Characterization of the Effect of Melt Treatments on Melt Quality in Al-7wt %Si-Mg Alloys
Abstract
:1. Introduction
2. Background
3. Materials and Methods
4. Results and Discussion
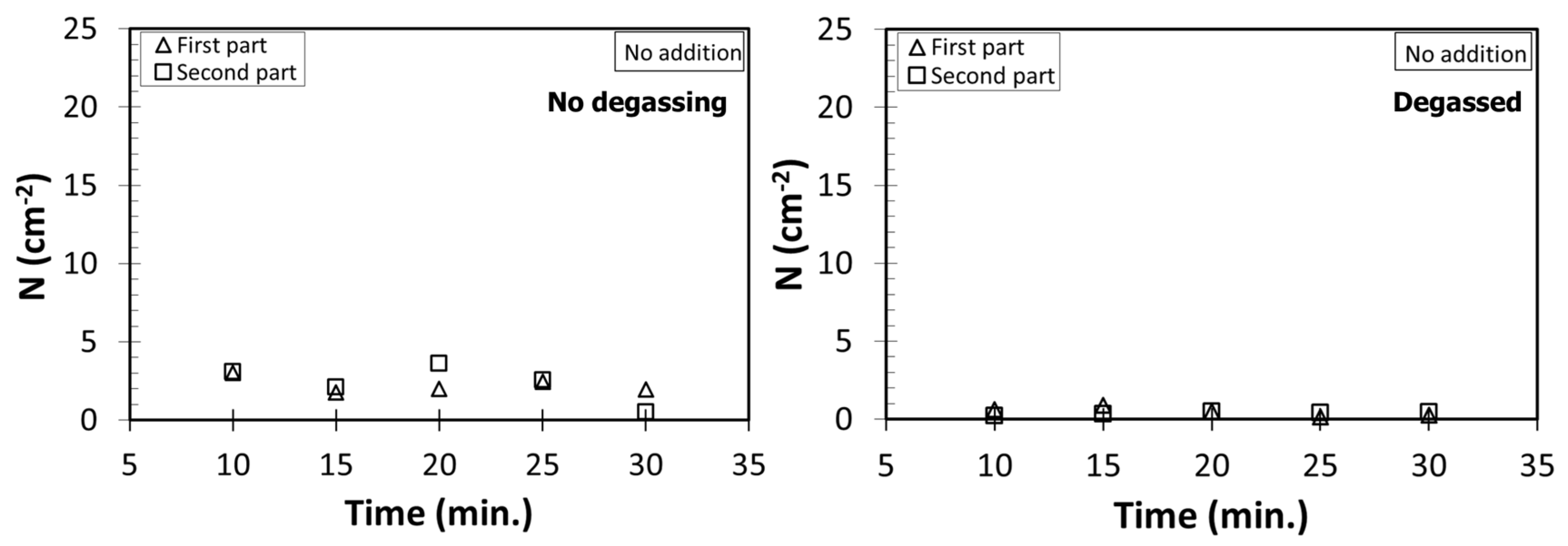
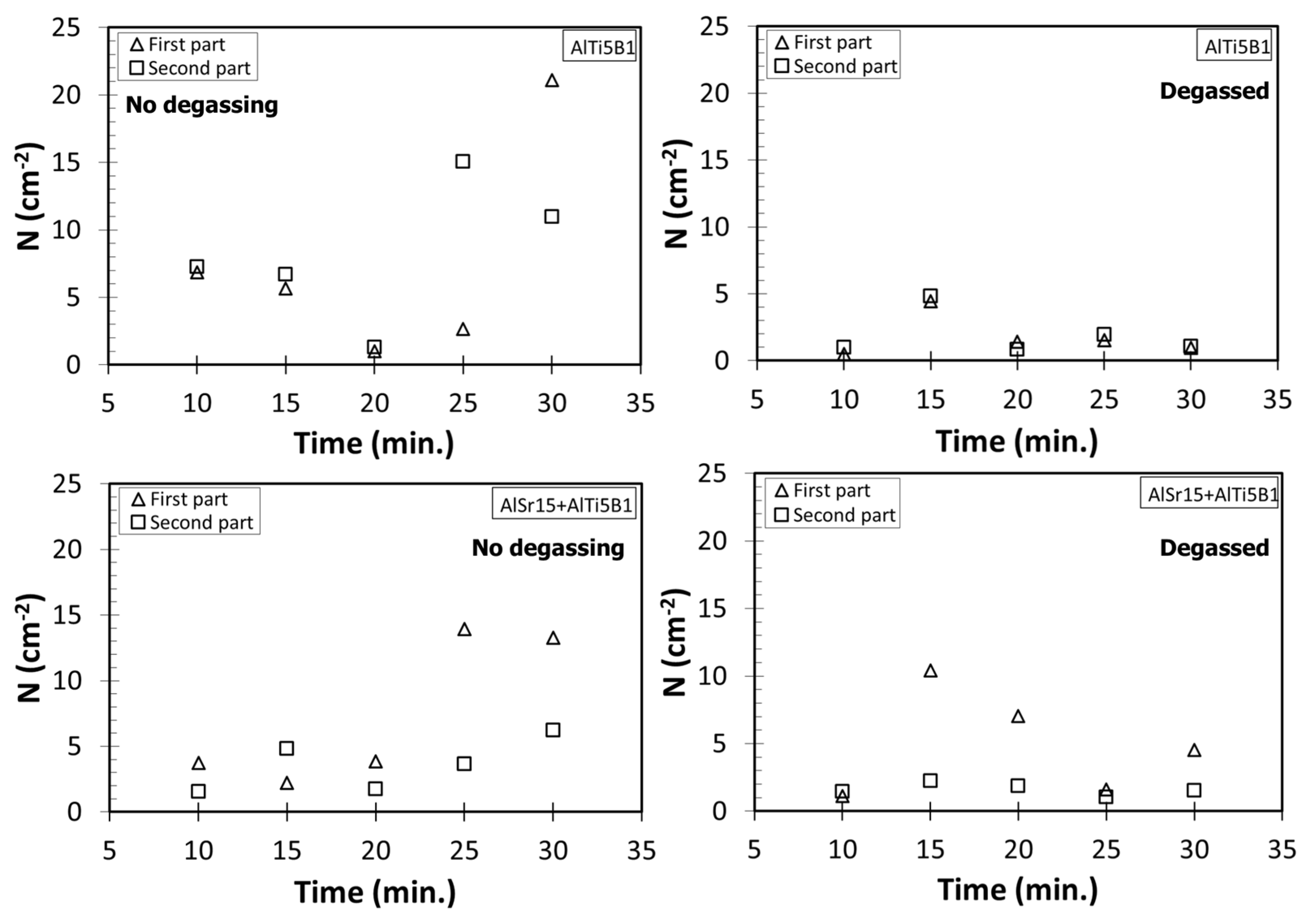
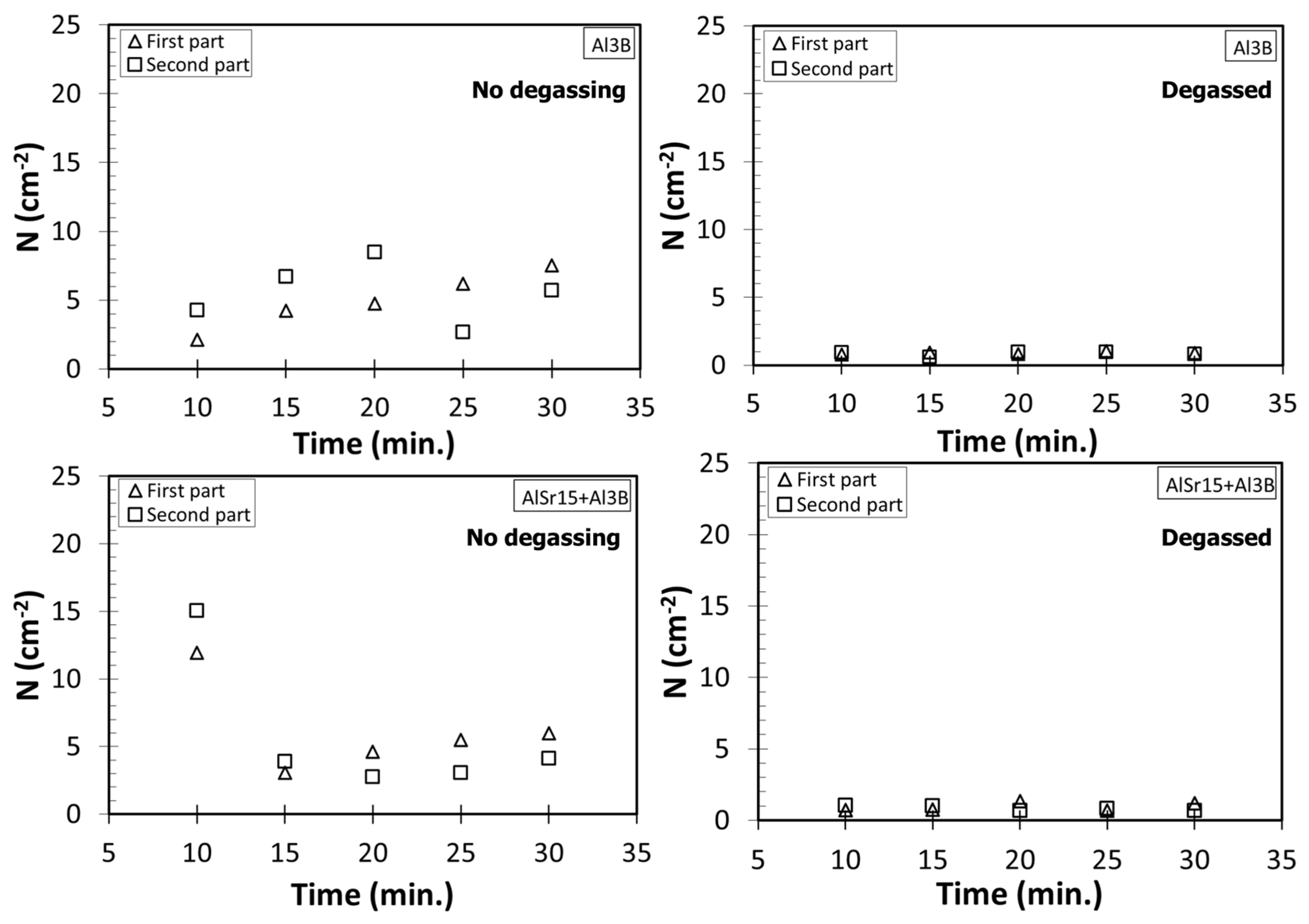
4.1. Number Density Measurements
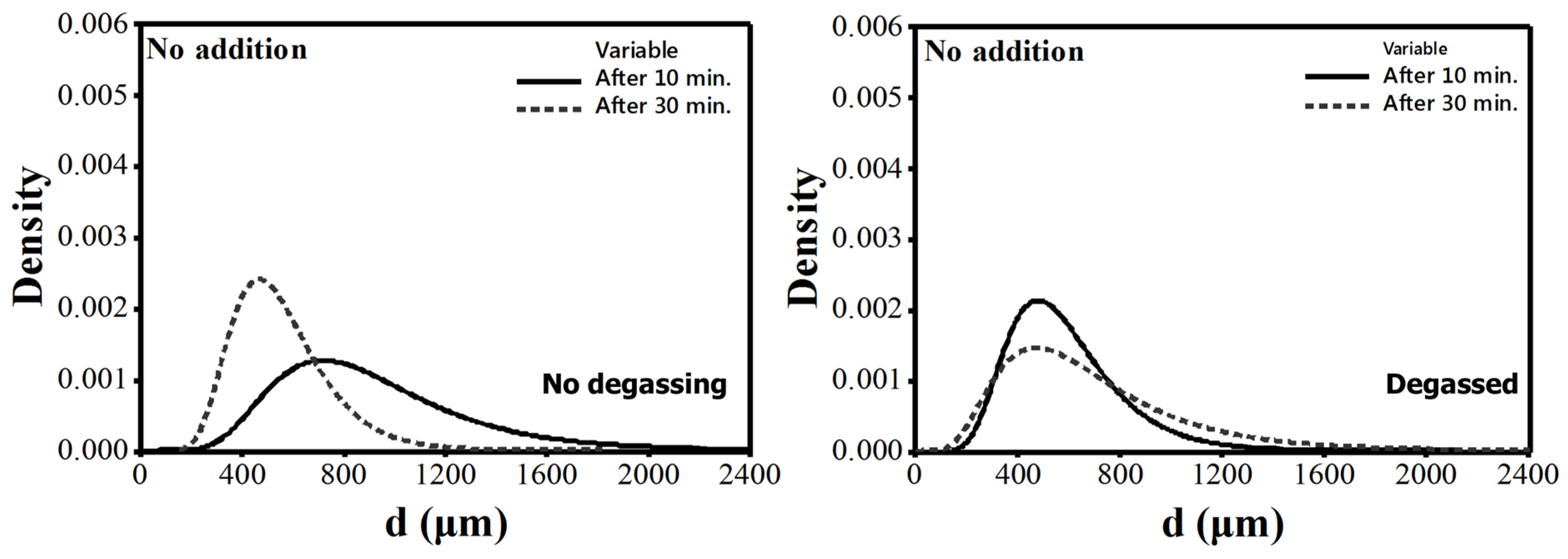
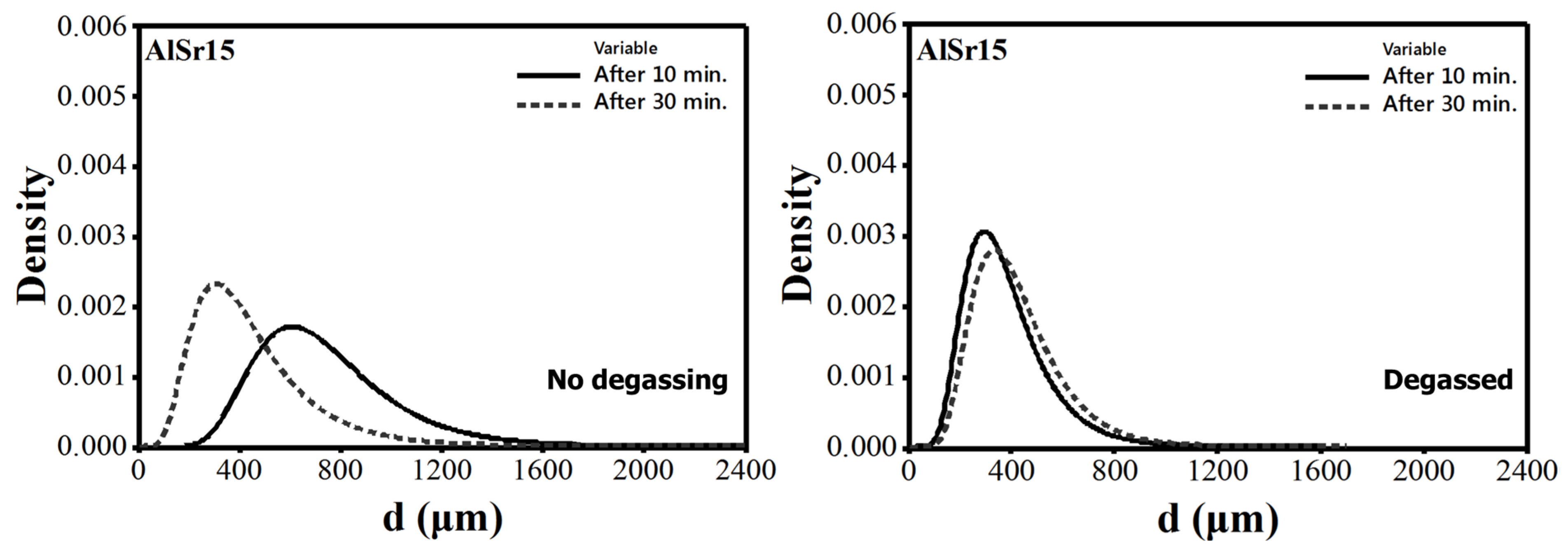
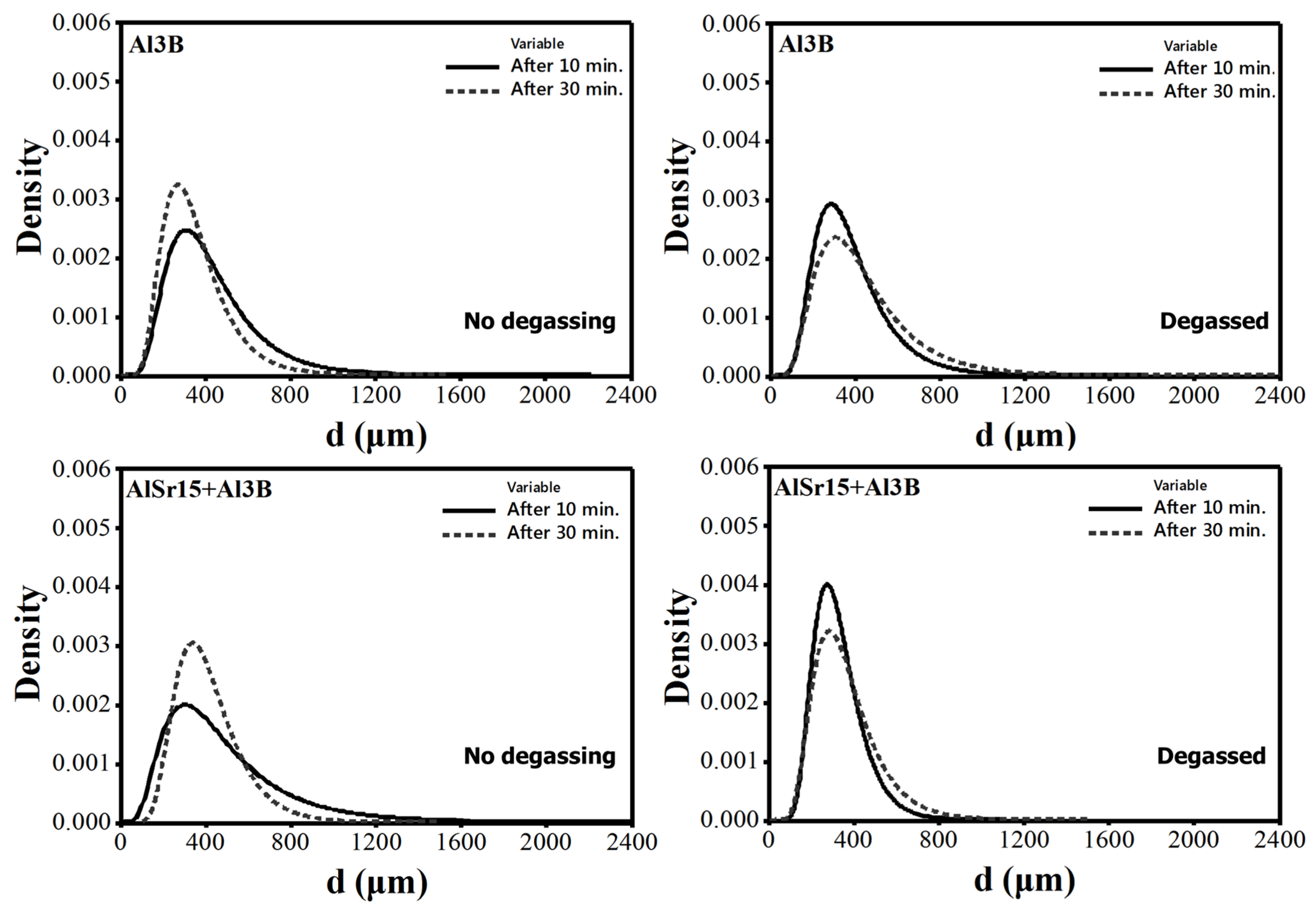
4.2. Size Distribution of Pores
- In non-degassed castings, N before Sr addition can be taken as 6.10 cm−2, the value for “no addition” in Table 5. After Sr addition, N is almost doubled to 11.62 cm−2. Almost the same magnitude of increase takes place in degassed melts, from 0.86 to 6.51 cm−2, after the addition of Sr. This increase is of course due to entrainment of new surface oxides, as stated by Campbell [5], and at least partially due to the reaction of Sr with Al2O3 oxide, as suggested Liu et al. [57].
- That Sr addition increases mean pore diameter in non-degassed melts but has essentially no effect in degassed melts are in agreement with the findings of Iwahori et al. [26]. When N is low, mean pore diameter does not change with hold time either, resulting in a stable melt quality.
- In non-degassed “no addition” melts, there is evidence for natural degassing (lower mean pore diameter) and floatation of bifilms to the surface of melt (lower N) with time.
- The strong increase in N with time with Ti additions in non-degassed as well as degassed melts can be interpreted as sedimentation of bifilms to the bottom of the ladle with holding time, as a result of Ti nucleating heterogeneously on bifilms. Because Ti is heavier than Al, these phases help bifilms sink to the bottom.
- The increase in N with holding time for Sr and B additions cannot be completely explained by sedimentation because these two elements have slightly lower density than Al. Therefore, additional research is needed to uncover the reasons for this finding.
- The absolute best melt quality (lowest N) is obtained when the melt is degassed and no addition is made. Moreover, there is virtually no change in N with holding time, although there is a decrease in mean pore diameter, due to natural degassing. Therefore, to save energy, it is sufficient to only have an effective degassing operation to clean Al-7%Si-Mg melts.
5. Conclusions
- The absolute best melt quality is obtained when the melt is degassed and no addition is made. Therefore, degassing operation can undoubtedly clean Al-7%Si-Mg melts.
- Comparing the degassing operation, non-treated alloy has the lowest levels of number density of defects, compared to other additions. It is concluded that all melt additions degrade melt quality.
- There is an evidence that natural degassing occurs where bifilms float to the surface of melt with time.
- Bifilms sediment to the bottom of the ladle with holding time when Ti is added to the melt. This reveals that Ti is heterogeneously nucleates on bifilms.
- Sr additions to A356 increased the number of bifilms in the melt and also resulted larger pores. Sr reacts with Al2O3 to form SrO.Al2O3 spinel, which results in fracturing of oxides into smaller pieces through breakaway oxidation.
- The increase in N with holding time for Sr and B additions cannot be completely explained by sedimentation because these two elements have slightly lower density than Al. Therefore, additional research is needed to uncover the reasons for this finding.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Campbell, J. Complete Casting Handbook: Metal Casting Processes, Metallurgy, Techniques and Design; Elsevier Science: Oxford, UK, 2011; p. 3. [Google Scholar]
- Anson, J.; Gruzleski, J. The quantitative discrimination between shrinkage and gas microporosity in cast aluminum alloys using spatial data analysis. Mater. Charact. 1999, 43, 319–335. [Google Scholar] [CrossRef]
- Brandt, F.; Bishop, H.; Pellini, W. Solidification at corner and core positions. AFS Trans. 1953, 61, 451. [Google Scholar]
- Campbell, J. Cast Met. Res. J. 1969, 4, 1. [Google Scholar]
- Campbell, J. Entrainment defects. Mater. Sci. Technol. 2006, 22, 127–145. [Google Scholar] [CrossRef]
- Dispinar, D.; Akhtar, S.; Nordmark, A.; di Sabatino, M.; Arnberg, L. Degassing, hydrogen and porosity phenomena in A356. Mater. Sci. Eng. A 2010, 527, 3719–3725. [Google Scholar] [CrossRef]
- Dispinar, D.; Campbell, J. Critical assessment of reduced pressure test. Part 2: Quantification. Int. J. Cast Met. Res. 2004, 17, 287–294. [Google Scholar] [CrossRef]
- Dispinar, D.; Campbell, J. Use of bifilm index as an assessment of liquid metal quality. Int. J. Cast Met. Res. 2006, 19, 5–17. [Google Scholar] [CrossRef]
- Dispinar, D.; Campbell, J. Effect of casting conditions on aluminium metal quality. J. Mater. Process. Technol. 2007, 182, 405–410. [Google Scholar] [CrossRef]
- Dispinar, D.; Campbell, J. Porosity, hydrogen and bifilm content in Al alloy castings. Mater. Sci. Eng. A 2011, 528, 3860–3865. [Google Scholar] [CrossRef]
- Dong, X.; He, L.; Huang, X.; Li, P. Effect of electromagnetic transport process on the improvement of hydrogen porosity defect in A380 aluminum alloy. Int. J. Hydrog. Energy 2015, 40, 9287–9297. [Google Scholar] [CrossRef]
- Drouzy, M.; Jacob, S.; Richard, M. Interpretation of Tensile Results by Means of Quality Index And Probable Yield Strength-Application to Al-Si7 Mg Foundry Alloys-France. Int. Cast Met. J. 1980, 5, 43–50. [Google Scholar]
- El-Sayed, M.; Hassanin, H.; Essa, K. Effect of casting practice on the reliability of Al cast alloys. Int. J. Cast Met. Res. 2016, 29, 350–354. [Google Scholar] [CrossRef]
- Liu, S.-G.; Cao, F.-Y.; Zhao, X.-Y.; Jia, Y.-D.; Ning, Z.-L.; Sun, J.-F. Characteristics of mold filling and entrainment of oxide film in low pressure casting of A356 alloy. Mater. Sci. Eng. A 2015, 626, 159–164. [Google Scholar] [CrossRef]
- Mutwil, J. An establish attempt of reasons of machining splinter formation in AC44200 alloy high pressure die castings. Arch. Foundry Eng. 2008, 8, 159–166. [Google Scholar]
- Zeng, J.; Li, D.; Kang, M.; He, H.; Hu, Z. Nanostructure of Aluminum Oxide Inclusion and Formation of Hydrogen Bubbles in Molten Aluminum. J. Nanosci. Nanotechnol. 2013, 13, 6948–6952. [Google Scholar] [CrossRef] [PubMed]
- Gruzleski, J.E.; Closset, B.M. The Treatment of Liquid Aluminum-Silicon Alloys; American Foundrymen’s Society, Inc.: Des Plaines, IL, USA, 1990; pp. 25–55. [Google Scholar]
- Harris, R.; Lipson, S.; Rosenthal, H. Tensile properties of aluminum–silicon magnesium alloys and the effect of sodium modification. AFS Trans. 1956, 64, 470–481. [Google Scholar]
- Shivkumar, S.; Ricci, S., Jr.; Steenhoff, B.; Apelian, D.; Sigworth, G. An experimental study to optimize the heat treatment of A356 alloy. AFS Trans. 1989, 97, 791–810. [Google Scholar]
- Fang, Q.; Granger, D. Porosity formation in modified and unmodified A356 alloy castings. AFS Trans. 1989, 97, 989–1000. [Google Scholar]
- Brosnan, M.; Shivkumar, S. Elevated-temperature tensile properties and fracture behavior of A 356 castings. Am. Foundrym. Soc. Inc. 1996, 727–737. [Google Scholar]
- Byczynski, G.; Cusinato, D. The effects of strontium and grain refiner additions on the fatigue and tensile properties of industrial Al-Si-Cu-Mg alloy castings produced using the Ford Motor Company—Cosworth precision sand process. Int. J. Cast Met. Res. 2002, 14, 315–324. [Google Scholar] [CrossRef]
- Gundlach, R.; Ross, B.; Hetke, A.; Valtierra, S.; Mojica, J. Thermal Fatigue Resistance of Hypoeutectic Aluminum-Silicon Casting Alloys (94–141). Trans. Am. Foundrym. Soc. Inc. 1994, 102, 205–224. [Google Scholar]
- Campbell, J.; Tiryakioğlu, M. Review of effect of P and Sr on modification and porosity development in Al–Si alloys. Mater. Sci. Technol. 2010, 26, 262–268. [Google Scholar] [CrossRef]
- Iwahori, H.; Yonekura, K.; Yamamoto, Y.; Nakamura, M. Occurring Behavior of Porosity and Feeding Capabilities of Sodium-and Strontium-Modified Al-Si Alloys. AFS Trans. 1990, 98, 167–173. [Google Scholar]
- Gruzleski, J.; Handiak, N.; Campbell, H.; Closset, B. Hydrogen measurement by Telegas in strontium treated A365 Metals. AFS Trans. 1986, 28, 147–154. [Google Scholar]
- Argo, D.; Gruzleski, J. Porosity in modified aluminum alloy castings. AFS Trans. 1988, 96, 65–74. [Google Scholar]
- Dinnis, C.; Dahle, A.; Taylor, J.; Otte, M. The influence of strontium on porosity formation in Al-Si alloys. Metall. Mater. Trans. A 2004, 35, 3531–3541. [Google Scholar] [CrossRef]
- Tiedje, N.S.; Taylor, J.A.; Easton, M.A. Feeding and distribution of porosity in cast Al-Si alloys as function of alloy composition and modification. Metall. Mater. Trans. A 2012, 43, 4846–4858. [Google Scholar] [CrossRef]
- Tiedje, N.S.; Taylor, J.A.; Easton, M.A. A new multi-zone model for porosity distribution in Al–Si alloy castings. Acta Mater. 2013, 61, 3037–3049. [Google Scholar] [CrossRef]
- Liao, H.; Wu, Y.; Fan, R.; Wang, Q. Effect of Sr content on porosity formation in directionally solidified Al-12.3 wt.% Si alloy. China Foundry 2014, 11, 435–439. [Google Scholar]
- Roy, N.; Zhang, L.; Louchez, P.; Samuel, F. Porosity formation in Al-9 wt% Si-3 wt% Cu-X alloy systems: Measurements of porosity. J. Mater. Sci. 1996, 31, 1243–1254. [Google Scholar] [CrossRef]
- Farhoodi, B.; Raiszadeh, R.; Ghanaatian, M.-H. Role of double oxide film defects in the formation of gas porosity in commercial purity and Sr-containing Al alloys. J. Mater. Sci. Technol. 2014, 30, 154–162. [Google Scholar] [CrossRef]
- El-Sayed, M.A.; Salem, H.A.; Kandeil, A.Y.; Griffiths, W. Effect of holding time before solidification on double-oxide film defects and mechanical properties of aluminum alloys. Metall. Mater. Trans. B 2011, 42, 1104–1109. [Google Scholar] [CrossRef]
- Haberl, K.; Schumacher, P.; Geier, G.; Stauder, B. Characterization of the melt quality and impurity content of an LM25 alloy. Metall. Mater. Trans. B 2009, 40, 812. [Google Scholar] [CrossRef]
- Kori, S.; Murty, B.; Chakraborty, M. Development of an efficient grain refiner for Al–7Si alloy and its modification with strontium. Mater. Sci. Eng. A 2000, 283, 94–104. [Google Scholar] [CrossRef]
- Yuan, L.; Chao, D.; Li, Y.-X. Grain refining mechanism of Al-3B master alloy on hypoeutectic Al-Si alloys. Trans. Nonferr. Met. Soc. China 2011, 21, 1435–1440. [Google Scholar]
- Dispinar, D.; Nordmark, A.; Voje, J.; Arnberg, L. Influence of Hydrogen Content And Bi-Film Index on Feeding Behaviour of Al-7Si. In Proceedings of the 138th TMS Annual Meeting, Shape Casting: 3rd International Symposium, San Francisco, CA, USA, 15–19 February 2009; pp. 63–70. [Google Scholar]
- Arnberg, L.; Bäckerud, L.; Klang, H. 1: Production and properties of master alloys of Al–Ti–B type and their ability to grain refine aluminium. Met. Technol. 1982, 9, 1–6. [Google Scholar] [CrossRef]
- Schaffer, P.L.; Dahle, A.K. Settling behaviour of different grain refiners in aluminium. Mater. Sci. Eng. A 2005, 413, 373–378. [Google Scholar] [CrossRef]
- Anyalebechi, P.N. Hydrogen-induced gas porosity formation in Al–4.5 wt% Cu–1.4 wt% Mg alloy. J. Mater. Sci. 2013, 48, 5342–5353. [Google Scholar] [CrossRef]
- Fakhraei, O.; Emamy, M.; Farhangi, H. The effect of Al–5Ti–1B grain refiner on the structure and tensile properties of Al–20% Mg alloy. Mater. Sci. Eng. A 2013, 560, 148–153. [Google Scholar] [CrossRef]
- Lee, J.-M.; Lee, S.-H.; Yoon, J.-H.; Kim, K.-H. Effects of Melt Treatments on Microstructures and Mechanical Properties of A357 Alloy. J. Korea Foundry Soc. 2003, 23, 69–76. [Google Scholar]
- Eskin, D.; Alba-Baena, N.; Pabel, T.; da Silva, M. Ultrasonic degassing of aluminium alloys: Basic studies and practical implementation. Mater. Sci. Technol. 2015, 31, 79–84. [Google Scholar] [CrossRef]
- Lee, C.; So, T.; Shin, K. Effect of Gas Bubbling Filtration Treatment on Microporosity Variation in A356 Aluminium Alloy. Acta Metall. Sin. 2016, 29, 638–646. [Google Scholar] [CrossRef]
- Puga, H.; Barbosa, J.; Seabra, E.; Ribeiro, S.; Prokic, M. The influence of processing parameters on the ultrasonic degassing of molten AlSi9Cu3 aluminium alloy. Mater. Lett. 2009, 63, 806–808. [Google Scholar] [CrossRef]
- Dispinar, D.; Campbell, J. Reduced Pressure Test (RPT) for Bifilm Assessment. In Proceedings of the Shape Casting: 5th International Symposium, San Diego, CA, USA, 16–20 February 2014; pp. 243–251. [Google Scholar]
- Akhtar, S.; Arnberg, L.; di Sabatino, M.; Dispinar, D.; Syvertsen, M. A comparative study of porosity and pore morphology in a directionally solidified A356 alloy. Int. J. Metalcast. 2009, 3, 39–52. [Google Scholar] [CrossRef]
- Akhtar, S.; Dispinar, D.; Arnberg, L.; di Sabatino, M. Effect of hydrogen content, melt cleanliness and solidification conditions on tensile properties of A356 alloy. Int. J. Cast Met. Res. 2009, 22, 22–25. [Google Scholar] [CrossRef]
- Çolak, M.; Kayikci, R.; Dispinar, D. Melt Cleanliness Comparison of Chlorine Fluxing and Ar Degassing of Secondary Al-4Cu. Metall. Mater. Trans. B 2016, 47, 2705–2709. [Google Scholar] [CrossRef]
- Koca, E.; Yuksel, C.; Erzi, E.; Dışpınar, D. Quality Assessment of A356 Ingots from Different Suppliers in Wheel Production. In Proceedings of the Shape Casting: 6th International Symposium, Nashville, TN, USA, 14–18 February 2016; pp. 77–83. [Google Scholar]
- Ludwig, T.; di Sabatino, M.; Arnberg, L.; Dispinar, D. Influence of oxide additions on the porosity development and mechanical properties of A356 aluminium alloy castings. Int. J. Metalcast. 2012, 6, 41–50. [Google Scholar] [CrossRef]
- Dispinar, D.; Istanbul University, Istanbul, Turkey. Unpublished research. 2017.
- Liu, L.; Samuel, A.; Samuel, F.; Doty, H.; Valtierra, S. Influence of oxides on porosity formation in Sr-treated Al-Si casting alloys. J. Mater. Sci. 2003, 38, 1255–1267. [Google Scholar] [CrossRef]
- Nateghian, M.; Raiszadeh, R.; Doostmohammadi, H. Behavior of Double-Oxide Film Defects in Al-0.05 wt pct Sr Alloy. Metall. Mater. Trans. B 2012, 43, 1540–1549. [Google Scholar] [CrossRef]
- Yao, L.; Cockcroft, S.; Reilly, C.; Zhu, J. Factors affecting the nucleation kinetics of microporosity formation in aluminum alloy A356. Metall. Mater. Trans. A 2012, 43, 1004–1016. [Google Scholar] [CrossRef]
- Dvorak, H.; Schwegtier, E. Statistical distribution of flaw sizes. Int. J. Fract. Mech. 1972, 8, 110–111. [Google Scholar] [CrossRef]
- Tiryakioğlu, M. Pore size distributions in AM50 Mg alloy die castings. Mater. Sci. Eng. A 2007, 465, 287–289. [Google Scholar] [CrossRef]
- Tiryakioğlu, M. On fatigue life variability in cast Al–10% Si–Mg alloys. Mater. Sci. Eng. A 2010, 527, 1560–1564. [Google Scholar] [CrossRef]
- Anderson, T.W.; Darling, D.A. A test of goodness of fit. J. Am. Stat. Assoc. 1954, 49, 765–769. [Google Scholar] [CrossRef]

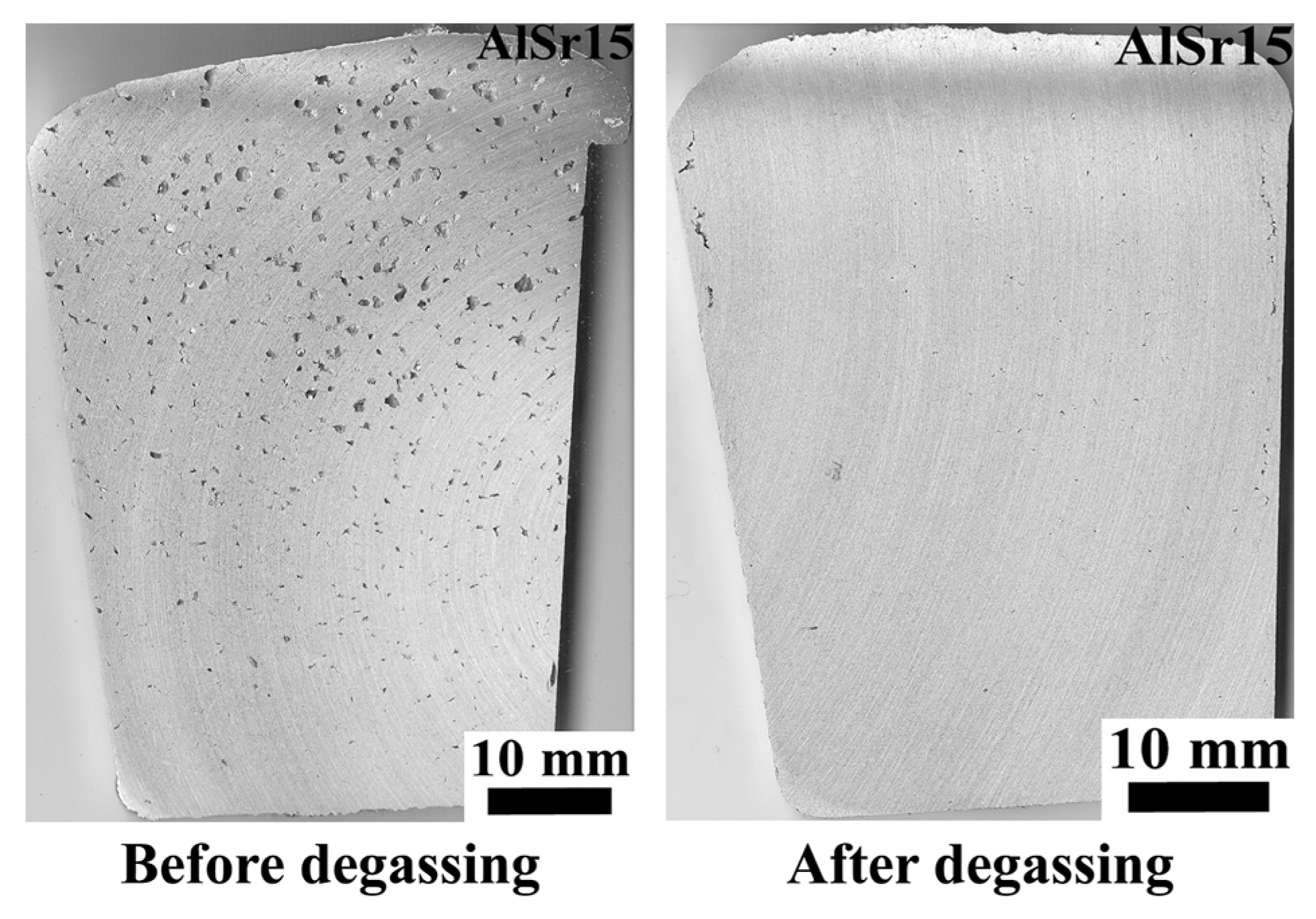
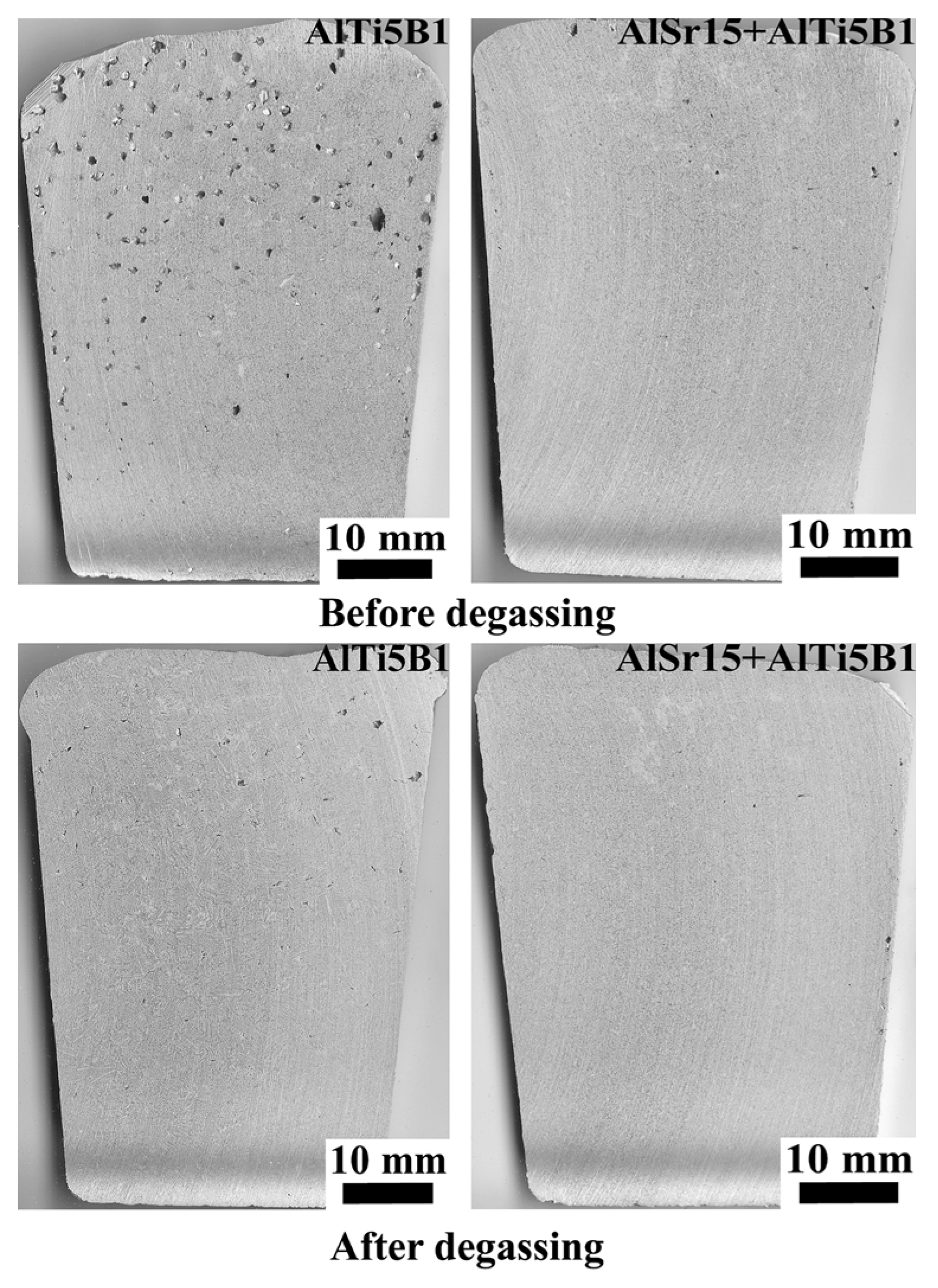
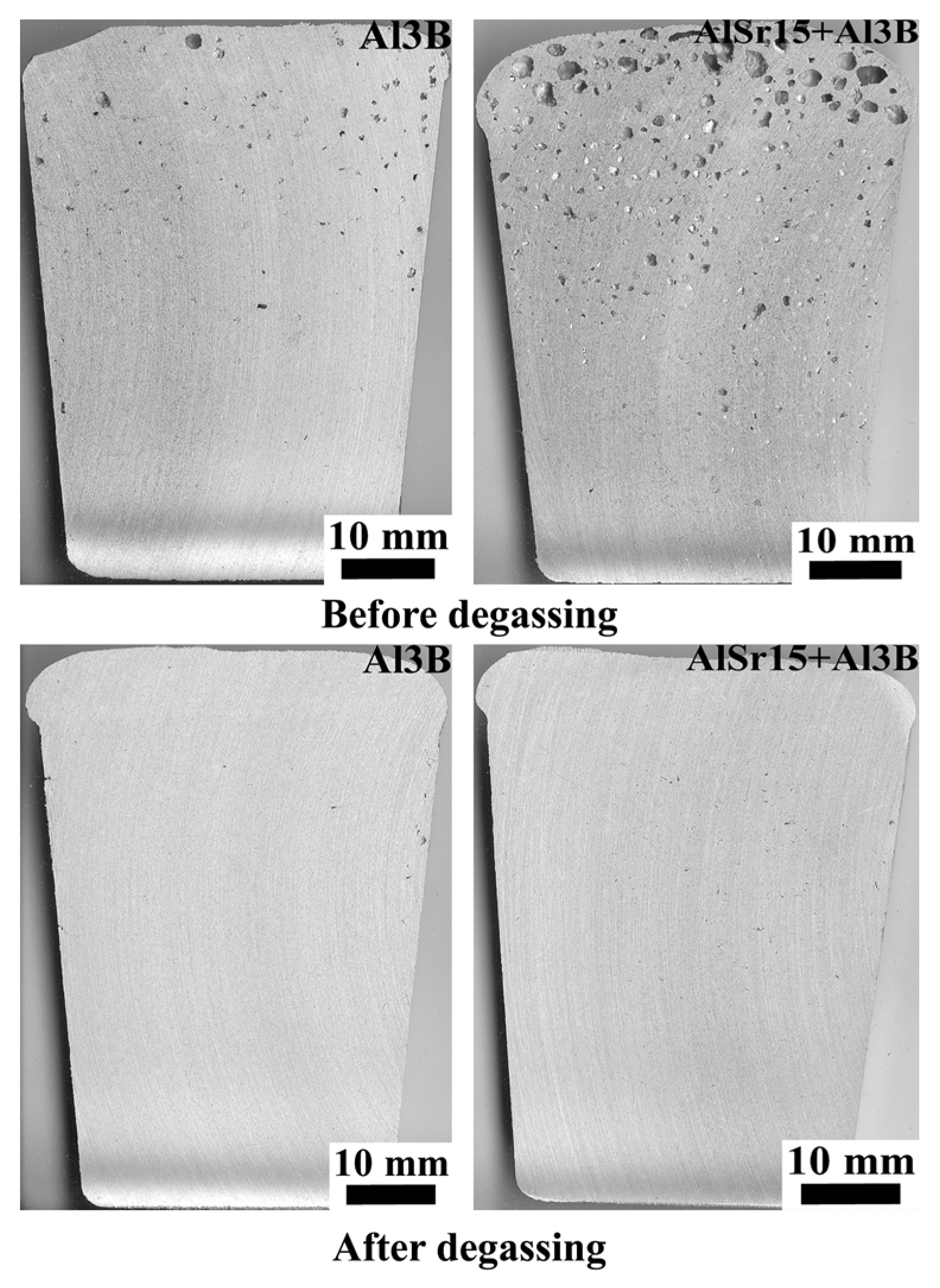

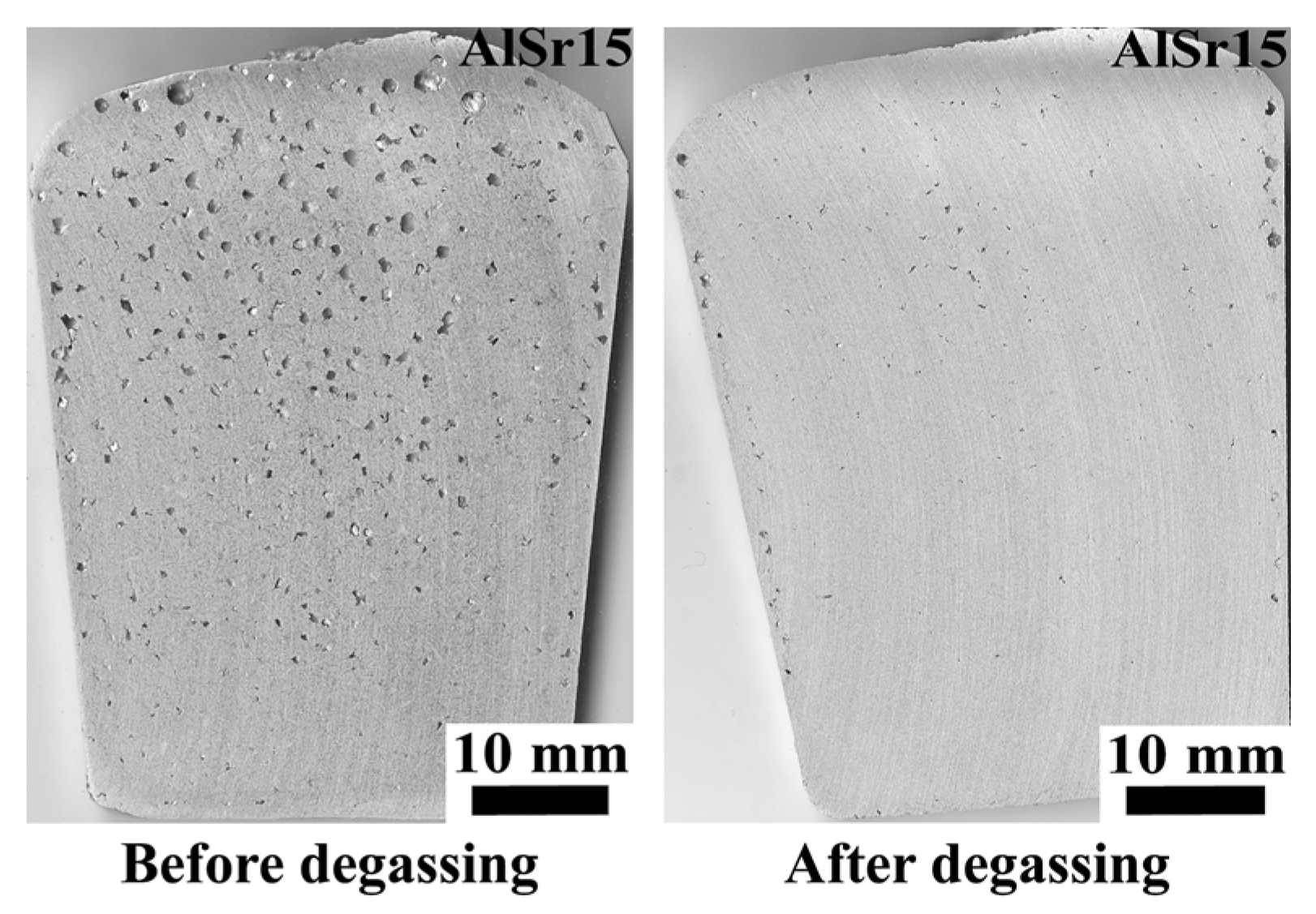
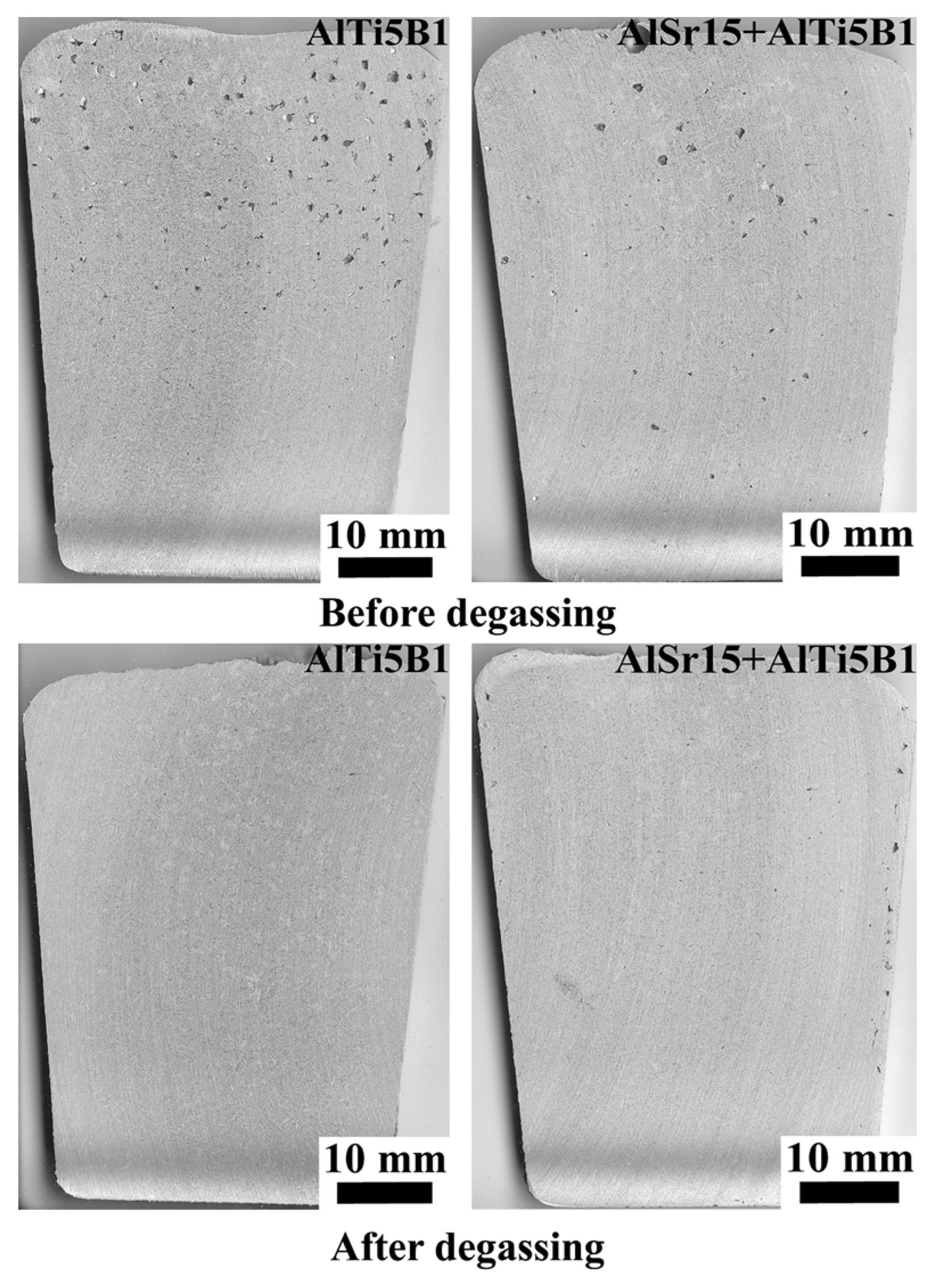
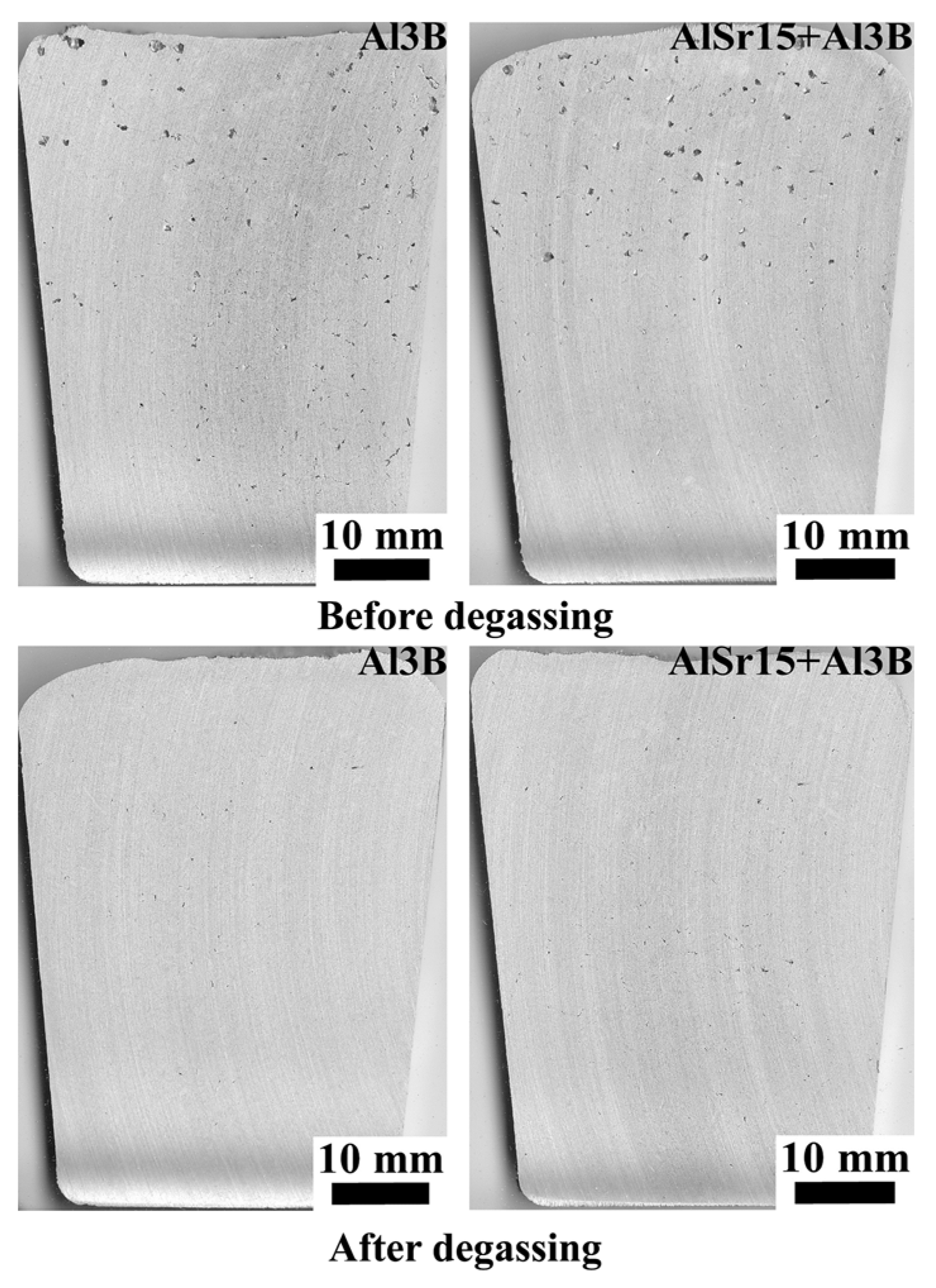


| Alloy | Si | Fe | Cu | Mn | Mg | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| A356 | 6.80 | 0.19 | 0.003 | 0.001 | 0.30 | 0.011 | 0.108 | Rem. |
| Master Alloys | Ti | Sr | B | Fe | Si | Ca | Al |
|---|---|---|---|---|---|---|---|
| AlSr15 | - | 14–15 | - | ≤ 0.2 | ≤ 0.2 | ≤ 0.2 | Rem. |
| AlTi5B1 | 5 | - | 1 | ≤ 0.2 | ≤ 0.2 | - | Rem. |
| Al3B | - | - | 2.5–3.5 | 0.3 | 0.2 | - | Rem. |
| Parameter | Levels |
|---|---|
| Degassing | No degassing; degassing |
| Holding time (min) | 10; 15; 20; 25; 30 |
| Melt additions | AlSr15; AlSr15 + AlTi5B1; AlTi5B1; Al3B; AlSr15 + Al3B |
| Degassing | Additions | μ | σ | ||
|---|---|---|---|---|---|
| 10 min | 30 min | 10 min | 30 min | ||
| No Degassing | No addition | 13.48 | 12.43 | 0.81 | 0.78 |
| AlSr15 | 13.08 | 11.96 | 0.71 | 1.02 | |
| AlSr15 + AlTi5B1 | 11.97 | 11.30 | 0.90 | 0.69 | |
| AlTi5B1 | 11.89 | 11.20 | 0.96 | 0.47 | |
| Al3B | 11.98 | 11.52 | 0.93 | 0.82 | |
| AlSr15 + Al3B | 12.06 | 11.90 | 1.15 | 0.72 | |
| Degassed | No addition | 12.59 | 12.67 | 0.73 | 0.79 |
| AlSr15 | 11.60 | 11.51 | 0.82 | 0.84 | |
| AlSr15 + AlTi5B1 | 11.94 | 11.24 | 1.03 | 0.67 | |
| AlTi5B1 | 11.79 | 11.56 | 0.93 | 1.32 | |
| Al3B | 11.70 | 11.91 | 0.82 | 1.00 | |
| AlSr15 + Al3B | 11.46 | 11.62 | 0.70 | 0.84 | |
| Degassing | Additions | (cm−2) | |||
|---|---|---|---|---|---|
| 10 min | 30 min | 10 min | 30 min | ||
| No Degassing | No addition | 6.10 | 2.48 | 917 | 544 |
| AlSr15 | 11.62 | 17.19 | 739 | 454 | |
| AlSr15 + AlTi5B1 | 5.30 | 19.51 | 440 | 301 | |
| AlTi5B1 | 14.13 | 32.06 | 432 | 278 | |
| Al3B | 6.40 | 13.23 | 451 | 346 | |
| AlSr15 + Al3B | 26.96 | 10.09 | 490 | 409 | |
| Degassed | No addition | 0.86 | 0.75 | 579 | 645 |
| AlSr15 | 6.51 | 2.04 | 361 | 349 | |
| AlSr15 + AlTi5B1 | 2.59 | 6.04 | 449 | 295 | |
| AlTi5B1 | 1.49 | 2.00 | 406 | 402 | |
| Al3B | 1.73 | 1.73 | 388 | 438 | |
| AlSr15 + Al3B | 1.77 | 1.84 | 328 | 365 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uludağ, M.; Çetin, R.; Dispinar, D.; Tiryakioğlu, M. Characterization of the Effect of Melt Treatments on Melt Quality in Al-7wt %Si-Mg Alloys. Metals 2017, 7, 157. https://doi.org/10.3390/met7050157
Uludağ M, Çetin R, Dispinar D, Tiryakioğlu M. Characterization of the Effect of Melt Treatments on Melt Quality in Al-7wt %Si-Mg Alloys. Metals. 2017; 7(5):157. https://doi.org/10.3390/met7050157
Chicago/Turabian StyleUludağ, Muhammet, Remzi Çetin, Derya Dispinar, and Murat Tiryakioğlu. 2017. "Characterization of the Effect of Melt Treatments on Melt Quality in Al-7wt %Si-Mg Alloys" Metals 7, no. 5: 157. https://doi.org/10.3390/met7050157





