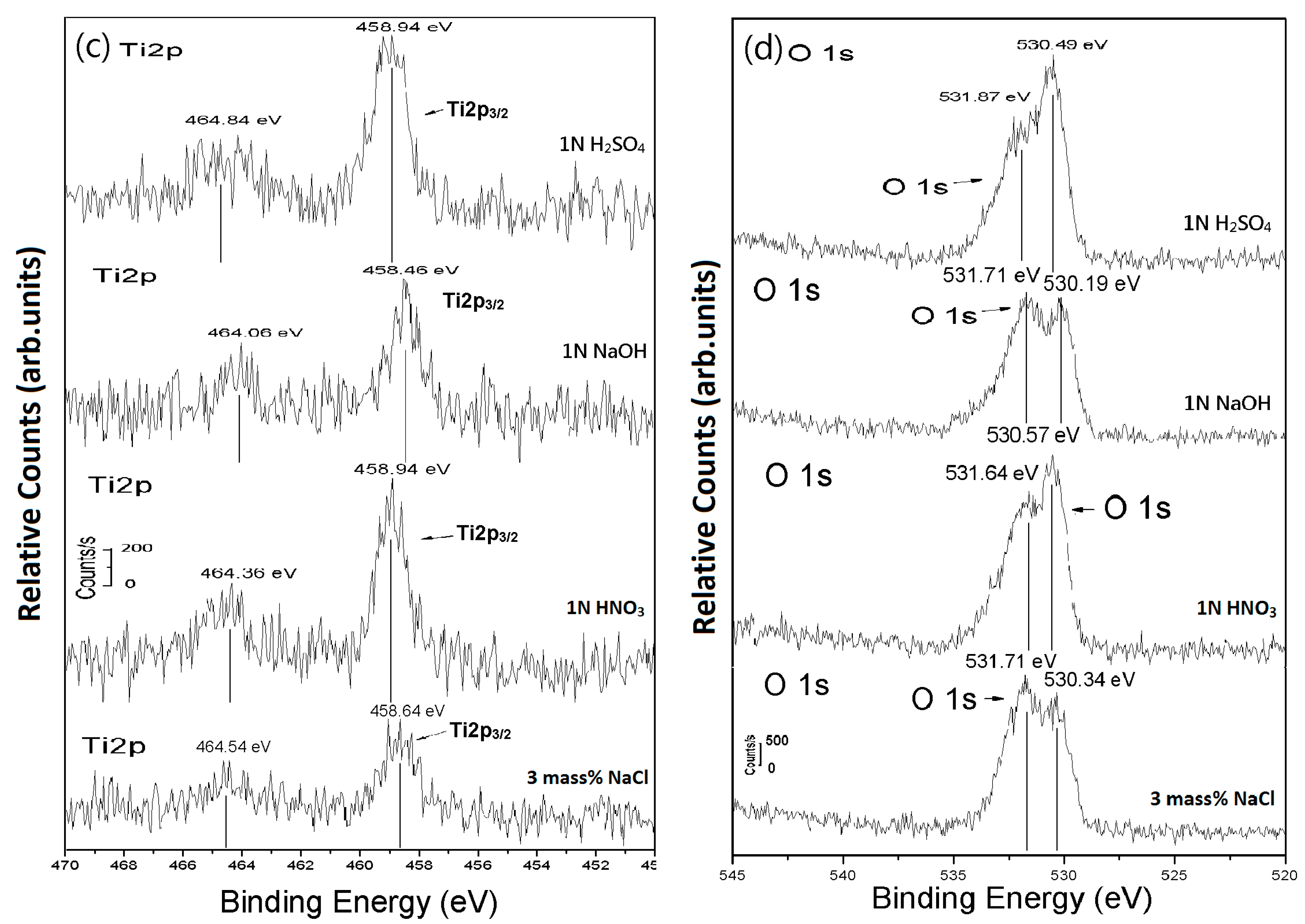

| Item | Pressure (GPa) | Ec (Volts) | ic (A/cm2) | Ep (Volts) | ip (A/cm2) | |
|---|---|---|---|---|---|---|
| Solution | ||||||
| 1 N H2SO4 | 0.72 | −0.05 | 8.4 × 10−6 | >0.5 V | 2.2 × 10−4 | |
| 0.96 | −0.05 | 6.9 × 10−6 | >0.5 V | 6.4 × 10−5 | ||
| 1.2 | −0.07 | 1.2 × 10−6 | >0.5 V | 2.2 × 10−5 | ||
| 1 N NaOH | 0.72 | −0.55 | 4.8 × 10−4 | >0.5 V | 1.8 × 10−3 | |
| 0.96 | −0.56 | 2.2 × 10−5 | >0.5 V | 6.5 × 10−5 | ||
| 1.2 | −0.52 | 1.6 × 10−5 | <0.5 V | 3.1 × 10−5 | ||
| 3 wt % NaCl | 0.72 | 0.27 | 4.9 × 10−6 | --- | --- | |
| 0.96 | −0.37 | 4.3 × 10−6 | --- | --- | ||
| 1.2 | −0.34 | 1.5 × 10−6 | <0.5 V | 1.1 × 10−3 | ||
| 1 N HNO3 | 0.72 | 0.04 | 2.8 × 10−4 | >0.5 V | 5.7 × 10−4 | |
| 0.96 | 0.03 | 7.9 × 10−5 | >0.5 V | 1.5 × 10−4 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.-Y.; Cheng, Y.-M.; Chen, J.-Y.; Hu, C.-J. Formation and Corrosion Behavior of Mechanically-Alloyed Cu–Zr–Ti Bulk Metallic Glasses. Metals 2017, 7, 148. https://doi.org/10.3390/met7040148
Lee P-Y, Cheng Y-M, Chen J-Y, Hu C-J. Formation and Corrosion Behavior of Mechanically-Alloyed Cu–Zr–Ti Bulk Metallic Glasses. Metals. 2017; 7(4):148. https://doi.org/10.3390/met7040148
Chicago/Turabian StyleLee, Pee-Yew, Yeh-Ming Cheng, Jyun-Yu Chen, and Chia-Jung Hu. 2017. "Formation and Corrosion Behavior of Mechanically-Alloyed Cu–Zr–Ti Bulk Metallic Glasses" Metals 7, no. 4: 148. https://doi.org/10.3390/met7040148




